ABSTRACT
The cyclin-dependent kinase inhibitor p27Kip1 is a tumor suppressor whose intrinsic activity in cancer cells correlates with tumor aggressiveness, invasiveness, and impaired tumor cell differentiation. Here we explore whether p27Kip1 indirectly influences tumor progression by restricting expansion and survival of effector memory T cell (TEM) populations in a preclinical model of spontaneous colitis-associated colorectal cancer (CAC). We show mRNA and protein expression of p27Kip1 to be significantly decreased in the colons of mice with a T cell-restricted deletion of the TGF-β intermediate, SMAD4 (Smad4TKO). Loss of p27Kip1 expression in T cells correlates with the onset of spontaneous CAC in Smad4TKO mice by 8 months of age. This phenotype is greatly accelerated by the introduction of a germline deletion of CDKN1b (the gene encoding p27Kip1) in Smad4TKO mice (Smad4TKO/p27Kip1-/-, DKO). DKO mice display colon carcinoma by 3 months of age and increased mortality compared to Smad4TKO. Importantly, the phenotype in DKO mice is associated with a significant increase in the frequency of effector CD4 T cells expressing abundant IFN-γ and with a concomitant decrease in Foxp3+ regulatory T cells, both in the intestinal mucosa and in the periphery. In addition, induction of inflammatory mediators (IFN-γ, TNF-γ, IL-6, IL-1β, iNOS) and activation of Stat1, Stat3, and IκB is also observed in the colon as early as 1–2 months of age. Our data suggest that genomic alterations known to influence p27Kip1 abundance in gastrointestinal cancers may indirectly promote epithelial malignancy by augmenting the production of inflammatory mediators from a spontaneously expanding pool of TEM cells.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths in western countries.Citation1 Genetic mutations involved in CRC influence cellular processes including proliferation, adhesion, apoptosis, and stem cell differentiation.Citation2–4 The majority of these gene mutations lead to activation of the Wnt pathway, with mutations in the adenomatous polyposis coli (APC) gene being the most common, including both germline mutations in familial CRC and acquired APC mutations that are found in at least two-thirds of sporadic cases of CRC. While sporadic mutations act principally in a tumor intrinsic manner, germline mutations leading to Wnt pathway activation could influence the proliferation and differentiated function of stromal cells in the tumor microenvironment (TME), and thereby act in a tumor extrinsic manner to promote tumor progression. Demonstrations of stromal APC haploinsufficiency support the notion that the consequences of Wnt pathway activation in stromal cells may be essential determinants of the cancer phenotype.Citation5
An important molecular target of Wnt pathway activation in cancer cells is the cyclin-dependent kinase (Cdk) inhibitor p27Kip1, a member of the Cip/Kip family of Cdk inhibitors.Citation6 Mitogen withdrawal, treatment of cells with TGF-β, and cadherin-mediated cell-cell contact each lead to increased p27Kip1 binding to cyclin E/Cdk2 and cyclin A/Cdk2 complexes, and inhibition of G1/S progression In vitro.Citation7,Citation8 The binding of p27Kip1 to Cdk4 can also inhibit cyclin D/Cdk4 complex formation.Citation9,Citation10 In human cancers, reduced p27Kip1 expression is an unfavorable prognostic marker in tumors of the colon, stomach, breast, lung, prostate, and ovary.Citation11,Citation12 Loss of p27Kip1 within tumor cells is also correlated with increased tumor aggressiveness and depth of tumor cell invasion, as well as a poor state of differentiation.Citation13,Citation14 In colon cancer, low p27Kip1 expression is linked to a more advanced stage and to more poorly differentiated tumors.Citation15–18 Median five-year survival rates are also lower for CRC patients with reduced p27Kip1 expression.Citation19,Citation20 Relevant to the current study, low p27Kip1 expression has also been reported in colitis-associated CRC (CAC); however, mechanisms underlying this loss of p27Kip1 and its contribution to cancer development in CAC remain to be elucidated.Citation21
These observations suggest the contribution of p27Kip1 to the maintenance of the proliferation, differentiation, and function of stromal cells and mucosal immune cells is particularly important.Citation22 Abnormal CD4 T cell activation is associated with the development of colitis, which ultimately contributes to the pathogenesis of CAC. This aberrant effector CD4+ T cell activity leads to tissue accumulation of pro-inflammatory cytokines that enhance the risk for mutations in oncogenes and tumor suppressor genes and contribute to genomic instability via various mechanisms.Citation23–25 Recent reports have demonstrated a central role for tissue-resident memory T cells (TRM cells) as well as for intestinal memory T cell trafficking in the pathogenesis of inflammatory bowel disease (IBD) and CAC.Citation26,Citation27 Additionally, a reduction in the number and function of regulatory T cells (Tregs) can contribute to the development of inflammatory disease and CAC progression in the gastrointestinal tract as a reduction in Treg frequency underlies the abnormal activation and expansion of pathogenic CD4 T cells.Citation24,Citation28 Our group previously reported that p27Kip1 deficiency reduces the requirement for CD28-mediated co-stimulation in naïve CD8+ T lymphocytes and that p27Kip1 acts in synergy with p21 Cip1 to alter the sensitivity of naïve T cells to TGF-β-mediated G1 arrest through modulation of IL-2 responsiveness.Citation29,Citation30 Similarly, we have shown that a reduction in expression of the TGF-β-receptor-activated intermediate Smad3 cooperates with the loss of p27Kip1 to promote spontaneous T-cell leukemogenesis in mice.Citation31 More recently, others have shown a critical role for p27Kip1 as a negative regulator of the proliferation and expansion of effector/memory subsets of both CD8+ and CD4+ T cells.Citation22,Citation32–34 The impact of p27Kip1 expression on T cell fate has also been evidenced by the increased disease severity observed in p27Kip1 knockout (p27Kip1-/- or p27KO) mice in models of experimental arthritis, a phenotype attributed to a reduction in Foxp3+ Tregs.Citation35
Here we explore the potential that stromal cell expression of p27Kip1 might influence disease progression in an established model of CAC in which a T cell-restricted loss of the SMAD4 gene (Smad4co/co;Lck-cre, Smad4TKO) leads to spontaneous CAC.Citation36 Smad4TKO mice exhibit mucosal epithelial hyperplasia that is accompanied by increased expression of Cyclin D1, pRB, PCNA, and by a significant reduction in the expression of p27Kip1. Introduction of the Smad4TKO conditional deletion onto a background with a germline deletion of CDKN1b, the gene encoding p27Kip1 led to rapid acceleration and increased severity of the cancer phenotype in mice harboring both deletions (Smad4TKO/p27Kip1-/- or DKO). Mechanisms involved in this process include not only enhanced mucosal epithelial proliferation, but also a dramatic expansion of pathogenic effector CD4+ T cells and a reduction in the frequency of mucosal Treg cells. These data provide evidence that the common genomic alterations in CRC may impact cancer phenotype through a tumor extrinsic function in stromal cells, and particularly lymphocytes, within the TME.
