ABSTRACT
Anti-epidermal growth factor receptor (EGFR) monoclonal antibody is a standard treatment of metastatic colorectal cancer (mCRC) and its most common adverse effect is a papulopustular acneiform rash. The aim of the CUTACETUX study was to characterize the skin inflammatory response associated with this rash and its relation to treatment efficacy.
This prospective study included patients with mCRC treated with first-line chemotherapy plus cetuximab. Patients underwent skin biopsies before the initiation of cetuximab (D0) and before the third infusion (D28), one in a rash zone and one in an unaffected zone. Expression of Th17-related cytokines (IL-17A, IL-21, IL-22), antimicrobial peptides (S100A7 and BD-2), innate response-related cytokines (IL-1β, IL-6, TNF-α and OSM), T-reg-related cytokines (IL-10 and TGF-β), Th1-related cytokine (IFN-γ), Th2-related cytokine (IL-4), Thymic stromal lymphopoietin and keratinocyte-derived cytokines (IL-8, IL-23 and CCL20) were determined by RT-PCR.
Twenty-seven patients were included. Levels of most of the cytokines increased at D28 in the rash zone compared to D0. No significant association was observed between variations of cytokines levels and treatment response in the rash zone and only the increase of IL-4 (p = .04) and IL-23 (p = .02) levels between D0 and D28 in the unaffected zone was significantly associated with treatment response. Increased levels of IL-8 (p = .02), BD-2 (p = .02), IL-1β (p = .004) and OSM (p = .02) in the rash zone were associated with longer progression-free survival.
Expression of Th2-related and keratinocyte-derived cytokines in the skin was associated with anti-EGFR efficacy. If this inflammatory signature can explain the rash, the exact mechanism by which these cytokines are involved in anti-EGFR tumor response remains to be studied.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide, with 1.5 million new cases each year and 700.000 deaths.Citation1 Nowadays, for patients with metastatic CRC (mCRC), median overall survival (OS) is around 30 months for those harboring a RAS wild-type status and 26 months for those harboring a RAS mutation.Citation2–5 For mCRC, chemotherapy is associated with a biological agent, an anti-vascular endothelial growth factor or an anti-epithelial growth factor receptor (anti-EGFR) monoclonal antibody (mAb), remains the standard of care.Citation6,Citation7
EGFR is a transmembrane glycoprotein, one of the four members of the ErbB family of tyrosine kinase receptors, over-expressed in many cancers, especially in 60–80% of mCRC.Citation8–12 EGFR is an inactive monomer on its own and is activated after ligand binding and dimerization. This activates the intracellular tyrosine kinase region of EGFR, resulting in the initiation of a signaling pathway involved in cell differentiation, proliferation, migration, angiogenesis, apoptosis and metastatic spread.Citation13 Cetuximab is a chimeric IgG1 mAb that binds specifically to the external domain of EGFR and blocks ligand binding and receptor activation.Citation13 Several studies have shown the efficacy of cetuximab in mCRC, as monotherapy or combined with chemotherapy, but only in RAS wild-type tumors.Citation8,Citation14,Citation15 Cetuximab can cause mostly manageable infusion-related hypersensitivity reactions in approximately 15% of patients,Citation16 but the most common adverse event is a papulopustular rash.Citation17 This skin reaction is found in 60% to 80% of patients treated with an anti-EGFR mAb with 8% to 17% having grade 3 or 4 skin toxicity.Citation7,Citation8,Citation18 Other forms of skin reaction include dry skin, pruritus, erythema and paronychia. The papulopustular rash generally occurs between one and three weeks after treatment initiation and usually involves the face and upper torso.Citation17 Several studies have demonstrated a positive correlation between the papulopustular rash and tumor response and/or survival in mCRC patients treated with anti-EGFR therapy.Citation8,Citation19 For example, Cunningham et al. showed that the response rate to cetuximab in mCRC was 55.2% when the skin reaction was severe compared to 6.3% in the absence of skin reaction.Citation8
Histological analyses of affected skin biopsies have revealed a neutrophilic infiltrate in the dermal tissue and a thinner layer of the stratum corneum of the epidermis. EGFR is essentially expressed in normal proliferating keratinocytes in the basal layer of the epidermis and the outer layers of the hair follicle.Citation20 The EGF pathway is involved in keratinocyte survival and proliferation. Inhibiting this pathway blocks proliferation, accelerates differentiation, decreases migration and induces apoptosis of keratinocytes.Citation21 Moreover, the EGF pathway is involved in the skin immune response. EGFR activation downregulates the levels of Tumor Necrosis Factor-alpha (TNF-α) and Interferon-gamma (IFN-γ)-induced expression of a cluster of chemokines release by the keratinocytes.Citation22,Citation23 By contrast, anti-EGFR agents induce the liberation of pro-inflammatory chemokines such as monocyte chemotactic protein-1 (MCP-1) and chemokine (C-C motif) ligand 5 (CCL5) and attract T cells and neutrophils (interleukin 8 [IL-8 or chemokine (C-X-C motif) ligand 8]) which results in skin inflammation.Citation20,Citation22 Anti-EGFR agents also induce the production of pro-inflammatory cytokines by the cancer cells, thus possibly influencing tumor response.Citation23
The pathophysiology of these skin reactions as well as their relationship with treatment efficacy are not fully understood. The purpose of the CUTACETUX study was to characterize the inflammatory profile of the skin reactions in patients treated with cetuximab and to explore the association between this inflammatory profile and treatment efficacy.
