ABSTRACT
Breast cancer is the most common form of cancer in women worldwide. Although the survival among breast cancer patients has improved, there is still a large group of patients with dismal prognosis. One of the most important prognostic factors for poor prognosis is lymph node metastasis. Increasing knowledge concerning the lymph nodes of breast cancer patients indicates that they are affected by the primary tumor. In this study we show that presence of CD169+ subcapsular sinus macrophages in contact with lymph node metastases in breast cancer patients, is related to better prognosis after adjuvant tamoxifen treatment, but only in patients with PDL1+ primary tumors. This is in contrast to the prognostic effect of CD169+ primary tumor-associated macrophages (TAMs). We further show that CD169+ macrophages were spatially associated with expression of PDL1 on nearby cells, both in primary tumors and metastatic lymph node, although PDL1 expression in metastatic lymph node as such did not have further prognostic impact. Our data suggest that CD169+ resident lymph node macrophages have a unique function in targeting immune responses against breast cancer and should be further investigated in detail.
Introduction
Breast cancer is the most common type of cancer among women and is divided into different subtypes depending on the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki67, and histological grade.1 Whereas breast cancers with a hormone receptor-positive status (ER+PR+) have a beneficial short-term prognosis, those that lack all three receptors (ER−PR−HER2−; triple-negative breast cancers; TNBC) have a worse prognosis.Citation1 Still, for all breast cancer subtypes, the dissemination of tumor cells to the lymph nodes is one of the most significant prognostic factors associated with worse prognosis.Citation2
Lymph nodes are secondary lymphoid organs where immune responses are mounted.Citation2 It is here that the tumor-associated antigens are transported to be recognized by the adaptive immune response, so that a tumor-specific immune attack can be started. In the lymph nodes, cells of the innate immune response are present, with various functions, but one important function is to act as antigen-pesenting cells (APCs); to phagocytose and present foreign substances (antigens) to the adaptive lymphocytes (T cells and B cells). The most important APC for the activation of naïve T cells are dendritic cells (DCs), while macrophages can induce activation of effector or memory T cells and naïve B cells.Citation3 Tumor antigens are mutated proteins that are present in the malignant cells. Evidence suggest that tumor-draining lymph nodes are affected by the tumor, and that the immune balance in the lymph node affects the anti-tumor immune response.Citation4,Citation5
Conventional tumor-associated macrophages (TAMs) located in the primary tumor are mostly associated with a worse prognosis in cancer patients.Citation6–9 In lymph nodes however, there are resident macrophages, that are subdivided into specific populations. One subtype of lymph node resident macrophages is the subcapsular sinus macrophages (CD169+).Citation10,Citation11 These specialized CD169+ macrophages surround the lymphoid follicles in lymph nodes and act as gate-keepers for antigen delivery.Citation3 In mice, they have been proposed to be involved in the activation of B, T and NK cells, but also in regulating overt immune responses and Tregs.Citation10,Citation12–15 The CD169+ macrophages have also been shown to be specialized in phagocytosing and bringing distant tumor cell antigens to the lymph nodes in mice.Citation16 In humans, the presence of CD169+ macrophages in metastasis-free lymph nodes of cancer patients with endometrial, bladder, prostate, and colorectal cancer has previously been correlated to an improved prognosis.Citation17–20 In contrast, a similar study on breast cancer patients showed that presence of lymph node CD169+ macrophages, in lymph nodes without metastasis, correlated to early tumor stage, but not to prognosis.Citation21 High expression of SIGLEC1 (CD169) in primary breast tumors, on the other hand, is associated with shorter disease-specific survival in public datasets derived from tumor samples from breast cancer patients.Citation22
During the last years, immune checkpoint inhibitors have revolutionized clinical care in oncology.Citation23 Antibodies targeting CTLA4, PD1, and PDL1 have been evaluated with therapeutic success in many types of cancer. In breast cancer however, the success is hitherto more limited.Citation24 The only example in breast cancer is the positive effect of anti-PDL1 (atezolizumab) – nab-paclitaxel combination therapy in advanced TNBC.Citation25 The reason to this is unknown and more information is needed to understand breast cancer-induced immune responses.Citation24,Citation26 PDL1 is expressed on both APCs and tumor cells.Citation26 In cervical cancer patients, PDL1-expressing macrophages with immunosuppressive character have been found surrounding metastatic tumor cells in lymph nodes with metastasis,Citation27 and this correlated with non-responsive, tolerogenic lymphocytes.Citation28 Interestingly, CD169+ macrophages are responsible for induction of PDL1 expression via local type I IFN production in viral infections, which lead to a local T cell exhaustion.Citation12 Whether PDL1 is co-expressed with CD169, in vicinity of, or on the subset of CD169+ subcapsular sinus macrophages in lymph nodes with metastases and primary tumors of cancer patients, and what the consequences this would have on immune escape, is not known.
