ABSTRACT
Expressed by cancer stem cells of various epithelial cell origins and hepatocellular carcinoma (HCC), CD133 is an attractive therapeutic target for HCC. The marker CD133 is highly expressed in endothelial progenitor cells (EPC). EPCs circulate in increased numbers in the peripheral blood of patients with highly vascularized HCC and contribute to angiogenesis and neovascularization. This phase II study investigated CD133-directed chimeric antigen receptor (CAR) T (CART-133) cells in adults with HCC. Patients with histologically confirmed and measurable advanced HCC and adequate hematologic, hepatic, and renal functions received CART-133 cell infusions. The primary endpoints were safety in phase I and progression-free survival (PFS) and overall survival (OS) in phase II. Other endpoints included biomarkers for CART-133 T cell therapy. Between June 1, 2015, and September 1, 2017, this study enrolled 21 patients who subsequently received CART-133 T cells across phases I and II. The median OS was 12 months (95% CI, 9.3–15.3 months) and the median PFS was 6.8 months (95% CI, 4.3–8.4 months). Of 21 evaluable patients, 1 had a partial response, 14 had stable disease for 2 to 16.3 months, and 6 progressed after T-cell infusion. The most common high-grade adverse event was hyperbilirubinemia. Outcome was correlated with the baseline levels of vascular endothelial growth factor (VEGF), soluble VEGF receptor 2 (sVEGFR2), stromal cell-derived factor (SDF)-1, and EPC counts. Changes in EPC counts, VEGF, SDF-1, sVEGFR2, and interferon (IFN)-γ after cell infusion were associated with survival. In patients with previously treated advanced HCC, CART-133 cell therapy demonstrates promising antitumor activity and a manageable safety profile. We identified early changes in circulating molecules as potential biomarkers of response to CART-133 cells. The predictive value of these proangiogenic and inflammatory factors as potential biomarkers of CART-133 cell therapy in HCC will be explored in prospective trials. This study is registered at ClinicalTrials.gov (NCT02541370)
Introduction
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death globally; It causes nearly one million deaths of patients every year, and 50% of the world’s new HCC patients each year come from China.Citation1 Despite some systemic therapy has improved the survival of patients with advanced HCC,Citation2 the outcomes of most patients remain poor.Citation3–7 Thus additional treatment options for advanced HCC are clearly needed.
One characteristic feature of HCC is abundant angiogenesis and tumor neovascularization. Emerging evidence supports the role of angiogenesis in hepatocarcinogenesis and suggests the potential for inhibiting this pathway as a therapeutic strategy in HCC.Citation8–11 Tumor angiogenesis is dependent on the recruitment and proliferation of endothelial cells from surrounding existing vessels or the systemic mobilization of bone marrow-derived endothelial progenitor cells (EPCs), which home to sites of angiogenesis.Citation12–14 In the clinic, the numbers of circulating EPCs are positively correlated with the advanced, invasive stages of HCC,Citation13,Citation15,Citation16 and mobilized EPCs participate in HCC vasculogenesis.Citation16,Citation17 A reduced number of circulating EPCs after CART-133 cell therapy may indicate a better clinical outcome.
CD133 is frequently expressed in HCC,Citation18–20 and CD133 overexpression in HCC often corresponds with a poor patient outcome.Citation21,Citation22 Moreover, CD133 is considered a cancer stem cell marker and a more specific EPC marker.Citation23–25 Therefore, CD133 is a novel and promising target in the treatment of HCC, and several drugs have been developed to selectively target CD133.Citation26 The present study is a phase II, open-label, single-arm prospective examination of the effect of CD133-directed chimeric antigen receptor (CAR) T (CART-133) cells in patients with advanced HCC.
Methods
Study design
This was a single-center, open-label, phase II clinical trial (ClinicalTrials.gov identified, NCT02541370) at the Chinese PLA General Hospital. The study protocols were approved by the institutional review board at the Chinese PLA General Hospital, and the patients provided written informed consent. This clinical investigation was conducted in accordance with good clinical practice and the Declaration of Helsinki. There was no commercial support for this study.