Patients and methods
Study design and patients’ selection
This bicentric (Poitiers and Tours university hospitals) prospective study was approved by the French “Comité de protection des personnes Ouest III” (2010–019837-85), the “Commission nationale de l’informatique et des libertés” and was registered in clinicaltrials.gov (NCT01292356). The study was performed according to the principles of the Declaration of Helsinki and all participants have given written consent.
Inclusion criteria were as follows: patients over 18 y old, histologically proven mCRC with wild-type (K)RAS genes (wild-type KRAS before 2013 and wild-type RAS after 2013), cetuximab treatment in first-line combined with FOLFOX (5-fluorouracil (5-FU) plus oxaliplatin) or FOLFIRI (5-FU plus irinotecan) chemotherapy, patients having signed an informed consent form and affiliated to the Social Security scheme. Exclusion criteria were as follows: patients taking an immunosuppressive therapy, patients with severe, concomitant and progressive skin pathology, the absence of measurable lesion according to RECIST 1.1 criteria (Response Evaluation Criteria in Solid Tumors), patients with a contraindication to the use of cetuximab or FOLFOX and FOLFIRI, severe impairment of cardiac or respiratory function and patients deprived of their liberty by judicial or administrative decision.
Cetuximab was administered at 500 mg/m2 every 14 d during a 2-hour infusion for the first cycle and a one-hour injection thereafter. Premedication with corticosteroids and antihistamines was mandatory before every injection.
Immunological profile of skin biopsies
Two skin biopsies (6 mm punch) were undertaken from the upper back region of patients by a dermatologist before initiation of the treatment. One was frozen in nitrogen and used for RNA extraction and analysis. The second one was embedded in paraffin for pathological analyses. At day 28 (D28), before the third chemotherapy/cetuximab cycle, three 6 mm biopsies were performed, two in the rash zone (if it was present, one frozen and one embedded in paraffin) and one in an unaffected zone (frozen).
We selected a panel of different cytokines or indicators representative of skin inflammation in order to determine an immune signature of anti-EGFR induced skin rash. The selection of these inflammatory factors is based on literature data on anti-EGFR induced skin rash (i.e. TNF-α, IFN-γ and IL-8) but also cytokines frequently involved in inflammatory skin diseases like psoriasis and atopic dermatitis (i.e. IL-8, IL-17A, IL-22, Oncostatin M, Thymic stromal lymphopoietin (TSLP), IL-1α, and TNF-α) and antimicrobial peptides such as BD-2 and S100A7.Citation20,Citation22–26 Indeed, expression of the Th17-related cytokines (IL-17A, IL-21, IL-22), antimicrobial peptides (S100A7 and BD-2), the innate response-related cytokines (IL-1β, IL-6, TNF-α and oncostatin-M (OSM)), the T-reg-related cytokines (IL-10 and TGF-β), the Th1-related cytokine (IFN-γ), the Th2-related cytokine (IL-4), TSLP and keratinocyte-derived cytokines (IL-8, IL-23 and CCL20) were determined by reverse transcription PCR (RT-PCR).Citation24 Briefly, total RNA from skin biopsies (including epidermis and dermis) was isolated using a NucleoSpin® RNA II kit (Macherey-Nagel, Hoerdt, France) and reverse-transcribed with SuperScript® II reverse transcriptase (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was conducted using a Light Cycler-FastStart DNA MasterPlus SYBR® Green I kit and a LightCycler 480 system (Roche Diagnostics, Meylan, France). The reaction components consisted of 1x DNA Master Mix and 0.5 µM HPLC-purified (High Performance Liquid Chromatography) sense and anti-sense oligonucleotides purchased from Eurogentec (Eurogentec France, Angers, France) and designed using Primer3® software. The mathematical model delta Ct was used to determine the relative quantification of target genes compared to the housekeeping genes G3PDH and RPL13.Citation27
To characterize the skin rash and determine the immune cells infiltrates, immunohistochemistry analyses (IHC) were carried out using CD3 (T cells), CD4 (helper T cells), CD8 (cytotoxic T cells), Granzyme (cytotoxicity), CD15 (neutrophils), CD68 (monocytes/macrophages), CD20 (B cells), CD30 (usually expressed on activated B cells and T cells), CD56 (Natural killer cells), PD1 (usually expressed on T cells), FOXP3 (regulatory T cells) and CD1a (usually expressed on Langerhans cells and dendritic cells). The level of EGFR expression was also evaluated. IHC was performed using a BenchMark® automated staining system (Ventana Medical System, Tucson, AZ, USA) according to the manufacturer’s instructions.
Serum concentration of cytokines
Blood samples were also collected for the analysis of circulating cytokines at D0, D28, 3 and 6 months. Serum was collected, aliquoted and kept frozen at −20°C until analysis. The concentration of circulating cytokines (IL-4, IL-6, IL-8, IL-10, IL-17A, IL-21 and IL-23) was quantified with Luminex 200 platform® (Luminex xMap Technology) by using Milliplex MAP® kit, human cytokine/chemokine Magnetic Bead Panel (Millipore Corporation, Billerica, MA, USA) according to the manufacturer’s instruction.