This study included patients with primary breast cancer who received 2 years of adjuvant tamoxifen. We retrieved tissue samples from primary tumors and synchronous lymph nodes with metastases. We investigated whether CD169+ subcapsular sinus lymph node macrophages, present in direct contact with cancer cells in lymph node metastases, as compared to CD169+ macrophages located in primary tumor (TAMs), would be a prognostic factor for breast cancer patients or not. We further investigated whether CD169+ lymph node and CD169+ primary tumor-associated macrophages were associated with PDL1 expression in breast cancer patients, as they are in viral infections,Citation12 and how this correlated to prognosis.
Materials and methods
Patients
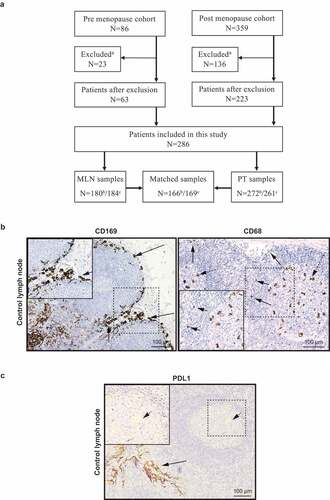
This study was based on a representative cohort of primary stage 2 breast cancer patients (N = 445) from two prospective-randomized clinical trials that included patients from the South-Swedish Health Care Region during 1985–1994.Citation29–31 At that time neither adjuvant chemotherapy nor anti-HER2 therapy were included in general treatment guidelines for primary breast cancer in the South Swedish Health Care Region. Only patients treated with 2 years of tamoxifen were included. Two of the premenopausal patients received adjuvant chemotherapy in addition to tamoxifen. 159 patients were excluded due to loss of primary tumor and metastatic lymph node tissue, leaving 286 for the present study. 272 samples were annotated for CD169 and PDL1 expression in primary tumor and 180 for metastatic lymph node. Matched primary tumor and lymph node samples were obtained from 166 patients ()). For CD68 staining, 261 samples were annotated for primary tumor and 184 for metastatic lymph node. Matched samples were obtained from 169 patients. For PD1 staining, 263 samples were annotated for primary tumor and 177 for metastatic lymph node. Matched samples were obtained from 159 patients. Patient and tumor characteristics for the patients included, as well as those excluded, are summarized in . Ethical approval for the use of retrospective breast cancer and lymph node specimens (Dnr 240–01), IHC control lymph node (Dnr 2010/477), and IHC control tonsil (Dnr 2017/941) was obtained from the Regional Ethics Committee in Lund, Sweden, and have been handled all in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Table 1. Patient characteristics and clinicopathological features
Figure 1. Cohort flow diagram and immunohistochemical staining of biomarkers in lymph node. (a) Cohort flow diagram for biomarker evaluation. aExcluded = both primary tumor (PT) and metastatic lymph node (MLN) material missing. b CD169 and PDL1 evaluation. c CD68 evaluation. (b) Positive staining control for CD169 (left) and CD68 (right) macrophages in a metastasis free control lymph node from a breast cancer patient. Arrows point to Subcapsular sinus macrophages (CD169+) surrounding the lymphoid follicles. The CD68 staining was titrated to show differences in intensity of CD68 in the various macrophage compartments in human lymph node, where black arrows point to subcapsular sinus macrophages with weak CD68 expression and dashed arrows point to germinal center tingible body macrophages with a strong CD68 expression.Citation32 (c) Positive staining control for PDL1 in a human tonsil. Arrows point to epithelial crypt cells (black arrows) and to a small extent and of weak expression in the germinal center macrophages in lymphoid follicles (dashed arrows) as previously described.Citation33
Tissue microarray and immunohistochemistry
The expression levels of ER, PR, Ki67, and HER2 had been reevaluated on whole sections or tissue microarrays (TMAs) from paraffin-embedded tumor material as previously described.Citation31,Citation34,Citation35 The experimental biomarkers in the present study were analyzed on TMAs. All cores were 1.0 mm in diameter.