Patients were treated with 0.5 × 106 to 2 × 106 autologous CAR T cells per kilogram of body weight. The dose was selected based on the previous dose escalation study. Patients were eligible to receive an additional cell treatment cycle unless there were new tumor lesions or intolerable toxicities. The second treatment cycle was performed at least 4 weeks after the first infusion. The initial results from the first 14 patients treated in this study were previously published.Citation27 The current report describes the longer-term clinical outcomes of these patients and an additional 7 treated subjects.
Computed tomography (CT) with contrast or magnetic resonance imaging (MRI) was used to assess the tumor response to treatment 1 month after the cell infusion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Adverse events were graded in accordance with the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, and the causal association with study drug was determined by the investigators. Cytokine release syndrome (CRS) graded and managed based on a previous report by Lee et al.Citation28
Patient eligibility
Eligible patients were at least 18-y old and had biopsy-proven HCC that was not amenable to curative treatment. Eligible patients showed disease progression after at least one systemic treatment for HCC. Primary exclusion criteria were severe organ dysfunction, a history of, or active systemic autoimmune/immunodeficiency disease, and a treatment history of immunosuppressive agents or glucocorticoids within a month of the study enrollment.
Study objectives
The primary endpoint of this study was progression-free survival (PFS). Secondary objectives were overall survival and safety and tolerability using the NCI CTCAE version 5.0. Exploratory efficacy endpoints were to evaluate the ability of biomarkers to predict PFS and OS.
CAR T cell production
CAR T cells were produced by directly adding the anti-CD3 monoclonal antibody OKT3 to whole PBMCs suspended in culture medium containing interleukin (IL)-2 as described in previously.Citation27 Lentiviral transduction was performed as described.Citation27
Correlative studies
Peripheral blood (PB) was obtained from all enrolled patients to evaluate early changes in circulating proangiogenic and proinflammatory molecules and cells. We characterized live EPC by exclusion of a viability dye and a CD45low CD146+ CD133+ CD31+ CD34+ phenotype.Citation29 Blood samples were collected in EDTA-containing tubes before cell therapy 7 and 14 d after the first cell infusion. bFGF, PDGF BB, sVEGFR-2, VEGF, and SDF-1 levels were measured using commercially available sandwich enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN). Biomarker levels measured on quantitative scales were log-transformed and changes were calculated as the ratios of on-study to baseline values.
Statistical analyses
Published historical outcomes of recurrent HCC included a median PFS of 5.2 months and a median OS of 10.2 months.Citation4 This phase II study was designed to detect an increase in 6-months PFS from 40% to 50%.
Changes in biomarker levels, expressed as ratios of on-study to baseline values, were tested using exact paired Wilcoxon test. Survival curves were estimated using the Kaplan–Meier method. Associations of biomarkers with OS and PFS were assessed using univariate Cox regression models. The safety, tolerability, and adverse events were summarized using descriptive statistics. Statistical analysis was performed with SPSS (IBM Statistics, version 25.0). A two-side P value of less than 0.05 indicates statistical significance.
Results
Demographics
Baseline characteristics of the enrolled patients are summarized in and Supplement Table 1. All patients had received prior systemic therapy including 16 patients who had received prior sorafenib. Three patients (14.3%) had stage III disease and 18 patients (85.7%) had stage IV disease. Nine patients (42.9%) had Child-Pugh score A and 12 (57.1%) had a Child-Pugh B. All patients had BCLC stage C.
Table 1. Clinical characteristics of patients
Safety and in vivo detection of CAR T cells
Twenty-one patients received a total of 39 infusions, with 9 patients receiving multiple infusions (Supplement Table 2). Grade 3 toxicities lasting 3 weeks with hyperbilirubinemia (direct bilirubin) occurred in four patients who had an obstructed of the biliary tract accompanied and a high bilirubin before cell infusion. All adverse events are summarized in . Hematologic toxicities generally occurred 3 to 5 d after cell infusion and self-resolved within 1 week. The highest frequency of CART-133 cells was observed between 7 and 21 d after infusion in nearly all patients. As previously reported,Citation27 PCR signals derived from CAR T cells were declined by 8 weeks after infusion; 10 of 13 samples were positive at 3 months, 8 of 10 samples were positive at 6 months, 5 of 6 samples were positive at 9 months, 2 of 4 samples were positive at 12 months, and no samples were positive at 18 or 24 months. These results indicate that CART-133 cells can persist at a low frequency for 1 y (Figure. S1).