Outcomes
Skin toxicity was evaluated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events v4.0 (NCI-CTCAE) at each chemotherapy/cetuximab cycle (every 14 d). Treatment response was assessed radiologically and evaluated according to RECIST 1.1 criteria, i.e. progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR), at first evaluation at about 3 months (M3) and second evaluation at about 6 months (M6). Depth of response was defined as the best percentage of tumor shrinkage compared to baseline according to RECIST 1.1 criteria. Median progression-free survival (PFS) and OS were calculated using the Kaplan–Meier method.
The main objective was to identify an association between skin immune marker mRNA expression (RT-PCR) and treatment efficacy using objective response rate (ORR) according to RECIST 1.1 criteria. ORR is defined as the percentage of patients with a response of either PR and CR. The secondary objectives were to identify an association between the skin immune marker mRNA and PFS, and also an association between circulating cytokine concentrations and ORR and PFS.
Statistical analysis
No sample calculation was made since it was a pilot exploratory study and we arbitrarily planned to recruit 30 patients. Quantitative data were described by means and standard deviations (SD), or medians and interquartile ranges (IQR). Categorical data were presented as absolute frequencies and percentages.
To study variations of cytokines between D0 and D28, ratios of levels between D28 (unaffected zone or rash zone) and D0 were calculated and a non-parametric signed rank test was used to compare the ratio to 1.
Comparisons between subgroups were performed using chi-square test or Fisher’s exact test for qualitative variables and using the student t test or Wilcoxon signed-rank test for quantitative variables. Correlations between quantitative variables were estimated by the Spearman correlation coefficient (Rho).
Survival curves were estimated using the Kaplan–Meier method and described using medians and 95% confidence intervals (CI). The log rank test was used to compare survival curves. Hazard ratios were calculated based on Cox proportional hazards model.
All statistical tests were two-sided and of exploratory nature, thus no alpha-level adjustment for multiple testing was considered. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and R statistical software version 3.6.0.
Results
Patients’ characteristics, skin toxicity and outcomes
A total of 27 patients were enrolled in this study between 2011 and 2016. Patients’ median age was 66 y. These patients were mainly men (78%) and presented a WHO performance status of 0 or 1 (89%) (Supp Table 1). Most patients had liver metastases (89%) and the majority received the FOLFIRI plus cetuximab regimen as first-line chemotherapy (67%).
Twenty-three patients (85%) displayed a skin reaction at D14 (whatever the type) and 24 (89%) at D28, mostly grade 2 (63% at D14 and 78% at D28) and as a papulopustular rash (78% at D14 and 70% at D28) (). Grade 3 skin reactions were found in only 2 patients at D14 and none at D28. Due to skin reactions, some patients were treated with oral doxycycline (63%) and/or metronidazole cream (48%) at D14. Three patients had serious adverse events (cetuximab-induced anaphylaxis).
Table 1. Skin reactions at days 14 and 28
Response rates according to RECIST version 1.1 were 44% (n = 12) PR, 44% (n = 12) SD and 7% (n = 2) PD (one patient was non-evaluable) at the first evaluation (M3) and 50% (n = 10) PR, 20% (n = 4) SD and 30% (n = 6) PD (seven patients were non-evaluable) at the second evaluation (M6). Mean depth of response were −26% (min-max, −80%; +35%) at the first evaluation and −29% (min-max, −70%; +40%) at the second evaluation.
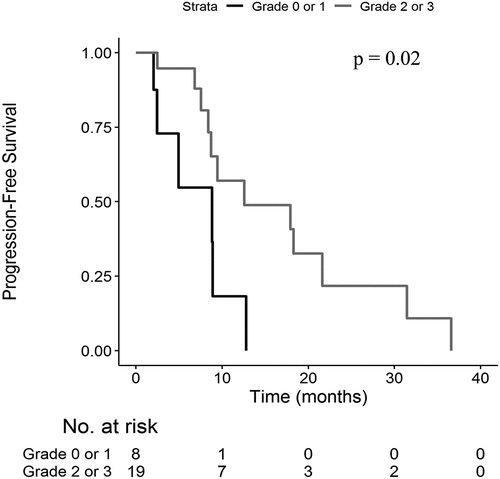
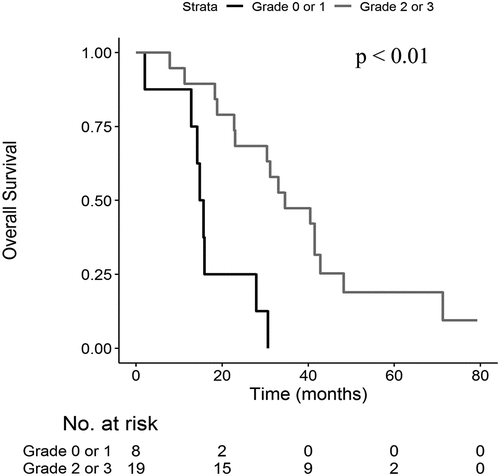
The end of the study was in December 2018. Median PFS (Supp Figure 1) was 9.5 months (95% CI 8.7–21.6) and median OS (Supp Figure 2) was 30.4 months (95% CI 18.8–41.5).
Skin toxicity and treatment response
No associations between objective response rate at the first evaluation and grade of skin reactions (grade 0 or 1 versus grade 2 or 3) at D14 or D28 were detected (Supp Table 2).