Methods and materials
Antibodies
Anti-p27Kip1 (C-19), anti-p21 Cip1 (N-20), anti-phospho-iκB (B-9) and anti-iNOS (M-19) were purchased from Santa-Cruz biotechnology. Anti-phospho-Akt (Ser473) (#4060), anti-phospho-Rb (Ser807/811) (D20B12), anti-cyclin D1 (DCS6) (#2926), anti-PCNA (D3H8P), anti-phospho-Foxo1/3, anti-phospho-Stat1 (Tyr701) (58D6), and anti-phospho-Stat3 (Thyr705) (D3A7) were purchased from Cell Signaling. Anti-CD3, anti-CD28, anti-CD44, anti-CD62L, anti-IFN-γ, and anti-TNF-α were purchased from BD Biosciences. Anti-Foxp3 antibody was purchased from eBioscience. Anti-Ki-67 was purchased from Abcam.
Animals
T cell-restricted deletion of the SMAD4 gene (Smad4co/co;Lck-cre, Smad4TKO) in mice has been described previously.Citation36,Citation37 The model characterized by germline deletion of p27Kip1 (p27Kip1-/-, p27KO) was kindly provided by Dr. Koff (Memorial Sloan-Kettering, New York, NY).Citation38 The p27KO mice express a truncated 20-kDa protein that is devoid of any cyclin/Cdk inhibitory activity. To generate mice deficient for both p27Kip1 germline and for Smad4 in the T cell lineage only, p27KO males (p27KO females are infertile) were crossed with Smad4TKO females. The resulting F1 heterozygotes were then bred to generate all genotypes. Mice were housed in a pathogen-free facility. All animal experiments were performed in accordance with institutional guidelines and with approval of the Institutional Animal Care and Use Committee at Case Western Reserve University.
Assessment of neoplasia and colitis
The colon was excised from the ileocecal junction to the anal verge, flushed with phosphate-buffered saline (Gibco), and opened longitudinally. Gross examination was performed to measure colon length and colon weight and to evaluate tumor size and number. The thickening of the intestinal mucosa was assessed by measurement of the colon length to colon weight ratio. The incidence (defined as the number of mice with tumors/total mice in the group), the mean number of tumors/mouse ± standard deviation, and the mean tumor size ± standard deviation were calculated for each group. Tumor size was determined by image analysis using imaging software (ImageJ). Images were taken with a scale bar and lengths were measured in pixels and correlated to the known distance in scale bars. Colonic tissues as well as colon tumors were processed for histopathological evaluation and further biochemical analyses.
Nitrite assay
Serum Nitric oxide (NO) levels were measured by photometric analysis by using a nitrite/nitrate assay kit (Cayman Chemical) according to the manufacturer’s instructions.
Quantitative RT-PCR analysis
Colon mucosa was obtained from scrapings of full-length colon and total RNA was isolated using Trizol reagent (Invitrogen). For reverse transcription-PCR (RT-PCR), cDNA was synthesized using a High Capacity cDNA synthesis kit (Applied Biosystems). Quantitative RT-PCR was performed using a BioRad CFX96 Real-Time System C1000 Thermal Cycler. The expression of target genes was normalized to the expression of housekeeping gene β-actin. The relative gene level was expressed as 2−ΔΔCt, in which ΔΔCt equals ΔCt of the experimental sample (p27KO, Smad4TKO, or DKO mouse sample) minus ΔCt of the control sample (WT mouse sample).
Western blotting
For Western blot, colon mucosa was obtained from scrapings of full-length colon and lysed by incubation in lysis buffer (150 mM NaCl, 20 mM Tris-Cl, pH 7.5, 1 mM PMSF, 1 mM Na3VO4, 25 mM NaF, 1% aprotinin, 10 μg/ml leupeptin) on ice for 30 min. About 20 μg aliquots of proteins were separated by electrophoresis in 10% SDS/PAGE minigels and transferred to nitrocellulose membrane (Invitrogen). Following blocking, membranes were incubated in a buffer containing the primary antibody, followed by washing and incubation for 1 hr at room temperature with horseradish peroxidase-conjugated secondary antibodies. Immunostaining was visualized by ECL.
Histology
For hematoxylin and eosin staining (H&E), excised colons were washed with PBS and fixed in 10% formalin. Samples were embedded in paraffin wax, sectioned, stained with H&E, and examined by light microscopy. For immunohistochemistry (IHC), slides were deparaffinized, and rehydrated and heat-induced epitope retrieval was performed prior to blocking with Peroxidazed 1 (BioCare, PX968) and Rodent Block M (BioCare, RBM961). Slides were incubated with primary antibodies, Ki-67, p27Kip1, and CD3 for 1 hour at room temperature. Antibodies were detected using Rabbit-on-Rodent HRP polymer (BioCare, RMR622) and visualized using the Betazoid DAB chromogen kit (BioCare, BDB2004). The percentage of Ki67 positive cells were assessed on six randomly selected field using a digital eyepiece.
FACS analysis
Cell suspensions were prepared from spleens or colon lamina propria by filtering through a nylon mesh (40-µm diameter). Erythrocytes were lysed using ACK lysis buffer (BioWhittaker) and cells were washed twice in RPMI 1640 supplemented with 10% heat-inactivated FBS, 50 µM 2-ME, penicillin, and streptomycin (GIBCO/Life Sciences). Viable cells were counted using trypan blue exclusion and a hemocytometer. All Antibodies used in FACS analyses were purchased from BD Pharmingen. Pan T cells were purified from the spleen and lymph node using a Pan T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions (purity greater than 95%).
Regulatory T cell induction and intracellular cytokine staining
Splenocytes from each genotype were activated in vitro by plate-bound anti-CD3 and anti-CD28 antibodies in 24-well plates, in the absence or presence of TGF-β for 72 h. The cells were harvested, washed with PBS, stained with anti-CD4 and CD8 antibodies, and stained for intracellular Foxp3 using a Foxp3 staining Kit (eBioscience) according to the manufacturer’s instructions. For intracellular staining for cytokines, lymphocytes from spleen and colon lamina propria were activated with plate-bound anti-CD3 and anti-CD28 antibodies for 48 h and re-stimulated with PMA and ionomycin for the last 5 h in the presence of Golgi stop solution prior to washing and staining with antibodies for CD4 and CD8. Intracellular staining for IFN-γ and TNF-α were performed using an intracellular staining kit (BD Biosciences) according to the manufacturer’s instructions.
Statistical evaluation
Data are expressed as means ± SE. Statistical significance was determined by 1-way ANOVA with Tukey–Kramer Multiple Comparisons Test. The Fisher’s Exact Probability test was used for comparison of the incidence of lesions between the two groups. Statistical significance was accepted to be a p-value less than or equal to 0.05, with *p< .05, **p< .01, ***p< .001.