Following antibodies and dilutions were used: anti-CD169 (dilution 1:500, Spring M5160), anti-PDL1 (dilution 1:500, Cell Signaling 29122), anti-CD68 (dilution 1:1500, DAKO M0876) chosen at a dilution and time to highlight the variations in intensity between macrophages located in different areas of a human lymph node as previously discussed by us in a recent review,Citation36 anti-PD1 (dilution 1:100, Abcam 137132). For control staining of metastasis-free lymph node and human tonsil see . TMA-sections were automatically pre-treated using the PT Link system and then stained in an Autostainer Plus (DAKO) at pH9 with an overnight staining protocol. As secondary antibody-staining protocol, a Double Stain Polymer Kit from Nordic Biosite (anti-mouse HRP (brown) and anti-rabbit AP (pink)) was used according to the manufacturer´s guidelines. The glass slides were fixed and mounted using xylene and Cyto Seal (DAKO).
Biomarker evaluation
CD169, CD68, and PDL1 staining was scored independently by three of the authors (FGB, NA and KL) and discordant scorings were discussed until consensus was reached. The density of CD169+ or CD68+ macrophages or PDL1+ cells, either in the primary tumor or in direct contact with the cancer cells in lymph node metastases of breast cancer patients, was scored as 0 (absent), 1 (<10%) or 2 (≥10%). If at least one of two cores was positive for biomarker expression, this tumor was classified as positive. For statistical analysis, these categories were dichotomized into absent (0) or present (1–2) biomarker expression. and show examples of immunohistochemical (IHC) staining of CD169, CD68, and PDL1. In addition to individual biomarker scoring, samples were also scored positive for CD169 and PDL1 co-expression (CD169+PDL1+), but only if the cells expressing the markers were in close proximity or both markers were expressed on the same cell. We also evaluated PD1 to visualize PD1 expressing lymphocytes in relation to PDL1 expression and macrophage distribution, and found PD1 to be expressed in the T cell zone, lymphoid follicles and germinal centers mainly () right). In the primary tumor and metastatic lymph node specimens, PD1 was scored as 0 (absent), 1 (<10%), 2 (≥10-25%) or 3 (>25%), whereby categories were dichotomized into low (0–2) or high (3) biomarker expression.
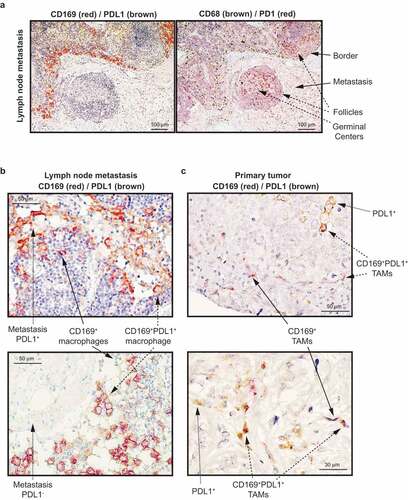
Figure 2. Immunohistochemical staining of CD169, PDL1 and CD68 in primary human breast tumors with paired lymph node metastases. (a) CD169 expression (red) and PDL1 expression (brown) (left) and CD68 expression (brown) and PD1 expression (red) (right) in lymph node metastases of breast cancer patients. Arrows point to the indicated histological structures. (b) CD169 expression (red) and PDL1 expression (brown) in lymph node metastases of breast cancer patients. The images show two metastases representing a PDL1+ (upper) and a PDL1− (lower) metastasis. Arrows point to single PDL1+ malignant cells, or co-expressing CD169+ PDL1+ macrophages. (c) CD169 expression (red) and PDL1 expression (brown) in primary tumor. Arrows point to single PDL1+ malignant cells, single CD169+ tumor associated macrophages (TAMs) or co-expressing CD169+ PDL1+ TAMs. The images show two representative primary tumors
Statistical analysis
The association between primary tumor (PT) and metastatic lymph node (MLN) expression of CD169, CD68, PDL1, and PD1, and different patient and tumor characteristics was analyzed using Fisher’s exact test, logistic regression, or Mann–Whitney U test, where appropriate. When planning the study, 5-year distant recurrence-free interval (DRFi) was chosen as endpoint for the prognostic analyses of the experimental markers. Longer follow-up could have been used, but prognostic effects of biomarkers, measured at the time of diagnosis, tend to weaken with follow-up time leading to non-proportional hazards. DRFi was defined as the time from surgery of the primary tumor until radiological and/or biopsy-verified