Patients with grade 2 or 3 skin reactions at D14 showed a longer median PFS (12.6 months versus 8.8 months; p = .02 ()) and OS (34.6 months versus 15.2 months; p < .01) compared to patients with grade 0 or 1 skin reaction (). Median PFS was 12.6 months versus 8.8 months (p = .12) and median OS was 33.0 months versus 15.3 months (p = .01) in patients with grade 2 or 3 skin reactions at D28 compared to patients with grade 0 or 1 skin reactions.
Immunological profile of skin biopsies
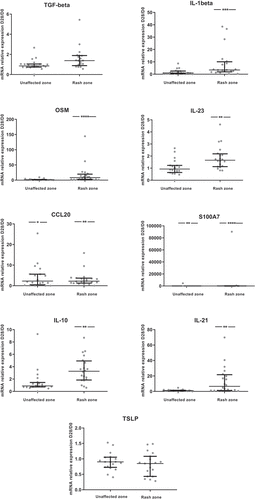
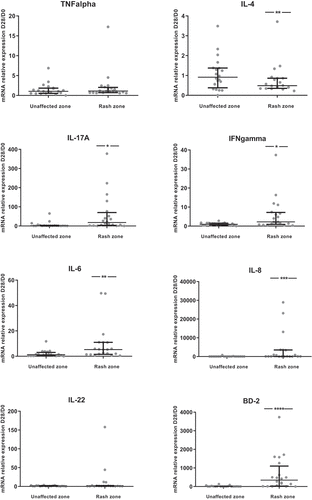
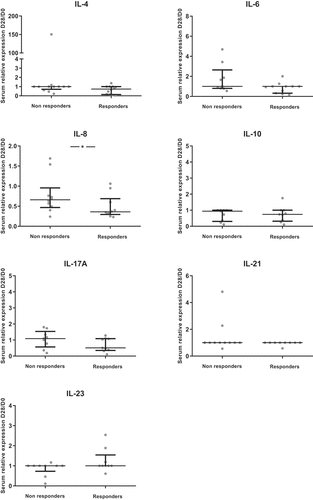
Only 20 patients had available and exploitable pre-therapeutic and post-therapeutic biopsies (7 patients with early death or degradation of skin biopsies’ RNA). The levels of cytokines or inflammatory factors were increased between skin biopsies at D28 in an unaffected zone and pre-therapeutic biopsies at D0 for only CCL20 and S100A7 (). However, the levels between skin biopsies at D28 in a rash zone and biopsies at D0 were increased for most cytokines/inflammatory factors studied (IL-1ß, IL-6, IL-8, IL-10, IL-17A, IL-21, IL-23, OSM, IFN-γ, S100A7, CCL20, BD-2), except for IL-4, whose level was lower at D28 compared to D0, and TNF-α, IL-22, TGF-ß and TSLP which remained unchanged ().
Figure 3. Ratios of skin cytokines levels between D28 and D0 (N = 20).The ratio D28/D0 is the ratio of cytokine levels between skin biopsies at D28 and pre-therapeutic biopsies at D0. Results are available for the unaffected zone and the rash zone with medians and interquartile ranges. A non-parametric signed rank test was used to compare the ratio with 1.*: p < .05; **: p < .01; ***: p < .001; ****: p < .0001
We have compared cytokine expression between patients with (n = 13) and without (n = 7) oral doxycycline. We identified an increase in the ratios of cytokines’ levels between D28 and D0 in the oral doxycycline group compared to the group without oral doxycycline in the rash zone only for TGF-ß (p = .01) and IL-23 (p = .02). We have also compared positive Gram staining on D28 skin biopsies in the rash zone (n = 3/17) between patients with (n = 12) and without (n = 5) oral doxycycline used before D28 and no association was observed (p = .22).
The ratios of cytokines between D28 and D0 were positively correlated with the skin reaction grade at D28 for TNF-α (rho = 0.45; p < .05), CCL20 (rho = 0.52; p = .02), S100A7 (rho = 0.58; p = .01), TSLP (rho = 0.56; p = .02) in the unaffected zone and BD-2 (rho = 0.46; p = .04), CCL20 (rho = 0.55; p = .02), S100A7 (rho = 0.49; p = .03), IL-21 (rho = 0.45; p < .05), TSLP (rho = 0.53; p = .03) in the rash zone (Supp Table 3).
Immunological profile of skin biopsies according to treatment effectiveness
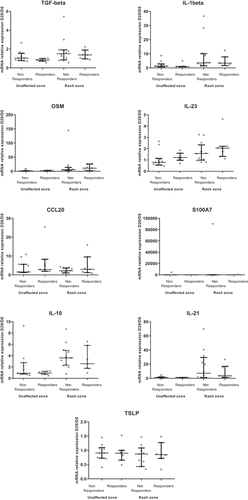
Most of the cytokines/inflammatory factors evaluated as ratios of levels between D28 (unaffected zone or rash zone) and D0 showed no difference between patients with and without objective response (). Only the ratio of IL-4 between the unaffected zone at D28 and D0 was higher in responders compared to non-responders (p = .04) (). When looking at the depth of response, there was a moderate correlation between higher of depth of response at M3 and an increase of the ratio of IL-23 between D28 and D0 in the unaffected zone, as well as in the rash zone (Rho = −0.56; p = .02 for both zones) (Supp Table 4). In addition, there was no impact on oral doxycycline use on objective response rate (p = .34).