Results
Proliferation of intestinal epithelial cells is increased while p27Kip1 expression is suppressed in the mucosal epithelial cells and T cells in the colon of Smad4TKO mice
We previously established a novel murine model of colitis-associated colorectal cancer (CAC) through the creation of a T cell-specific deletion of the Smad4 gene in mice.Citation36 In this model, selective loss of Smad4-dependent signaling in T cells (Smad4co/co;Lck-cre, Smad4TKO) leads to spontaneous intestinal inflammation-induced cancer throughout the gastrointestinal tract. Smad4TKO mice invariably develop CAC after 8 months of age, with inflammatory cell infiltration of the mucosa, loss of body weight, and bloody diarrhea. To examine the status of cell proliferation in mice exhibiting CAC symptoms, we evaluated Ki-67 expression by immunohistochemistry (IHC) analysis of colon tissue. Ki-67 positive cells in the intestinal crypts of Smad4TKO mice (85%) were significantly greater than that of wild type (WT) mice (40%) at 6 months of age (). Increased proliferation of colon epithelial cells in Smad4TKO mice correlated with an increase in expression of the proliferating cell nuclear antigen (PCNA) in the colonic mucosa of Smad4TKO mice as determined by Western blot analysis (). Increased mucosal epithelial cell proliferation is also associated with a consistent, significant increase in the expression of Cyclin D1 and an abundance of the phosphorylated retinoblastoma protein (pRb) (). Synthesis of cyclin D is initiated during the G1 phase of the cell cycle and drives the G1/S phase transition, a step tightly regulated by the Rb protein. These data indicate that epithelial cell proliferation in the colon of Smad4TKO mice is increased relative to that of WT mice and is associated with changes in expression of cell-cycle regulatory proteins that act to enhance G1-S phase transition.
Figure 1. Proliferation of mucosal epithelial cells is increased and p27kip1 expression is decreased in the colon and T cells of Smad4TKO
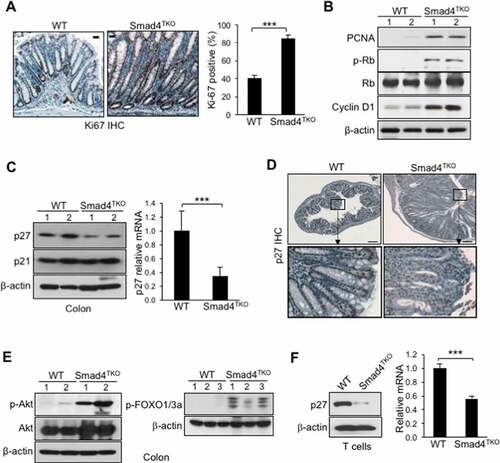
The CIP/KIP family proteins p21Cip1 and p27Kip1 have essential roles regulating the proliferation, differentiation, and viability of mucosal epithelial cells.Citation39–41 Interestingly, while we found no change in the abundance of p21Cip1 expression in the colonic mucosa of Smad4TKO mice relative to WT control mice, there was a significant reduction in the level of p27Kip1 protein expression when compared to colonic mucosa from WT mice, as observed by Western blot (). A similar reduction in p27Kip1 mRNA was also observed in the colon of Smad4TKO mice by real-time PCR (). To examine whether suppressed p27Kip1 in the Smad4TKO mouse is localized to either epithelial cells or infiltrating lymphocytes, colon cross-sections were analyzed for intracellular p27Kip1 expression by IHC. Normal colon tissue from WT mice demonstrated nuclear p27Kip1 protein expression in terminally differentiated epithelial cells in the uppermost one-third of crypts as well as in the infiltrating lymphocytes in the lamina propria. These phenomena are markedly reduced in Smad4TKO mice (). Previous reports have shown that p27Kip1 expression is transcriptionally regulated by the family of forkhead transcription factors (FOXO) which play an essential role in cellular proliferation, apoptosis, and differentiation.Citation42 PI3 kinase (PI3K) activates Akt through phosphorylation and activated Akt, in turn, phosphorylates FOXOs directly, which results in their nuclear exclusion and inhibition of FOXO mediated gene expression.Citation43 To investigate the mechanism underlying the decreased expression of p27Kip1 in Smad4TKO mice, we examined the status of Akt and FOXO activation in the colon of Smad4TKO mice. Western blotting analysis shows high phosphorylation of Akt in the colons of Smad4TKO mice while there was no change in the total Akt protein expression, indicating activation of Akt (). Phosphorylation of FOXO1/3 was also observed in the colons of Smad4TKO mice, but not in WT mice, indicating a mechanistic role for the PI3K-Akt pathway in suppression of p27Kip1 expression ().
This reduction in the expression of p27Kip1 was closely associated with the accumulation of mucosal T lymphocytes, as enumerated by CD3 immunoreactivity. The accumulation of CD3+ T cells in the mucosa of Smad4TKO mice relative to WT mice indicates a link between the abundance of p27Kip1 expression in T cells and the development of the CAC phenotype observed in Smad4TKO mice (Supplementary Figure 1). We investigated this relationship further by examining the expression of p27Kip1 in splenic T cells by Western blot and real-time PCR analysis. Protein expression of p27Kip1 was significantly decreased in T cells of Smad4TKO spleen compared to that of WT mice, and decreased mRNA expression of p27Kip1 of Smad4TKO was confirmed by quantitative RT-PCR (). These results indicate that the reduction of p27Kip1 expression in T cells is an important event that contributes to the accumulation of mucosal T lymphocytes and that this reduction in p27Kip1 is associated with the pathogenesis of CAC in Smad4TKO mice.
Germ line deficiency of p27Kip1 accelerates colitis-associated colorectal tumorigenesis in Smad4TKO mice
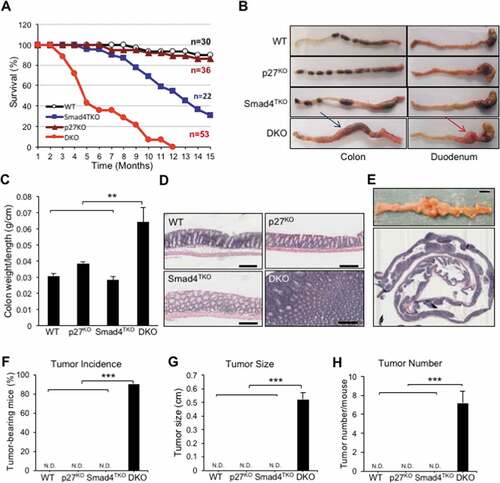
Based on the data described above, we hypothesized that the loss of T cell expression of p27Kip1 undermines the maintenance of both intestinal epithelial and mucosal immune homeostasis. Therefore, to directly demonstrate the contribution of p27Kip1 in the pathogenesis of inflammation-driven colon cancer in Smad4TKO mice, we introduced the lineage-restricted SMAD4 deletion (Smad4TKO) onto a background with a germline CDKN1b deletion (p27KO) to generate a ‘double knockout’ (Smad4TKO/p27KO, DKO) model. DKO mice harboring the T cell-restricted deletion of the tumor suppressor SMAD4 and a germline deletion of cell-cycle regulator CDKN1b developed CAC and inflammatory infiltration of the mucosa as early as 3 months of age, at which point the mortality rate of DKO mice began to increase. The survival rate of DKO was only 40% at the age of 5 months, compared to 98% for Smad4TKO mice (). Clinical features of systemic illness (lethargy, hunched posture, disheveled fur) became evident by 3 months of age and necropsy studies at this age revealed significant gastrointestinal pathology as a cause of clinical symptoms. The colon (blue arrow) and duodenum (red arrow) of DKO mice were thicker than those of either the Smad4TKO or p27KO mice () and the significant increase in colon thickness, as measured by colon weight-to-length ratio, was evident in DKO mice at 3 months of age (). Histological analysis by hematoxylin and eosin (H&E) staining of intestinal sections from DKO mice showed a clear difference in histopathology including the disrupted villus architecture with regions of epithelial atypia, as well as adenomas and invasive carcinomas compared to the colon histology of either the WT, p27KO, or Smad4TKO mice (). Necropsy and mucosal histology showed that DKO mice developed tumors in the colon at 3 months of age (), whereas the other genotypes had no lesions and displayed normal gastrointestinal villus architecture. At this age, tumor incidence in DKO mice was more than 80% (). The average tumor size was 0.5 cm in DKO mice () and tumor multiplicity was more than 6 tumors/mouse in DKO mice (). The phenotypic evaluation of DKO mice showed a similar but accelerated disease presentation as the Smad4TKO, presenting at the earlier age of 3 months versus 8 months of age in Smad4TKO mice, and clearly demonstrating that germline p27Kip1 deletion accelerates CAC development and tumorigenesis in the Smad4TKO mice.