Table 2. Adverse events within the first 4 weeks after CART-133 cell infusion
Outcome
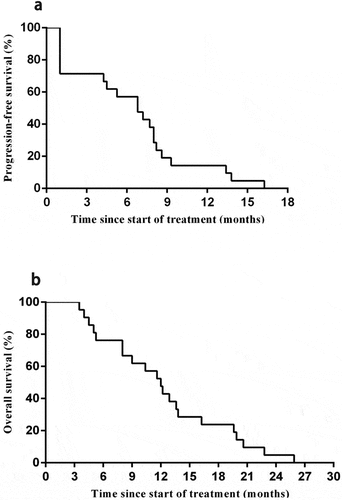
As of the cutoff date of Oct 1, 2019, 21 patients assessable for activity in phase 2 were followed up. One (4.8%; patient 8) had a partial response, and 14 (66.7%) had a stable disease after the first cell infusion. For the entire study cohort, the median PFS was 6.8 months ((95% CI, 4.3–8.4 months) ()), and the median OS was 12 months (95% CI, 9.3–15.3 months) after the first CART-133 cell infusion ()).
Correlative studies
In line with the result of a previous study,Citation27 there was a significant increase in the level of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IFN-γ at 1 and 2 weeks after the first cell infusion (Figure S2, ). During the first 4 weeks of treatment, VEGF and SDF-1 levels significantly increased from baseline, whereas sVEGFR2 and PDGF BB levels and EPCs count significantly decreased ().
Table 3. Evolution of biomarkers on CART-133 treatment
At baseline, higher plasma levels of sVEGFR2 and SDF-1 were significantly correlated with a longer OS and the higher EPC counts were significantly correlated with a shorter OS after the first cell infusion. In addition, a higher VEGF level was significantly correlated with a longer OS and PFS (). The association between CART-133 cell treatment outcome measures (OS and PFS) and biomarkers was explored based on baseline levels and early changes. The extent of the increase in SDF-1 and EPC counts was significantly associated with longer OS. The smaller the decrease in sVEGFR2 was the longer OS (). In addition, PFS was directly associated with changes in circulating VEGF, whereas PFS was inversely associated with changes in IFN-γ (). None of the other serum markers, including free PDGF BB, IL-6, and IL-8, showed any correlation with PFS or OS (data not shown).
Table 4. Significant correlations between overall survival (OS) and progression-free survival (PFS) after CART-133 cell treatment in advanced HCC patients with pretreatment and on-treatment changes (normalized to baseline values) in biomarkers (in bold text)
Discussion
The result from this phase II study provides preliminary evidence that CART-133 cells have antitumor activity and a manageable safety profile in advanced HCC. The median OS was 12 months, and the median PFS was 6.8 months, which are an encouraging findings for this advanced-stage population with a high incidence of extrahepatic disease and prior local treatment. In addition, the data support the manageable safety profile of CART-133 cells.
Chimeric antigen receptor T-cell therapies are an attractive strategy to improve the outcomes of patients with HCC. Our findings agree with those of two other studies, in which T cells modified with an anti-CEA CAR were shown to be safe in patients with HCC without significant adverse effects and to produce objective tumor responses.Citation30,Citation31 However, our studies demonstrated that CART-133 cell therapy improved the clinical outcomes of patients with advanced HCC compared with the treatment regimen in these two previous studies. The difference in the observed outcomes may be a result of several factors. In this study, we used CD133 as the target, administered a higher maximum dose of cells, observed CAR T cell expansion, and used the CD137 signaling domain.