Figure 4. Comparison of D28/D0 ratios of skin cytokine levels according objective response at first evaluation (M3) (N = 19)
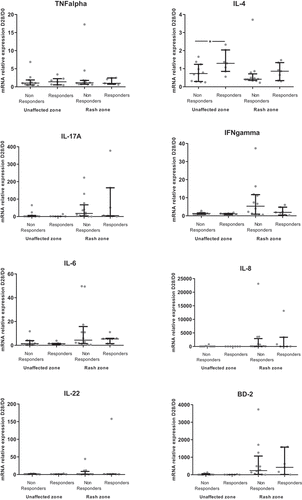
The D28/D0 ratio of IL-23 in the unaffected zone was higher for responders than non-responders at the second evaluation at M6 (p = .03), all other cytokines showing no difference between responders and non-responders (Supp Figure 3). When looking at the depth of response at the second evaluation, there were good correlations between the D28/D0 ratios of IL-4, IL-23 and CCL20 in the unaffected zone and the depth of response (Supp Table 5).
Higher values of the D28/D0 ratios for IL-8 and IL-22 in the unaffected zone and for IL-8, BD-2, IL1-ß and OSM in the rash zone were associated with better PFS whereas lower values of TSLP in the unaffected zone were associated with better PFS (Supp Table 6). Higher values of D28/D0 ratios for IL-21 in the unaffected zone and for IL1-ß in the rash zone were associated with better OS (data not shown).
Correlation of circulating cytokines with skin cytokines and outcomes
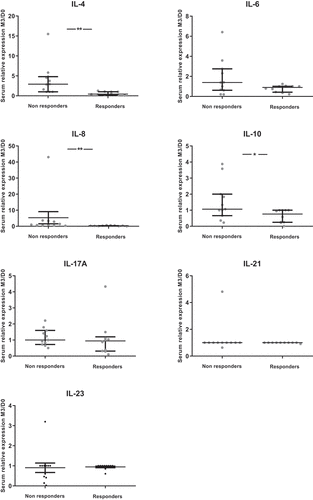
Concerning circulating cytokines, we observed a decrease from D0 to D28 for IL-4, IL-8 and IL-10 serum levels. This decrease was also observed from D0 to M6 for IL-8 (Supp Figure 4). We observed no significant correlation between circulating cytokines and skin cytokines except for IL-23 (rho = 0.62; p < .01) (data not shown).
We observed lower D28/D0 ratios of circulating IL-8 in responders compared to non-responders at M3 and M6 ( and Supp Figure 5). Moreover, lower ratios of circulating IL-8 were correlated with the depth of response at M3 (rho = 0.51; p = .01) and M6 (rho = 0.59; p = .03) (data not shown). A lower D28/D0 ratio of circulating IL-8 was also associated with a longer PFS (p < .01).
Figure 5. Comparisons of the ratios of circulating cytokine levels according to objective response at first evaluation (M3) (N = 26)
Immune cells infiltrates
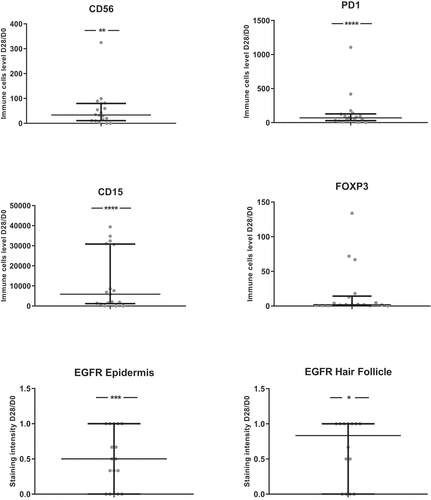
To characterize the immune cells involved in the skin reactions to anti-EGFR therapy, we performed IHC analyses. The number of most immune cells or levels of markers of immune cells activation (CD3, CD4, CD8, CD20, CD68, Granzyme, CD1a, CD15, CD56 and PD1) were increased between skin biopsies at D28 compared to pre-therapeutic biopsies at D0 ( and Supp Figure 6). In contrast, EGFR expression decreased between D0 and D28.
Figure 6. Ratios of immune cells levels between D28 and D0 (N = 18)
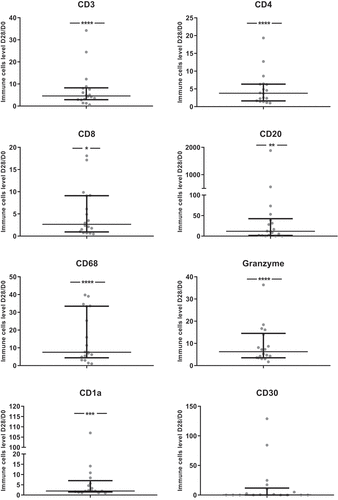
For CD4, CD8 and CD68 D28/D0 ratios, a significant correlation with D28 skin toxicity grade was found (data not shown). For all immune cells evaluated as ratios of levels between D28 and D0, no significant difference between responders and non-responders was found. Higher values of D28/D0 ratios for CD4 + T cells were associated with shorter PFS (p = 0.03, data not shown).