Figure 2. Deletion of p27kip1 in Smad4TKO mice accelerates colitis-associated colon cancer. A) Survival curves of WT, Smad4TKO, p27KO and Smad4TKO/p27Kip1-/- (DKO) mice. B) Photographs of colon, stomach and duodenum from each genotype at 3 months of age. C) Colon weight per length (g/cm) (n = 9). D) Hematoxylin and eosin (H&E) staining of the colon of each genotype at 3 months of age. Scale bar = 100 µm. E) Photograph and mucosal histology of the colon from DKO mice at 3 months of age. Paraffin-embedded sections were stained with H&E. Scale bar = 1 cm. F) Percentage of tumor-bearing mice (n = 10). G) Tumor size (n = 7). H) Tumor numbers per mouse at 3 months of age were determined using a digital eyepiece and an imageJ (n = 7). Error bars indicate S.E.; ***P < .001, **P < .01 compared with each genotype such as WT, p27KO and Smad4TKO
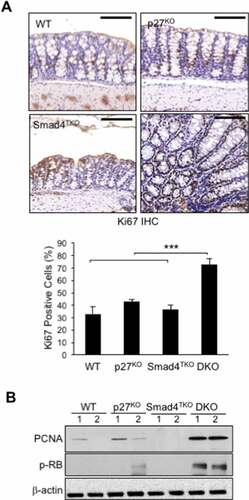
To evaluate the status of epithelial cell proliferation in symptomatic DKO mice, we examined intracellular Ki-67 staining by IHC. The percentage of Ki-67 positive cells in the intestinal crypts of DKO (74%) was significantly greater than that of wild type (WT) (32%), p27KO (41%), and Smad4TKO mice (35%) at 3 months of age (). We also found significantly elevated expression levels of PCNA protein in the colonic mucosa of DKO mice compared to those of WT, p27KO, or Smad4TKO mice at 3 months of age (). There was also significant phosphorylation of Rb protein in the colons of DKO mice at this young age (). As expected, we did not observe increased PCNA expression or Rb phosphorylation in Smad4TKO mice compared to WT mice. Our observations indicate that in DKO mice, the germline p27Kip1 deletion accelerates tumorigenesis in Smad4TKO mice, in part, by increasing colonic epithelial cell proliferation.
Figure 3. Proliferation of mucosal epithelial cells is increased in the colon of DKO mice. A) Immunohistochemistry (IHC) analysis for Ki-67 in the colon of WT, p27KO, Smad4TKO and DKO mice. The percentage of Ki-67 positive cells among all colon epithelial cells was determined in the colon at 3 months of age using a digital eyepiece. Data represent average ± S.E. (n = 4). B) Expression of PCNA and, p-Rb, and β-actin was measured by Western blot. Results shown are representative of 3 separate experiments. ***P < .001. Scale bar = 100 µm
Effector memory CD4 T cell population is increased by p27Kip1 deficiency in Smad4TKO mice
While the data above confirm a known role for p27Kip1 regulating epithelial proliferation, the accelerated tumorigenesis in the DKO model may also be linked to a more rapid expansion of tissue-resident memory T cells, which are known to require TGF-β for their differentiation and accumulate in the mucosa of patients with IBD.Citation26,Citation44 The function of CD4+ effector memory T (TEM) cells in cancer is complex, and is often dysregulated within the TME.Citation24,Citation25,Citation45 Abnormal CD4 T cell activation is associated with the development of colitis, which ultimately contributes to the pathogenesis of CAC. Although p27Kip1 markedly limits the abundance of memory CD4 T cells, the relevance of p27Kip1 activity in T cells and the progression of CAC has not been explored. Thus, we examined the role of p27Kip1 in T cell differentiation and function in the Smad4TKO mouse model of spontaneous CAC. We observed that the number of total spleen cells of DKO mice was significantly increased, compared with that of other genotypes (). We also observed a significant increase in both the percentage and number of total CD4+ T cells in the spleen of both p27KO and DKO mice at 4 months of age, when compared with that of either WT or Smad4TKO mice (). Populations of CD4+ TEM cells expressing CD44High and CD62LLow were examined in the spleen from each genotype at 4 months of age by FACS analysis (Supplementary Figure 2a, b). The proportion of CD4+ TEM cells in p27KO mice was diminished when compared with that of WT mice, while the absolute number of memory CD4+ T cells was greater. Notably, both the proportion and the absolute number of CD4+ TEM cells were greatly increased in the spleen of DKO mice, compared with that of either WT, p27KO, or Smad4TKO mice (). The population of CD4+ TEM cells was not significantly increased in the spleen of Smad4TKO at 6 months of age (Supplementary Figure 3a).