recurrence or breast cancer-related death. Kaplan–Meier graphs were used to illustrate differences in 5-year DRFi according to CD169, CD68, PDL1, and PD1 expression and log-rank tests used to quantify the evidence against the null hypotheses of equality. Cox regression models were used for estimation of hazard ratios (HR) with 95% confidence interval (CI) according to CD169 expression in metastatic lymph node in both uni- and multivariable analysis. Proportional hazards assumptions were checked graphically. The established prognostic factors tumor size, histological grade, ER, PR, Ki67, HER2, and age, were included in multivariable Cox analyses. Statistical calculations were performed using IBM SPSS Statistics (version 26.0). All P values presented are two-sided and should in general be regarded as continuous measures of evidence, but following Benjamin et al., two thresholds will be used throughout this paper: suggestive evidence for P values between 0.05 and 0.005 and significant evidence for P < 0.005.Citation37
Results
Distribution and characterization of CD169+ macrophages
To investigate CD169+ lymph node macrophages and CD169+ TAMs, antibodies were chosen that recognize resident subcapsular sinus CD169+ macrophages surrounding the lymphoid follicles in lymph nodes ()), and the pan-macrophage marker CD68 used at a concentration and time to visualize the various staining intensities that macrophages have in different locations of human lymph node ()).Citation36 A PDL1 antibody that recognized cells primarily in the epithelial crypt cells of human tonsil and to a small extent and of weak expression in the germinal center macrophages as previously shown,Citation33 was chosen and verified () and left).
In lymph node with metastasis, the investigated lymph node CD169+ macrophages were located in direct contact with lymph node metastases, mostly surrounding and not preferentially infiltrating () left). Lymph node macrophages in general (CD68+), and PD1+ lymphocytes, were present also in the metastases and in lymphoid follicles () right). When PDL1 expression was present in the lymph node metastases, it was found primarily in the malignant cells per se or co-expressed on CD169+ macrophages () left and )). In the primary tumor, CD169+ tumor-associated macrophages (CD169+ TAMs) were often associated near or in direct contact with PDL1+ malignant cells, and co-expression of CD169+PDL1+ on macrophages was also observed () right).
Association between the experimental biomarkers and clinicopathological parameters
Presence of CD169+ macrophages in primary tumor (CD169+ PT) showed evidence of correlation with high Ki67 in the primary tumor, as well as with premenopausal status (). Presence of CD169+ cells in metastatic lymph node (CD169+ MLN) on the other hand, correlated with smaller primary tumor size, and to a lesser degree with PR-positivity (PR+) of the lymph node metastasis ().
Table 2. Odds ratios of presence of CD169+ and CD68+ macrophages in metastatic lymph node (MLN) and primary tumor (PT) by patient and tumor clinicopathological features
Just like CD169+ macrophages, presence of any CD68+ TAMs in the primary tumor in general (CD68+ PT), correlated with high Ki67 in the primary tumor and to premenopausal status. It further correlated with ER-negativity (ER−) of the primary tumor and higher histological grade. Interestingly, presence of CD68 in metastatic lymph node (CD68+ MLN) only showed evidence of correlation with high Ki67 in primary tumor and to some extent with high Ki67 in lymph node metastasis ().
PDL1 expression in the primary tumor (PDL1+ PT) showed evidence for correlation with ER− in both primary tumor and lymph node metastases, as well as to a TNBC primary tumor subtype (). It further correlated with high Ki67 in both primary tumor and lymph node metastases and PR-negativity (PR−) in the primary tumor (). PDL1 expression in metastatic lymph node (PDL1+ MLN) correlated both with younger age and a premenopausal status ().