Discussion
The CUTACETUX study provides new relevant data concerning the anti-EGFR mAb related skin reaction. Many cytokines/inflammatory factors in the skin increased during cetuximab treatment, and the increased levels of some of these were correlated with the grade of the skin reaction. Alteration of the amount of immune skin infiltrating cells was also associated with skin reactions. The main objective was to study the association between increased cytokines/inflammatory protein levels and treatment response and that was the case for IL-4 and IL-23, but only in the unaffected zone. Moreover, increased levels of IL-8, BD-2, IL-1β and OSM in the rash zone and decreased levels of TSLP in the unaffected zone were associated with longer PFS. The levels of circulating cytokines were not correlated with those of skin cytokine transcripts, except for IL-23. However, a decrease of circulating IL-8 was associated with treatment response and longer PFS. Finally, an increase of CD4 + T cells in the skin was associated with shorter PFS.
It has to be noted that we had some difficulties to include patients and this led to the study being stopped before recruiting the 30 patients planned. More than half of the patients to whom we proposed a participation in this study refused to do so due to the multiple invasive skin biopsies. However, our studied population seems representative of a standard mCRC population treated with chemotherapy plus anti-EGFR therapy. Median PFS (9.5 months) and OS (30.4 months) are in accordance with results observed in clinical trials.Citation2–4 Moreover, the rates and grades of skin reactions to anti-EGFR therapy are those usually observed in other trials (89% and mostly grade 1–2).Citation7,Citation8,Citation18 No correlation was observed between the grade of skin reactions and radiological response, probably due to the low number of patients.Citation8,Citation19 Nevertheless, we observed a correlation between the grade of skin reactions and PFS and OS, as previously described.Citation8
So far, skin inflammatory response due to anti-EGFR therapy has been poorly studied as skin biopsies have never been performed in clinical practice in mCRC patients under anti-EGFR treatment. The EGF pathway has been shown to be critical in the upregulation of keratinocyte GM-CSF expression under cytokine stimulation.Citation28 EGFR-driven activities on the immune/inflammatory functions of keratinocytes included upregulation of Toll-like receptor 5 (TLR5) and TLR9, antimicrobial peptides BD-2 and BD-3, and IL-8.Citation29 In contrast, the activation of the EGF pathway in the skin induces a decrease of pro-inflammatory cytokines released by the keratinocytes in different skin diseases such as atopic dermatitis and psoriasis.Citation22,Citation23 The mechanism of the papulopustular rash induced by anti-EGFR therapy is linked to the blockade of the EGF pathway in keratinocyte, associated to a poorly characterized pro-inflammatory response.Citation20,Citation22
The design of the CUTACETUX study included skin biopsies in the unaffected zone and in the rash zone to identify the cytokines/inflammatory factors variations related to anti-EGFR-induced skin reactions. Indeed, we observed in the unaffected zone a significant increase of CCL20 and S100A7, produced among others by the keratinocytes. In the rash zone, we observed a significant increase in most of the cytokines/inflammatory factors studied, especially superior to 10-fold for Th17-related cytokines (IL-17A and IL-21), OSM, antimicrobial peptides (S100A7 and BD-2), and IL-8. Interestingly, IL-17, OSM and IL-21, which are produced by skin immune cells, induced morphologic changes of the skin epithelium (thickened skin with confluent parakeratotic scale and loss of the granular cell layer) and S100A7, BD-2, IL-8 production by keratinocytes are over expressed in skin diseases such as psoriasis.Citation24–26 By using IHC, we identified cells associated with anti-EGFR therapy-related skin reactions. Most of the immune cells increased in the rash zone are T lymphocytes, monocytes/macrophages and NK cells.Citation30 By defining a cytokine signature, these results could help to better understand the mechanism of anti-EGFR therapy-related skin reactions and support the development of new specific topical treatments. Nonetheless, we currently use topical and/or systemic antibiotics like tetracyclines to treat skin rash due to anti-EGFR therapy. The wide spectrum of anti-inflammatory effects related to tetracyclines is probably due to their ability to interfere with the synthesis or the activity of several mediators of inflammation, such as TNF-α, IL-1β, and IL-6.Citation31–34 However, this involves a risk of emerging resistance with the use of antibiotics on the long term, and this is not recommended.Citation35 In addition, recent advances in the field of anti-EGFR induced skin rash point to an involvement of the commensal microbiota.Citation36 Moreover, antibiotic’s use has recently been negatively correlated with treatment response in many cancers, especially in non-small cell lung cancer.Citation37 In our study, oral doxycycline had low effect on skin cytokines/inflammatory factors and was only associated with an increase of TGF-ß and IL-23 in the skin rash zone between D0 and D28. Nevertheless, oral doxycycline had no impact on cancer treatment efficacy. Furthermore, in our study, oral doxycycline use had no impact on bacterial involvement in the skin sections at D28 using Gram staining.