Figure 4. The population of effector memory CD4+ cells in each genotype. A) Analysis of total spleen cells and CD4+ T cells of each genotype at 4 months of age. Cell suspensions were prepared from spleens by filtering through nylon mesh. The cell numbers were counted by trypan blue exclusion assay. The population and number of CD4 T cells in each genotype. Splenocytes were stained with antibodies for CD4 and CD8. Events are gated on the live lymphocyte gate, based on forward and side light scatter (FSC X SSC) by FACS analysis. Bar graphs show the number of spleen cells and the proportion and absolute number of CD4 T cells, respectively. B) Analysis of memory markers (CD44High and CD62LLow) in CD4+ T cell compartment of each genotype. The bar graphs show the proportion and absolute number of CD4 TEM cells from the same experiment depicted in Supplementary Figure 2B. C) Analysis of effector CD4+ T cells expressing pro-inflammatory cytokines of each genotype. Splenocytes per genotype were collected, stained on cell surface with antibody for CD4 and intracellularly with IFN-γ or TNF-γ antibody, and analyzed on CD4+ T cells by FACS. The bar graphs are from the same experiment depicted in Supplementary Figure 2 C. D) IHC staining for CD3+ T cells in colon of each genotype. E) Colon lamina propria per each genotype at 4 months of age were collected, stained with antibodies for CD4 and CD8, and analyzed for effector memory markers (CD44High and CD62LLow) in CD4+ T cell compartment. Bar graphs show the proportion and absolute number of CD4 TEM cells and effector CD4 T cells producing IFN-γ, respectively. ***P < .001, **P < .01, *P < .05. Scale bar = 200 µm
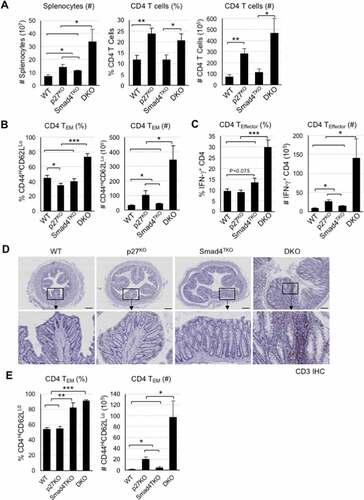
We next analyzed effector CD4+ T cells producing pro-inflammatory cytokines known to promote genomic instability via various mechanisms. The number of effector T cells producing IFN-γ was increased in p27KO and Smad4TKO mice, compared with that of the WT group, while the proportion was not altered. Whereas in DKO mice, both the proportion as well as the number of effector T cells producing IFN-γ were greatly increased, compared with those of either WT, p27KO, or Smad4TKO mice at 4 months of age (, Supplementary Figure 2c). In addition, the population of TNF-α producing T cells was also slightly increased in the spleen of the DKO mice (Supplementary Figure 2c).
To specifically investigate the CD4+ TEM cell population in the target organ of each genotypes, we further performed FACS analysis on colonic lamina propria from WT, p27KO, Smad4TKO, and DKO mice. CD3 immunohistochemistry analysis confirmed the substantial accumulation of T cells in the lamina propria and within the TME of the colons of DKO mice (). In addition, we also observed that the population of total CD4+ T cells was significantly increased in the colonic mucosa of DKO mice, compared with that of other genotypes, while the percentage was increased in p27KO and DKO mice (Supplementary Figure 2d). The proportion of CD4+ TEM cells was greatly increased in the colonic mucosa of both the Smad4TKO and DKO mice, compared with that of either WT or p27KO mice (). The absolute number of the CD4+ TEM cells was also greatly increased in DKO mice, compared with that of all other genotypes, including p27KO mice, which similarly showed a significant increase in effector memory CD4+ T cells when compared with WT mice. Taken together, these data suggest that p27Kip1 deficiency accelerates the expansion of a population of pathogenic Smad4-deficient CD4+ TEM cells, whose production of Th1 pro-inflammatory cytokines, such as IFN-γ and TNF-α, contributes to the pathogenesis of CAC in this model.
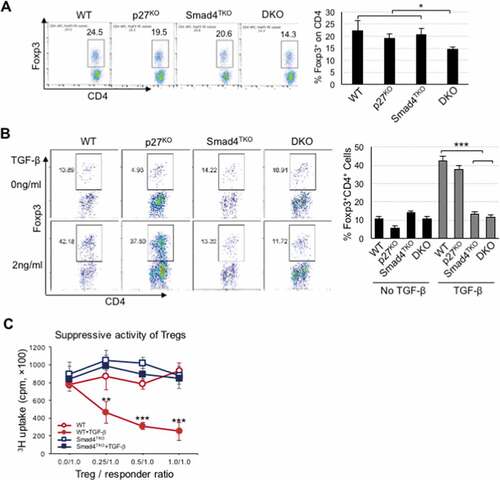
The population of Foxp3+ regulatory T cells is decreased in DKO mice
Tregs play a key role in the maintenance of mucosal immune homeostasis. A deficiency of Tregs leads to inflammation in the gastrointestinal tract and is associated with the pathogenesis of CAC.Citation24 Therefore, we next investigated the profile of natural Foxp3+ Treg (nTreg) in the spleen and colonic mucosa of each genotype. The proportion of Foxp3+ nTregs was decreased in the spleen and colon of DKO mice at 4 months of age, when compared with that of all other genotypes (, Supplemental Figure 3c). We also observed that Foxp3+ nTregs were significantly decreased in the colon of Smad4TKO mice at 6 months (Supplemental Figure 3b) and in the spleen of aged p27KO mice (15 months; data not shown), which is consistent with previous reports.Citation35,Citation46 In an inducible Treg (iTreg) assay, exogenous recombinant TGF-β induced the expression of Foxp3 in naïve CD4+ T cells of spleen from either WT or p27KO mice. However, TGF-β failed to induce Foxp3 expression in naïve CD4+ T cells isolated from either Smad4TKO or DKO mice (), indicating that a decrease in the nTreg population and a deficiency of iTreg induction are observed only in the DKO mouse model. In addition, while TGF-β-induced CD4+CD25+ Treg from WT mice suppressed naïve T cell proliferation in the Treg suppression assay, CD4+CD25+ Treg isolated from Smad4TKO mouse did not (). These data imply that p27Kip deficiency may also enhance inflammatory conditions by predisposing to a decline in the mucosal Treg population in Smad4TKO mice.