CD133 is currently the most mature surface marker on EPCs,Citation25 which circulate in increased numbers in the PB of patients with highly vascularized HCC and contribute to angiogenesis and neovascularization.Citation32 Antiangiogenic therapy has become a standard and widely used treatment modality for cancers, but inducing a prolonged OS with angiostatic or vascular targeted drugs is still a challenge;Citation33 antigangiogenesis strategies are not efficient and appear to be prone to drug-induced resistance, as the most prominent targets are the tumor cells themselves.Citation34 Therefore, we posit that a better strategy would be to directly target not only the tumor cells but also the vascular lining through specific EPC markers. In agreement with this hypothesis, we observed a marked decrease in the number of EPCs after cell infusion, and a lower number of EPCs after the first cell infusion indicated a longer PFS. Targeting CAR T cells toward the tumor vasculature of solid tumors rather than the tumor directly may serve as another method to target multiple tumor types.Citation35,Citation36 Compared to GPC3-targeted CAR T cells,Citation37,Citation38 CD133-targeted CAR T cells may not only compromise the nutrient supply of the tumor, it might also serve as an approach for improving infiltration of the tumor site for small-molecule drugs and other therapies that can be used in combination with the CAR T cells, even if only transiently. The combination therapy of CAR T cells with vaccines, biomaterials, and oncolytic viruses in preclinical models have achieved desired outcomes,Citation39 indicating the potential for translation into clinical applications, and more clinical studies to evaluate the effects of combinatorial treatment strategies are needed in the future.
The expansion of CART-133 cells in PB is inconsistent with previous reportsCitation40,Citation41 on the infusion of CAR T cells into patients with solid tumors. Conditioning chemical or radiological depletion of lymphocytes can lead to the enhanced engraftment of infused cells; the patients enrolled in our study did not receive conditioning treatment. Other studies have observed CD19-directed CAR T-cell expansion without prior lymphodepletion.Citation42,Citation43 The observed CART-133 cell expansion in this study may in part be attributable to the presence of the target cells in PB. CD133 is expressed by EPCs in PB,Citation44,Citation45 which may provide additional activation and expansion signals to CART-133 cells.
We evaluated EPCs and multiple plasma molecules that have been implicated in tumor angiogenesis and found significant associations between plasma biomarkers and outcome; these findings are consistent with previous data on antiangiogenic therapy.Citation46,Citation47 At baseline, high levels of VEGF and SDF-1, possibly reflecting hypoxia, and a low level of sVEGFR2 seem to be associated with a worse outcome. These data indicate that tumor hypoxia may be detectable in circulation based on the expression of hypoxia-induced factors such as VEGF, sVEGFR2, and SDF-1, which could be biomarkers of poor prognosis in HCC. Finally, an increase in circulating IFN-γ was significantly associated with a longer PFS. This finding suggests the possibility of an antitumor immune response elicited by CART-133 cell therapy which is consistent with previous results after administering anti-CEA CAR T cells to advanced HCC patients.Citation31 These observations are consistent with biomarker data from studies of sunitinib in HCC and cediranib in glioblastoma,Citation46,Citation47 and should be validated in preclinical studies and larger clinical studies. Albeit promising, these exploratory results need to be confirmed in larger randomized studies, which should also establish if any of these biomarkers have prognostic or predictive value.
Immune checkpoint inhibitors-based treatments have recently achieved promising outcome in unresectable HCC patients. However, despite promising results from clinical studies, only few patients benefit from immune checkpoint inhibitors.Citation48,Citation49 Combining immune checkpoint inhibitors with CAR T cell therapy provides a synergetic approach to optimizing the rate, depth, and durability of clinical responses;Citation50 future trials to test such approaches are under development.