Table 3. Odds ratios of presence of PDL1+ cells and co-expressing CD169+PDL1+ cells in metastatic lymph node (MLN) and primary tumor (PT) by patient and tumor clinicopathological features
Since most patients had PD1+ cells present in the primary tumor, and all had PD1+ cells present in the metastatic lymph node, high infiltration of PD1+ immune cells (PD1high) was used for statistical evaluation. As shown in Supplementary Table 1, PD1high in the primary tumor correlated with ER−, high Ki67, and a TNBC subtype in the primary tumor. PD1high in metastatic lymph node did not correlate with any of the clinicopathological features.
Correlation of CD169+ macrophages with PDL1, and PD1 expression
We next investigated whether CD169 expression would correlate with PDL1 expression as previously shown in viral infections.Citation12 Indeed, CD169 expression correlated positively with PDL1 expression both in primary tumor (OR = 8.4, 95% CI: (3.8–18.6), P < 0.001) and metastatic lymph node (OR = 3.6, 95% CI: (2.1–6.4), P < 0.001), although PDL1 expression was mostly present on adjacent cells, and only occasionally on the same cell (CD169+PDL1+) ()). Co-expression of CD169 and PDL1 in the primary tumor (CD169+PDL1+ PT) correlated with ER− in primary tumor and lymph node, PR− in primary tumor, high Ki67 in primary tumor, and positively with a TNBC primary tumor subtype, the same clinicopathological features that correlated with PDL1 expression alone in primary tumor, with the exception of high Ki67 in lymph node. Co-expression of CD169 and PDL1 in primary tumor showed evidence for further correlation with higher histological grade and HER2+ in primary tumor (). Co-expression of CD169 and PDL1 in metastatic lymph node (CD169+PDL1+ MLN) only showed evidence for a correlation with age, just as younger age correlated with PDL1 expression alone in metastatic lymph node ().
CD169 expression did not correlate with PD1 expression in the primary tumor (OR = 1.77, 95% CI: (0.70–4.48), P = 0.22) or in the metastatic lymph node (OR = 2.08, 95% CI: (0.68–6.38), P = 0.19), while PDL1 expression correlated with PD1 expression both in the primary tumor (OR = 9.44, 95% CI: (2.21–40.40), P < 0.001) and the metastatic lymph node (OR = 4.14, 95% CI: (1.37–12.52), P = 0.007).
The prognostic importance of the experimental biomarkers when analyzed individually
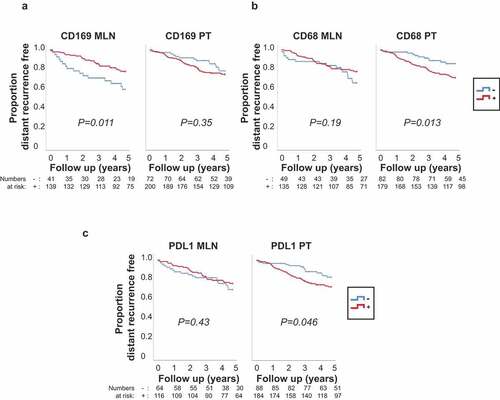
In univariable analyses, suggestive evidence for an association to better prognosis was seen for patients with CD169+ macrophages in metastatic lymph node compared to patients with no CD169+ macrophages in metastatic lymph node () left; HR = 0.46, 95% CI: (0.25–0.85), P = 0.013). This association was not seen when considering CD169 macrophages in the primary tumor () right; HR = 1.32, 95% CI: (0.73–2.41), P = 0.35). In contrast, patients with CD68+ macrophages in the primary tumor had worse prognosis compared to patients with no CD68+ macrophages in the primary tumor () right; HR = 2.24, 95% CI: (1.17–4.30), P = 0.016), an association not seen when considering CD68+ macrophages in the metastatic lymph node () left; HR = 0.67, 95% CI: (0.36–1.22), P = 0.19). Interestingly, suggestive evidence for the same survival trend as for CD68 was seen for PDL1 expression per se, with no association in the metastatic lymph node () left; HR = 0.79, 95% CI: (0.43–1.43), P = 0.44), but with an association to worse prognosis for patients with PDL1+ primary tumors () right; HR = 1.82, 95% CI: (1.00–3.29), P = 0.049). Suggestive evidence was also seen for an association between PD1high in the primary tumor and worse prognosis (Supplementary Fig. 1A right; HR = 2.01, 95% CI: (1.09–3.72), P = 0.025) in agreement with previous studies.Citation38,Citation39 This association was not seen in the metastatic lymph node (Supplementary Fig. 1A left; HR = 0.81, 95% CI: (0.34–1.93), P = 0.64).