Figure 5. Decreased percentage of natural Foxp3+ regulatory T cell (nTreg) and impaired TGF-β-induced Foxp3+ Treg (iTreg) in DKO mice. A) Analysis of nTreg population in CD4+ T cell compartment of each genotype mice. Splenocytes per genotype at 4 months of age were collected, stained on cell surface with antibody for CD4 and intracellularly with Foxp3 antibody, and analyzed on CD4+ T cells by FACS. Events are gated on the live lymphocyte gate, based on forward and side light scatter (FSC X SSC). Bar graph represents percent nTreg from each genotype at 4 months. B) Analysis of iTreg population in CD4+ T cell compartment of each genotype. Cells from Spleen per genotype at 2 months of age were collected and incubated with anti-CD3/CD28 for 72 hrs with (gray bar) or without (black bar) TGF-β (2 ng/ml) and intracellularly stained with Foxp3 antibody. Results are representative of three independent experiments using pooled spleens from two mice per genotype. Bar graph of percent iTreg from each group. C) Analysis of suppressive capacity of iTreg from CD4+ T cell compartment of WT and Smad4TKO. CD4+CD25+ iTregs from panel B were co-incubated with naïve CD4 T cells with 1 μg/ml of Con A for 3 days and the proliferation of naïve CD4 T cells was measured by H3 thymidine incorporation for the final 16 hrs of culture. ***P < .001, **P < .01, *P < .05
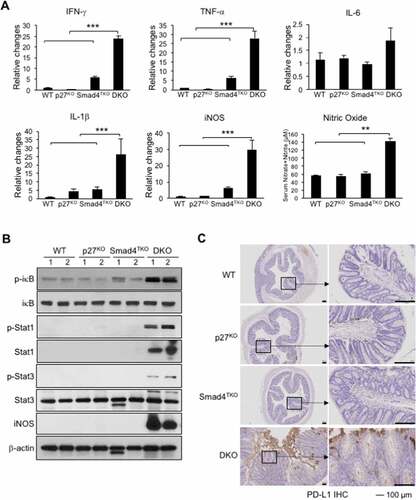
Mucosal inflammation in Smad4TKO mice is intensified by disruption of p27Kip1 expression
Cytokines including IFN-γ, TNF-γ, IL-6 and IL-1β, and many chemokines are known to promote inflammation and CAC development through the mechanisms that include deregulation of mucosal immune homeostasis and increased epithelial proliferation as well as through the induction of mutations in oncogenes and tumor suppressor genes.Citation23,Citation47–49 In order to elucidate the mechanism underlying the pathogenesis of CAC promoted by p27Kip1 deficiency in Smad4TKO mice, we examined expression levels of inflammatory mediators in the colon of WT, p27KO, Smad4TKO, and DKO mice. The mRNA levels of pro-inflammatory cytokines, such as IFN-γ, TNF-γ, and IL-1β were significantly elevated in the colons of DKO mice at 3 months of age, compared with those of either WT, p27KO, or Smad4TKO mice (). We observed a correlation between the expression of these inflammatory cytokines and the activation of the intracellular mediators of their response, including NF-κB, Stat1, and Stat3 in intestinal mucosa in DKO mice, as assessed by Western blot analysis (). The phosphorylation of Stat1 is greatly increased in the colons of DKO mice at 3 months of age, whereas it is barely detectable in either WT, p27KO, or Smad4TKO mice (). Stat3 and IκB phosphorylation was also significantly increased in colonic mucosal scrapings of DKO mice compared with those of other genotypes. Highly increased expression of iNOS is a common phenomenon during chronic inflammation. Inducible nitric oxide synthase (iNOS) mRNA expression in colonic mucosa and nitric oxide concentration in serum were also increased in DKO mice compared to other genotypes at this age (). While iNOS induction is not detected in the colon of WT, p27KO, or Smad4TKO mice, it is significantly increased in DKO mice (). iNOS and phospho-Stat1 were significantly increased in the colonic mucosa of DKO mice even at 1 month of age (Supplementary Figure 4), and therefore may represent important early oncogenic events linked to the inflammatory process in DKO mice. Considering the highly expressed IFN-γ in DKO colon mucosa as well as increased IFN-γ secretion observed in CD4+ effector T cells (), we next investigated immune checkpoint molecules in the colons of each phenotype. IFN-γ is known to induce programmed death ligand-1 (PD-L1) expression on tumor cells and immune cells that are also abundant in the DKO colon. We found that PD-L1 is significantly increased in colon mucosa of DKO mice, compared with those of other groups (). Thus, these data suggest that p27Kip1 deficiency in T cells is associated with the induction of an inflammatory disease state and that may confer protection of transformed or malignant epithelial cells from host anti-tumor immune response through mechanisms that include induction of tumor PD-L1 expression. The significance of this observation is supported by data demonstrating elevated mucosal epithelial expression of PD-L1 in patients with IBD, and by the observation that preexisting IBD predicts risk for severe adverse events in cancer patients treated with immune checkpoint inhibitors.Citation50–52
Figure 6. Increased inflammatory responses in the colon of DKO mice. A) IFN-γ, TNF-α, IL-6, IL-1β and iNOS were measured by real-time PCR in colon mucosa of WT, p27KO, Smad4TKO and DKO mice at 3 month of age. Nitric oxide (nitrate + nitrite) concentration in sera of each genotype at 3 months of age. B) Expression of phospho-iκb, phospho-Stat1, phospho-Stat3, and iNOS was determined in colon epithelia of each genotype (3 month old) by Western blot analysis. β-actin was used as the loading control. C) Immunohistochemistry (IHC) staining for PD-L1 in colons of each genotype. Scale bar = 100 µm
Discussion
In this study, we have demonstrated the distinct and essential contributions of both mucosal T cell and epithelial cell expression of p27Kip1 in the suppression of inflammation-related CRC. Our data indicate that a p27Kip1 deficiency accelerates gastrointestinal epithelial malignancy by increasing proliferation of epithelial cells and by promoting epithelial cell transformation in a manner dependent on enhanced production of pro-inflammatory mediators by tissue-resident CD4+ TEM cells, whose expansion is accompanied by a reduction of mucosal Treg cells. Our observations in the CAC mouse model resulting from T cell-restricted loss of TGF-β-dependent Smad4 (Smad4TKO) were validated by the introduction of the germline deletion of the CDKN1b (p27Kip1) gene in Smad4TKO mice (Smad4TKO/p27KO, DKO). Utilizing the DKO mouse model, we discovered that the CAC phenotype in Smad4TKO mice is linked to loss of p27Kip1 expression in lymphocytes, with the tremendous skewing of the mucosal CD4+ T cell repertoire toward an activated, effector memory phenotype. Consequently, the colonic epithelium of DKO mice exhibited an inflammation-driven oncogenic signature that includes a significant elevation in the expression of iNOS, p-Stat1, and p-Stat3.
While the tumor suppressor function of p27Kip1 is well recognized, the current study sheds new light on a unique mechanism through which a reduction in immune cell expression of p27Kip1 promotes epithelial carcinogenesis. We discovered that, through direct effects on epithelial cell-cycle regulation, p27Kip1 directly inhibits the initiation and progression of spontaneous epithelial carcinogenesis induced by inflammatory mediators, including iNOS, IFN-γ, TNF-α, and IL-6. The epithelial-intrinsic mechanisms underlying p27Kip1 suppression of CAC include the regulation of the abundance of p-Rb and of cyclin D1 and an inhibition of Akt-mediated FOXO phosphorylation, which also inhibits p27Kip1 expression.Citation53,Citation54 Our observations are consistent with prior studies that have indicated that p27Kip1 inhibits most cyclin/Cdk complexes and acts as a putative tumor suppressor for human cancer.Citation6,Citation11–14 There is a known inverse correlation between p27Kip1 protein levels and poor prognosis in CRC. Loda et al. first reported a low expression of p27Kip1 in tumor samples obtained from 149 patients who underwent surgery for CRC.Citation15 Nine out of 13 retrospective multivariate analyses of CRCs (n = 80–418) showed reduced p27Kip1 is associated with a 1.43–11 fold increase in relative risk of disease recurrence or death.Citation12,Citation15–20 Three out the four remaining studies showed a trend toward significance between low p27Kip1 protein levels and poor disease outcome. Our data are consistent with evidence linking the pathogenesis of CAC to low expression of p27Kip1.Citation21
Our data also suggest that the expansion of a pathogenic effector CD4+ T cell population is a direct consequence of a p27Kip1 deficiency, pointing toward an important mechanism through which p27Kip1 influences the progression of CAC. Specifically, this study demonstrates that p27Kip1 indirectly inhibits the initiation and progression of CAC through suppression of CD4+ T cell-mediated mucosal inflammation. Aberrant or dysregulated CD4+ T cell memory is suspected to contribute to multiple chronic or recurring inflammatory and immune-mediated disorders and to the progression of neoplastic disease.Citation55,Citation56 Here we show p27Kip1 deficiency increases the proportion and number of activated/memory CD44+CD62L− CD4+ T cells and effector CD4+ T cells producing IFN-γ in Smad4TKO mice. These results are consistent with a previous report that p27Kip1 negatively regulates the magnitude and persistence of CD4+ T cell memory by promoting apoptosis and contraction of effector CD4+ T cells.Citation34 Even though ubiquitination is the principal mechanism regulating p27Kip1 protein degradation, prior studies have shown that both IL-2 and IL-5 lead to inhibition of p27Kip1 mRNA expression in lymphocytes. Our present findings support the concept that p27Kip1 contributes to the maintenance of native, mucosal Tregs and acts to impair the expansion of pathogenic T cells. A recent report demonstrates that p27KO mice have more severe disease scores in preclinical models of arthritis, and that this is a consequence of a reduction in the abundance of Foxp3+ Tregs relative to WT control mice.Citation35 The latter indicates that p27Kip1 is involved in the maintenance of mucosal homeostasis of Foxp3+ Tregs. Indeed, p27Kip1 deficiency in aged C57BL/6 mice is linked to a decrease in the number and activity of Treg cells and is associated with the development of arthritis and mild lupus-like abnormalities.Citation35 Together, these observations point to p27Kip1 as a critical regulator of Treg cell differentiation and function through the positive modulation of TGF-β signaling strength in T cells.