New treatments of anti-EGFR-induced papulopustular rash are an important unmet need since skin reactions contribute to a significantly reduced quality of life for mCRC patients treated with anti-EGFR therapy.Citation38 Emerging topical JAK (Janus kinase) inhibitors in dermatology could be interesting since the inhibition of JAKs can simultaneously block the function of multiple cytokines such as IL-21, IL-23, OSM and IL-6, which were found in our study to be key cytokines involved in skin toxicity of anti-EGFR therapy.Citation39
As a correlation between anti-EGFR-induced skin reactions and treatment efficacy was described, we looked for a correlation between skin cytokine levels and the efficacy of anti-EGFR therapy. An increase of IL-4 and IL-23 levels between D28 and D0 only in unaffected skin zones was correlated with treatment response. In addition, some cytokines/pro-inflammatory factors were associated with PFS, especially IL-8, TSLP and IL-22 in the unaffected skin zone and IL-8, BD-2, IL1-ß and OSM in the rash zone. IL-8, IL-23, TSLP and BD-2 are mainly keratinocyte-derived factors. We hypothesize that EGFR blockade on keratinocytes is correlated with EGFR blockade on tumor cells and explain the observed association with the efficacy of anti-EGFR therapy. Moreover, anti-EGFR agents induce the production of pro-inflammatory cytokines by cancer cells, thus possibly influencing the tumor response.Citation40 Indeed, a link between cytokines involved in tumor response to anti-EGFR agents and cytokines involved in the development of skin reactions to anti-EGFR therapy is possible. In the pathogenesis of CRC, Th17 cell-mediated responses and production of IL-17A in the tumor microenvironment have been associated with worse outcomes for patients.Citation41 The increasing proportion of Th17 cells in peripheral blood and IL-17A, IL-6, IL-22, IL-23 levels in serum among CRC patients compared to control subjects were reported, but their association with treatment efficacy remains unknown.Citation42 In the CUTACETUX study, skin Th17 cytokines were not associated with the efficacy of anti-EGFR therapy. Skin CD4 + T cells were associated with shorter PFS. Indeed, in CRC, tumor infiltrating CD4 + T-cells have been associated with poor outcomes, especially Th2 CD4 + T-cells.Citation43
Clinical evidence suggests that local cutaneous inflammatory response has systemic repercussions because changes in circulating cytokines and chemokines were documented in patients treated with anti-EGFR therapy.Citation44–47 In the CUTACETUX study, we observed a significant decrease in the circulating levels of IL-4, IL-8 and IL-10 during anti-EGFR treatment. Moreover, there was an association between decreased levels of IL-8 and treatment efficacy, for both treatment response and PFS. This might reflect a decreasing pro-inflammatory condition in patients with an effective oncologic treatment rather than a direct effect of anti-EGFR treatment.Citation48,Citation49 Other CRC studies have shown that a decrease of circulating IL-8 was correlated with treatment efficacy.Citation48,Citation50,Citation51 IL-10 and IL-4 are T-reg-related and Th2-related cytokines both with anti-inflammatory properties usually associated with poor outcomes in CRC, but not in our study.Citation43 Inflammatory response in the skin, which is related to anti-EGFR treatment, has probably a different mechanism compared to the systemic inflammatory response in the blood related to both treatment and cancer condition. We could not establish a correlation between skin and systemic cytokines. If we consider the autocrine/paracrine action of cytokines in tissues and the fact that circulating cytokines are the inconsistent reflection of the tip of the iceberg, measuring cytokine expression in an inflamed tissue may be the most reliable way to evaluate a cytokinic signature associated with pathophysiology.
The main limitation of our study is the use of the combination of anti-EGFR plus chemotherapy, which can make it difficult to identify an association between the levels of skin cytokines and the efficacy of anti-EGFR therapy. Nevertheless, we included only patients treated in the first-line setting and not patients with previous chemotherapy that could have impacted skin and blood cytokine levels. Furthermore, it is difficult to grade the skin reactions, which is in part subjective, even though it was done by a dermatologist in the CUTACETUX study. Nonetheless, for the first time, this study collected prospective skin biopsies in patients treated with anti-EGFR therapy and identified relevant associations between skin cytokines, skin reactions and the efficacy of anti-EGFR therapy in mCRC.
To conclude, we described an original skin immune signature, i.e. cytokines/inflammatory factors and immune cells, of skin rash induced by anti-EGFR therapy and showed its possible association with skin toxicity grade. Moreover, levels of Th2-related (IL-4), innate response-related cytokines (IL-1β and OSM) and keratinocyte-derived cytokines (IL-8 and IL-23), as well as CD4 + T cells, in the skin seemed to be associated with the efficacy of anti-EGFR therapy. Nevertheless, we cannot establish a correlation between skin and systemic cytokines.
Disclosure of potential conflicts of interest
This work was supported in part by Merck Santé SAS, Lyon, France; an affiliate of Merck KGaA, Darmstadt, Germany. Merck KGaA, Darmstadt, Germany reviewed the manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
No other potential conflicts of interest were disclosed.
Supplemental Material
Download ()Acknowledgments
We thank Vanessa Le Berre, a research secretary, for her help in editing and formatting the manuscript. We thank the ImageUP platform for serum cytokines analysis.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Fact Sheets by Cancer [Internet]. [accessed 2018 Jul 9]. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–15.
- Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. 2017;20(317):2392–2401.
- Vincenzi B, Cremolini C, Sartore-Bianchi A, Russo A, Mannavola F, Perrone G, Pantano F, Loupakis F, Rossini D, Ongaro E, et al. Prognostic significance of K-Ras mutation rate in metastatic colorectal cancer patients. Oncotarget. 2015;6:31604–31612.
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342.
- Van Cutsem E, Köhne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345.