Although several patients had stable disease and one patient had a partial response after CART-133 cell infusion, no radiologic complete responses were observed, indicating that further manipulation of the immune system will be essential to achieve worthwhile benefits. One of the main therapeutic goals of immuno-oncology research is to convert cold tumors into immunogenic tumors.Citation51 Antiangiogenics and immunotherapy represent the main strategies for the treatment of advanced HCC.Citation52 One possibility is to combine CAR T cells with one or more checkpoint antibodies now becoming available or with cancer immunotherapy to potentially increase T cell activation and prolong persistence in vivo. Additionally, combination therapies involving antiangiogenics may be synergistic, because VEGF inhibition increases the intratumoral infiltration and survival of cytotoxic T lymphocytes and decreases regulatory T lymphocyte recruitment, resulting in a more favorable immune microenvironment for antitumor activity.Citation52
Conclusion
The results of this clinical trial inform the therapeutic potential of CART-133 cell therapy in patients with advanced HCC who have few existing treatment options, setting the stage for studies combining CAR T cells with other immunomodulatory and antiangiogenic approaches to improve clinical outcome. Exploratory studies further suggested potential response biomarkers of CART-133 cell therapy. Among the current targeted therapies, CART-133 cell therapy might provide favorable efficacy with a good safety profile.
Authors’ contributions
Study conception and design: Y. Wang, P. Shen, H. Dai;
Data collection: H. Dai, C. Tong. D. Shi;
Analysis and interpretation of data: M. Chen, X. Han, D. Chen;
Writing and critical revision of the draft: Y. Wang, H. Dai, P. Shen;
Administrative, technical, or material support: M. Chen, X. Han, D. Chen, Y. Guo, C. Tong;
Study supervision: H. Wang, Y. Wang, P. Shen.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Informed consent was obtained from all participants. They were reviewed and approved by the Ethics Review Committee at the Chinese PLA General Hospital (Beijing, China).
Supplemental Material
Download ()Acknowledgments
We thank the patients and their families who participated in this study. And we also thank all physicians, nurse, and other patient care providers involved in the care of these patients.
Data availability statement
All of the data and materials are available from the corresponding author upon reasonable ask.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- F. Bray, J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, A. Jemal. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–8. doi:10.3322/caac.21492.
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001.
- G.K. Abou-Alfa, T. Meyer, A.L. Cheng, A.B. El-Khoueiry, L. Rimassa, B.Y. Ryoo, I. Cicin, P. Merle, Y. Chen, J.W. Park, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002.
- J.M. Llovet, S. Ricci, V. Mazzaferro, P. Hilgard, E. Gane, J.F. Blanc, A.C. de Oliveira, A. Santoro, J.L. Raoul, A. Forner, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857.
- M. Kudo, R.S. Finn, S. Qin, K.H. Han, K. Ikeda, F. Piscaglia, A. Baron, J.W. Park, G. Han, J. Jassem, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1.
- J. Bruix, S. Qin, P. Merle, A. Granito, Y.H. Huang, G. Bodoky, M. Pracht, O. Yokosuka, O. Rosmorduc, V. Breder, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9.
- A.B. El-Khoueiry, B. Sangro, T. Yau, T.S. Crocenzi, M. Kudo, C. Hsu, T.Y. Kim, S.P. Choo, J. Trojan, T.H.R. Welling, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2.
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi:10.1038/nrc1782.
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631.
- De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9(6):789–795. doi:10.1038/nm871.
- F. Shojaei, X. Wu, A.K. Malik, C. Zhong, M.E. Baldwin, S. Schanz, G. Fuh, H.P. Gerber, N. Ferrara. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–920. doi:10.1038/nbt1323.
- Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004;8(3):294–300. doi:10.1111/j.1582-4934.2004.tb00319.x.
- J.W. Ho, R.W. Pang, C. Lau, C.K. Sun, W.C. Yu, S.T. Fan, R.T. Poon. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44(4):836–843. doi:10.1002/hep.21353.
- D. Gao, D.J. Nolan, A.S. Mellick, K. Bambino, K. McDonnell, V. Mittal. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi:10.1126/science.1150224.
- Y. Shaked, E. Henke, J.M. Roodhart, P. Mancuso, M.H. Langenberg, M. Colleoni, L.G. Daenen, S. Man, P. Xu, U. Emmenegger, T. Tang, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14(3):263–273. doi:10.1016/j.ccr.2008.08.001.
- D. Yu, X. Sun, Y. Qiu, J. Zhou, Y. Wu, L. Zhuang, J. Chen, Y. Ding. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13(13):3814–3824. doi:10.1158/1078-0432.CCR-06-2594.