Prior studies have shown that p27KO mice develop hyperplasia in multiple organs, resulting in a mouse roughly 20–30% larger than their WT counterparts.Citation38,Citation57,Citation58 Surprisingly, p27KO mice are relatively free of malignancy, with the exception of pituitary and prostatic hyperplasia that becomes increasingly severe with age.Citation59,Citation60 While these data indicate that p27Kip1 is a weak tumor suppressor, our data provide new evidence that p27Kip1 deficiency can greatly accelerate CAC by enabling rapid expansion of a population of tissue-resident, activated effector memory CD4+ T cells in a setting where Smad4-dependent TGF-β signaling is abrogated only in T cells. Germline mutations in SMAD4 are found in over 50% of the patients with familial juvenile polyposis (FJP), an autosomal dominant disorder characterized by a predisposition to hamartomatous polyps and gastrointestinal cancer.Citation61,Citation62 The histopathology of the intestines of Smad4TKO mice is in many respects a phenocopy of the pathology found in FJP, with a prominent stromal component.Citation36 The data presented here suggest that a reduction in the expression of p27Kip1 in both epithelial cells and lymphocytes may be a determinant of disease severity in FJP, including the number of polyps, recurrent polyps, polyp development at a young age, and the highly increased risk for malignancy. The relevance of this biology is further supported by the demonstration that PTEN deficiency, common in FJP, leads to a loss of p27Kip1 as PTEN is an important post-translational regulator of p27Kip1 protein stability.Citation55
To our knowledge, this is the first report describing dual, tandem mechanisms for tumor suppression by p27Kip1, which include constitutive restriction of epithelial cell proliferation and the concomitant suppression of the expansion of effector memory CD4+ T cells within the TME. It is notable that recent whole exome sequencing studies have identified a pathogenic variant of CDKN1b (p27Kip1) in familial colorectal cancer, Citation63 supporting the idea that the genetics and presentation of disease in the DKO mouse model are indeed relevant to CAC development in humans. Furthermore, we previously reported the value of the Smad4TKO mouse model for assessing the efficacy of potential cancer chemopreventive agents, Citation37 and our current data reveal accelerated disease progression in the colon of DKO mice, thereby improving the utility of this model for preclinical assessment of novel cancer chemopreventive agents. Accordingly, we have leveraged the DKO model to demonstrate potent suppression of carcinogenesis in DKO mice by a natural triterpenoid, celastrol.Citation64 Importantly, strategies designed to modulate the expression and activity of p27Kip1 in both the epithelial and stromal compartment in the TME may serve to concomitantly support the maintenance of mucosal epithelial homeostasis and suppress the expansion of pathogenic, tissue-resident effector T cells producing inflammatory cytokines, thereby forming a unique and effective approach to cancer chemoprevention.
Conflicts of interest
The authors declare no potential conflicts of interest.
Authors’ contributions
SHC, JJL, and BGK designed the studies and developed the methodology; SHC, KJG, and BGK performed and assisted with experiments; SHC, ECB, JJL, and BGK all assisted in interpreting the data and in writing the manuscript; JJL and BGK were responsible for overall project coordination and are co-senior authors.
Supplemental Material
Download ()Acknowledgments
We would like to acknowledge Janet K. Robinson for her work in making available the Smad4co/co;Lck-cre/p27Kip1-/- (DKO) mice for this study. We also wish to acknowledge Jane and Lee Seidman for their support of John Letterio through the Jane and Lee Seidman Endowed Chair in Pediatric Cancer Innovation. These studies were supported by NIH R01CA168586 (JJL) and T32 CA059366 (ECB).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Gill S, Blackstock AW, Goldberg RM. Colorectal cancer. Mayo Clin Proc. 2007;82:114–13.
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767.
- Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch JF, Lynch PM, Cavalieri RJ, Boland CR. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993;104:1535–1549.
- Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038.
- Stoddart A, Wang J, Fernald AA, Karrison T, Anastasi J and Le Beau MM. Cell intrinsic and extrinsic factors synergize in mice with haploinsufficiency for Tp53, and two human del(5q) genes, Egr1 and Apc. Blood. 2014;123:228–238.
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66.
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J Cell Biol. 1998;142:557–571.
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22.
- James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510.
- Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280.
- Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323.
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267.
- Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T. p27 expression and gastric carcinoma. Nat Med. 1997;3:593.
- Singh SP, Lipman J, Goldman H, Ellis FH Jr., Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res. 1998;58:1730–1735.
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234.
- Belluco C, Esposito G, Bertorelle R, Del Mistro A, Fassina A, Vieceli G, Chieco-Bianchi L, Nitti D, Lise M. Absence of the cell cycle inhibitor p27Kip1 protein predicts poor outcome in patients with stage I-III colorectal cancer. Ann Surg Oncol. 1999;6:19–25.
- Palmqvist R, Stenling R, Oberg A, Landberg G. Prognostic significance of p27(Kip1) expression in colorectal cancer: a clinico-pathological characterization. J Pathol. 1999;188:18–23.
- Manne U, Jhala NC, Jones J, Weiss HL, Chatla C, Meleth S, Suarez-Cuervo C, Grizzle WE. Prognostic significance of p27(kip-1) expression in colorectal adenocarcinomas is associated with tumor stage. Clin Cancer Res. 2004;10:1743–1752.
- Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58:114–122.
- Sarli L, Bottarelli L, Azzoni C, Campanini N, Di Cola G, Barilli AL, Marchesi F, Mazzeo A, Salvemini C, Morari S, et al. Loss of p27 expression and microsatellite instability in sporadic colorectal cancer. Surg Oncol. 2006;15(2):97–106.