- Messa C, Russo F, Caruso MG, Di Leo .EGF. TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–289.
- Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105–115.
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232.
- Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American joint committee on cancer stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92:1331–1346.
- Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970.
- Van Cutsem E, Lenz H-J, Köhne C-H, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700.
- Correale P, Cusi MG, Micheli L, Nencini C, Del Vecchio MT, Torino F, Aquino A, Bonmassar E, Francini G, Giorgi G. Chemo-immunotherapy of colorectal carcinoma: preclinical rationale and clinical experience. Invest New Drugs. 2006;24:99–110.
- Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2:e26333.
- Peréz-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–5246.
- Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417.
- Lièvre A, Bachet J-B, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379.
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812.
- Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol. 2002;20:110–124.
- Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–312.
- Pastore S, Mascia F. Novel acquisitions on the immunoprotective roles of the EGF receptor in the skin. Expert Rev Dermatol. 2008;3:525–527.
- Rabeony H, Petit-Paris I, Garnier J, Barrault C, Pedretti N, Guilloteau K, Jegou JF, Guillet G, Huguier V, Lecron JC, et al. Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin M. PloS One. 2014;9:e101937.
- Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol. 2007;178:4615–4622.
- Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J Immunol. 2010;184:5263–5270.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
- Mascia F, Lam G, Keith C, Garber C, Steinberg SM, Kohn E, Yuspa SH. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Transl Med. 2013;5:199ra110.
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374.
- Schön MP. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol. 2019;10:1764.
- Bahrami F, Morris DL, Pourgholami MH. Tetracyclines: drugs with huge therapeutic potential. Mini Rev Med Chem. 2012;12:44–52.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265.
- Dormán G, Cseh S, Hajdú I, Barna L, Kónya D, Kupai K, Kovács L, Ferdinandy P. Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs. 2010;70:949–964.
- Meyer-Hoffert U. Reddish, scaly, and itchy: how proteases and their inhibitors contribute to inflammatory skin diseases. Arch Immunol Ther Exp. 2009;57:345–354.
- Dréno B, Bettoli V, Ochsendorf F, Layton A, Mobacken H, Degreef H, et al. European recommendations on the use of oral antibiotics for acne. Eur J Dermatol. 2004;14:391–399.
- Klufa J, Bauer T, Hanson B, Herbold C, Starkl P, Lichtenberger B, Srutkova D, Schulz D, Vujic I, Mohr T, et al. Hair eruption initiates and commensal skin microbiota aggravate adverse events of anti-EGFR therapy. Sci Transl Med. 2019;11(11):522.
- Liu K, Zhang W, Tan Q, Jiang G, Jia J. Antibiotic use is a negative predictor of the efficacy and toxicity of epidermal growth factor receptor-targeted therapy in advanced non-small cell lung cancer. Oncol Lett. 2019;18:2677–2683.
- Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–1357.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Hoffmann TK, Schirlau K, Sonkoly E, Brandau S, Lang S, Pivarcsi A, Balz V, Müller A, Homey B, Boelke E, et al. A novel mechanism for anti-EGFR antibody action involves chemokine-mediated leukocyte infiltration. Int J Cancer. 2009;124:2589–2596.
- Hurtado CG, Wan F, Housseau F, Sears CL. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterol. 2018;155:1706–1715.
- Yan G, Liu T, Yin L, Kang Z, Wang L. Levels of peripheral Th17 cells and serum Th17-related cytokines in patients with colorectal cancer: a meta-analysis. Cell Mol Biol. 2018;64:94–102.
- Roxburgh CSD, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466.
- Argiris A, Lee SC, Feinstein T, Thomas S, Branstetter BF, Seethala R, Wang L, Gooding W, Grandis JR, Ferris RL, et al. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol. 2011;47:961–966.
- Debucquoy A, Haustermans K, Daemen A, Aydin S, Libbrecht L, Gevaert O, De Moor B, Tejpar S, McBride WH, Penninckx F, et al. Molecular response to cetuximab and efficacy of preoperative cetuximab-based chemoradiation in rectal cancer. J Clin Oncol. 2009;27:2751–2757.
- Kanazawa S, Yamaguchi K, Kinoshita Y, Komiyama Y, Muramatsu M, Nomura S. Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib. J Cancer Res Clin Oncol. 2006;132:719–725.
- Kimura H, Kasahara K, Sekijima M, Tamura T, Nishio K. Plasma MIP-1beta levels and skin toxicity in Japanese non-small cell lung cancer patients treated with the EGFR-targeted tyrosine kinase inhibitor, gefitinib. Lung Cancer. 2005;50:393–399.
- Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049.
- Malicki S, Winiarski M, Matlok M, Kostarczyk W, Guzdek A, Konturek PC. IL-6 and IL-8 responses of colorectal cancer in vivo and in vitro cancer cells subjected to simvastatin. J Physiol Pharmacol. 2009;60:141–146.
- Rubie C, Frick VO, Pfeil S, Wagner M, Kollmar O, Kopp B, Graber S, Rau BM, Schilling MK. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002.
- Gelfo V, Rodia MT, Pucci M, Dall’Ora M, Santi S, Solmi R, Roth L, Lindzen M, Bonafè M, Bertotti A, et al. A module of inflammatory cytokines defines resistance of colorectal cancer to EGFR inhibitors. Oncotarget. 2016;7:72167–72183.