- X.T. Sun, X.W. Yuan, H.T. Zhu, Z.M. Deng, D.C. Yu, X. Zhou, Y.T. Ding. Endothelial precursor cells promote angiogenesis in hepatocellular carcinoma. World J Gastroenterol. 2012;18(35):4925–4933. doi:10.3748/wjg.v18.i35.4925.
- W. Song, H. Li, K. Tao, R. Li, Z. Song, Q. Zhao, F. Zhang, K. Dou. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62(8):1212–1218. doi:10.1111/j.1742-1241.2008.01777.x.
- C. Won, B.H. Kim, E.H. Yi, K.J. Choi, E.K. Kim, J.M. Jeong, J.H. Lee, J.J. Jang, J.H. Yoon, W.I. Jeong, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62(4):1160–1173. doi:10.1002/hep.27968.
- L.M. Smith, A. Nesterova, M.C. Ryan, S. Duniho, M. Jonas, M. Anderson, R.F. Zabinski, M.K. Sutherland, H.P. Gerber, K.L. Van Orden, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99(1):100–109. doi:10.1038/sj.bjc.6604437.
- K. Kohga, T. Tatsumi, T. Takehara, H. Tsunematsu, S. Shimizu, M. Yamamoto, A. Sasakawa, T. Miyagi, N. Hayashi. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J Hepatol. 2010;52(6):872–879. doi:10.1016/j.jhep.2009.12.030.
- X.R. Yang, Y. Xu, B. Yu, J. Zhou, S.J. Qiu, G.M. Shi, B.H. Zhang, W.Z. Wu, Y.H. Shi, B. Wu, G.H. Yang, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59(7):953–962. doi:10.1136/gut.2008.176271.
- Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8(4):498–508. doi:10.1111/j.1582-4934.2004.tb00474.x.
- M. Peichev, A.J. Naiyer, D. Pereira, Z. Zhu, W.J. Lane, M. Williams, M.C. Oz, D.J. Hicklin, L. Witte, M.A. Moore, S. Rafii. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958.
- Y. Duan, S. Yu, P. Xu, X. Wang, X. Feng, Z. Mao, C. Gao. Co-immobilization of CD133 antibodies, vascular endothelial growth factors, and REDV peptide promotes capture, proliferation, and differentiation of endothelial progenitor cells. Acta Biomater. 2019;96:137–148. doi:10.1016/j.actbio.2019.07.004.
- Schmohl JU, Vallera DA. CD133, selectively targeting the root of cancer.
- Y. Wang, M. Chen, Z. Wu, C. Tong, H. Dai, Y. Guo, Y. Liu, J. Huang, H. Lv, C. Luo, et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology. 2018;7(7):e1440169. doi:10.1080/2162402X.2018.1440169.
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729.
- S.G. Kalathil, A.A. Lugade, R. Iyer, A. Miller, Y. Thanavala. Endothelial progenitor cell number and ERK phosphorylation serve as predictive and prognostic biomarkers in advanced hepatocellular carcinoma patients treated with sorafenib. Oncoimmunology. 2016;5(10):e1226718. doi:10.1080/2162402X.2016.1226718.
- F.C. Thistlethwaite, D.E. Gilham, R.D. Guest, D.G. Rothwell, M. Pillai, D.J. Burt, A.J. Byatte, N. Kirillova, J.W. Valle, S.K. Sharma, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66(11):1425–1436. doi:10.1007/s00262-017-2034-7.
- S.C. Katz, R.A. Burga, E. McCormack, L.J. Wang, W. Mooring, G.R. Point, P.D. Khare, M. Thorn, Q. Ma, B.F. Stainken, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421.
- Y.T. Shih, M.C. Wang, J. Zhou, H.H. Peng, D.Y. Lee, J.J. Chiu. Endothelial progenitors promote hepatocarcinoma intrahepatic metastasis through monocyte chemotactic protein-1 induction of microRNA-21. Gut. 2015;64:1132–1147.
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20:185–204.