- Walsh S, Murphy M, Silverman M, Odze R, Antonioli D, Goldman H, Loda M. p27 expression in inflammatory bowel disease-associated neoplasia. Further evidence of a unique molecular pathogenesis. Am J Pathol. 1999;155:1511–1518.
- Mohapatra S, Agrawal D, Pledger WJ. p27Kip1 regulates T cell proliferation. J Biol Chem. 2001;276:21976–21983.
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. e5
- Lin YC, Mahalingam J, Chiang JM, Su PJ, Chu YY, Lai HY, Fang JH, Huang CT, Chiu CT, Lin CY. Activated but not resting regulatory T cells accumulated in tumor microenvironment and correlated with tumor progression in patients with colorectal cancer. Int J Cancer. 2013;132:1341–1350.
- Kouidhi S, Elgaaied AB, Chouaib S. Impact of Metabolism on T-Cell Differentiation and Function and Cross Talk with Tumor Microenvironment. Front Immunol. 2017;8:270.
- Zundler S, Becker E, Spocinska M, Slawik M, Parga-Vidal L, Stark R, Wiendl M, Atreya R, Rath T, Leppkes M, et al. Hobit- and Blimp-1-driven CD4(+) tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol. 2019;20(3):288–300.
- Zundler S, Becker E, Schulze LL, Neurath MF. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut. 2019;68:1688–1700.
- Fujimoto H, Saito Y, Ohuchida K, Kawakami E, Fujiki S, Watanabe T, Ono R, Kaneko A, Takagi S, Najima Y, et al. Deregulated Mucosal Immune Surveillance through Gut-Associated Regulatory T Cells and PD-1(+) T Cells in Human Colorectal Cancer. J Immunol. 2018;200(9):3291–3303.
- Wolfraim LA, Letterio JJ. Cutting edge: p27Kip1 deficiency reduces the requirement for CD28-mediated costimulation in naive CD8+ but not CD4+ T lymphocytes. J Immunol. 2005;174:2481–2484.
- Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol. 2004;173:3093–3102.
- Wolfraim LA, Fernandez TM, Mamura M, Fuller WL, Kumar R, Cole DE, Byfield S, Felici A, Flanders KC, Walz TM, et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N Engl J Med. 2004;351(6):552–559.
- Singh A, Jatzek A, Plisch EH, Srinivasan R, Svaren J, Suresh M. Regulation of memory CD8 T-cell differentiation by cyclin-dependent kinase inhibitor p27Kip1. Mol Cell Biol. 2010;30:5145–5159.
- Jatzek A, Marie Tejera M, Plisch EH, Fero ML, Suresh M. T-cell intrinsic and extrinsic mechanisms of p27Kip1 in the regulation of CD8 T-cell memory. Immunol Cell Biol. 2013;91:120–129.
- Jatzek A, Tejera MM, Singh A, Sullivan JA, Plisch EH, Suresh M. p27(Kip1) negatively regulates the magnitude and persistence of CD4 T cell memory. J Immunol. 2012;189:5119–5128.
- Iglesias M, Postigo J, Santiuste I, Gonzalez J, Buelta L, Tamayo E, Merino J, Merino R. p27(Kip1) inhibits systemic autoimmunity through the control of Treg cell activity and differentiation. Arthritis Rheum. 2013;65:343–354.
- Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441(7096):1015–1019.
- Choi SH, Kim BG, Robinson J, Fink S, Yan M, Sporn MB, Markowitz SD, Letterio JJ. Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J Clin Invest. 2014;124:2472–2482.
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732.
- Tian JQ, Quaroni A. Involvement of p21(WAF1/Cip1) and p27(Kip1) in intestinal epithelial cell differentiation. Am J Physiol. 1999;276:C1245–58.
- Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014;289:581–593.
- Poole AJ, Heap D, Carroll RE, Tyner AL. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–8134.
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787.
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868.
- Sedda S, Marafini I, Dinallo V, Di Fusco D, The MG. TGF-beta/Smad System in IBD pathogenesis. Inflamm Bowel Dis. 2015;21:2921–2925.
- Marshall EA, Ng KW, Kung SH, Conway EM, Martinez VD, Halvorsen EC, Rowbotham DA, Vucic EA, Plumb AW, Becker-Santos DD, et al. Emerging roles of T helper 17 and regulatory T cells in lung cancer progression and metastasis. Mol Cancer. 2016;15(1):67.
- Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206(10):2131–2139.
- Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–7892.
- Yang GY, Taboada S, Liao J. Inflammatory bowel disease: a model of chronic inflammation-induced cancer. Methods Mol Biol. 2009;511:(193–233.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899.
- Beswick EJ, Grim C, Singh A, Aguirre JE, Tafoya M, Qiu S, Rogler G, McKee R, Samedi V, Ma TY, et al. Expression of programmed death-Ligand 1 by human colonic CD90(+) stromal cells differs between ulcerative Colitis and Crohn’s disease and determines their capacity to suppress Th1 Cells. Front Immunol. 2018;9:1125.
- Zhou H, Liu J, Zhang Y, Zhang L. Inflammatory bowel disease associated with the combination treatment of nivolumab and metformin: data from the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2019;83:599–601.
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51.
- Gopinath S, Malla RR, Gondi CS, Alapati K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Co-depletion of cathepsin B and uPAR induces G0/G1 arrest in glioma via FOXO3a mediated p27 upregulation. PLoS One. 2010;5:e11668.
- Ho WC, Pikor L, Gao Y, Elliott BE, Greer PA. Calpain 2 regulates Akt-FoxO-p27(Kip1) protein signaling pathway in mammary carcinoma. J Biol Chem. 2012;287:15458–15465.
- Caserta S, Borger JG, Zamoyska R. Central and effector memory CD4 and CD8 T-cell responses to tumor-associated antigens. Crit Rev Immunol. 2012;32:97–126.
- Gasper DJ, Tejera MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. 2014;34:121–146.
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85(5):733–744.
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720.
- Blain SW, Scher HI, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115.
- Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, et al. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90(17):1284–1291.
- Howe JR, Shellnut J, Wagner B, Ringold JC, Sayed MG, Ahmed AF, Lynch PM, Amos CI, Sistonen P, Aaltonen LA. Common deletion of SMAD4 in juvenile polyposis is a mutational hotspot. Am J Hum Genet. 2002;70:1357–1362.
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280(5366):1086–1088.
- Esteban-Jurado C, Vila-Casadesus M, Garre P, Lozano JJ, Pristoupilova A, Beltran S, Munoz J, Ocana T, Balaguer F, Lopez-Ceron M, et al. Whole-exome sequencing identifies rare pathogenic variants in new predisposition genes for familial colorectal cancer. Genet Med. 2015;17(2):131–142.
- Barker EC, Kim BG, Yoon JH, Tochtrop GP, Letterio JJ, Choi SH. Potent suppression of both spontaneous and carcinogen-induced colitis-associated colorectal cancer in mice by dietary celastrol supplementation. Carcinogenesis. 2018;39:36–46.