- J.R. van Beijnum, P. Nowak-Sliwinska, E.J. Huijbers, V.L. Thijssen, A.W. Griffioen. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev. 2015;67:441–461.
- Y.J. Xie, M. Dougan, N. Jailkhani, J. Ingram, T. Fang, L. Kummer, N. Momin, N. Pishesha, S. Rickelt, R.O. Hynes, H. Ploegh. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci U S A. 2019;116(16):7624–7631. doi:10.1073/pnas.1817147116.
- Akbari P, Huijbers EJM, Themeli M, Griffioen AW, van Beijnum JR. The tumor vasculature an attractive CAR T cell target in solid tumors. Angiogenesis. 2019;22(4):473–475. doi:10.1007/s10456-019-09687-9.
- D. Shi, Y. Shi, A.O. Kaseb, X. Qi, Y. Zhang, J. Chi, Q. Lu, H. Gao, H. Jiang, H. Wang, et al. Chimeric antigen receptor-glypican-3 T-Cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–3989. doi:10.1158/1078-0432.CCR-19-3259.
- D. Li, N. Li, Y.F. Zhang, H. Fu, M. Feng, D. Schneider, L. Su, X. Wu, J. Zhou, S. Mackay, et al. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology. 2020;158(8):2250–2265 e2220. doi:10.1053/j.gastro.2020.02.011.
- Huang M, Deng J, Gao L, Zhou J. Innovative strategies to advance CAR T cell therapy for solid tumors. Am J Cancer Res. 2020;10:1979–1992.
- N. Ahmed, V.S. Brawley, M. Hegde, C. Robertson, A. Ghazi, C. Gerken, E. Liu, O. Dakhova, A. Ashoori, A. Corder, et al. Human epidermal growth factor receptor 2 (her2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi:10.1200/JCO.2014.58.0225.
- N. Ahmed, V. Brawley, M. Hegde, K. Bielamowicz, M. Kalra, D. Landi, C. Robertson, T.L. Gray, O. Diouf, A. Wakefield, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. doi:10.1001/jamaoncol.2017.0184.
- D.L. Porter, B.L. Levine, M. Kalos, A. Bagg and C.H. June. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi:10.1056/NEJMoa1103849.
- B. Savoldo, C.A. Ramos, E. Liu, M.P. Mims, M.J. Keating, G. Carrum, R.T. Kamble, C.M. Bollard, A.P. Gee, Z. Mei, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi:10.1172/JCI46110.
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94(23):12425–12430. doi:10.1073/pnas.94.23.12425.
- A.H. Yin, S. Miraglia, E.D. Zanjani, G. Almeida-Porada, M. Ogawa, A.G. Leary, J. Olweus, J. Kearney, D.W. Buck. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. doi:10.1182/blood.V90.12.5002.
- L. Mauge, A. Mejean, L. Fournier, H. Pereira, M.C. Etienne-Grimaldi, E. Levionnois, A. Caty, S. Abadie-Lacourtoisie, S. Culine, S. Le Moulec, et al. Sunitinib prior to planned nephrectomy in metastatic renal cell carcinoma: angiogenesis biomarkers predict clinical outcome in the prospective phase II PREINSUT trial. Clin Cancer Res. 2018;24(22):5534–5542. doi:10.1158/1078-0432.CCR-18-1045.
- Batchelor TT, Duda DG, Di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi:10.1200/JCO.2009.26.3988.
- P. Federico, A. Petrillo, P. Giordano, D. Bosso, A. Fabbrocini, M. Ottaviano, M. Rosanova, A. Silvestri, A. Tufo, A. Cozzolino, B. Daniele. Immune checkpoint inhibitors in hepatocellular carcinoma: current status and novel perspectives. Cancers (Basel). 2020;12.
- Nishida N, Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res. 2018;48(8):622–634. doi:10.1111/hepr.13191.
- Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci. 2018 Jan 24;19(2):340. doi:10.3390/ijms19020340.
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218.
- M. Hilmi, C. Neuzillet, J. Calderaro, F. Lafdil, J.M. Pawlotsky, B. Rousseau. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7(1):333. doi:10.1186/s40425-019-0824-5.