Figure 3. Differences in 5-year distant recurrence-free interval (DRFi) according to CD169, CD68 and PDL1 expression in metastatic lymph node (MLN) and primary tumors (PT) of breast cancer patients. P value by log-rank test. (a) CD169 expression – in metastatic lymph node (CD169 MLN) (left) and primary tumor (CD169 PT) (right). (b) CD68 expression (-/+) in metastatic lymph node (CD68 MLN) (left) and primary tumor (CD68 PT) (right). (c) PDL1 expression (-/+) in metastatic lymph node (PDL1 MLN) (left) and primary tumor (PDL1 PT) (right)
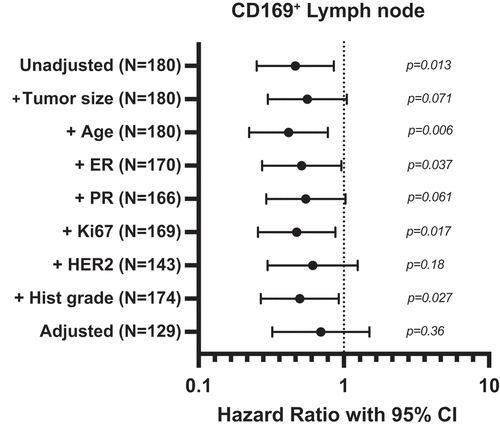
We next performed multivariable analyses. The suggestive evidence for a better prognosis for patients with CD169+ macrophages in the metastatic lymph node prompted us to investigate whether the lymph node CD169+ macrophages had an independent prognostic effect on 5-year DRFi. A series of Cox regression analyses adjusting for tumor size, histological grade, ER, PR, Ki67, HER2, and age, both individually and all together, were performed and summarized in a forest plot (). Unadjusted, presence of CD169+ macrophages in metastatic lymph node was associated to better prognosis (see above), but the association was considerably weaker after multivariable adjustment (HR = 0.70, 95% CI: (0.32–1.50), P = 0.36).
Figure 4. Forest plot showing results from Cox regression analysis on 5-year distant recurrence-free interval (DRFi) in breast cancer patients with CD169 expression in metastatic lymph node. Adjusted for tumor size, age, estrogen receptor (ER), progesterone receptor (PR), Ki67 expression, HER2 status and histological grade, both individually and all together. Dots indicate hazard ratios, horizontal lines indicate 95% confidence interval (95% CI). Note that the scale is logarithmic
Prognostic importance of experimental biomarker combinations
We continued investigating the prognostic importance of experimental biomarker combinations, starting within the primary tumor and metastatic lymph node, separately. When combining the individual scoring of CD169 and PDL1 expression in the metastatic lymph node () left), PDL1 expression did not add prognostic information for either the CD169+ group (red lines ) left; HR = 0.96, 95% CI: (0.41–2.25), P = 0.93), or the CD169− group (blue lines ) left; HR = 0.85, 95% CI: (0.29–2.44), P = 0.76). In contrast, in the primary tumor, there was a tendency that patients with PDL1− tumors had a better prognosis than patients with PDL1+ tumors in both the CD169+ group (red lines ) right; HR = 0.74, 95% CI: (0.37–1.48), P = 0.40) and the CD169− group (blue lines ) right; HR = 0.31, 95% CI: (0.10–1.00), P = 0.05). Based on these results, we decided to compare the two extreme groups. Patients lacking both CD169 and PDL1 expression in primary tumor (solid blue line ) right) had better prognosis compared to patients positive for both CD169 and PDL1 (red dashed line ) right; HR = 0.36, 95% CI: (0.13–1.00), P = 0.05).
Figure 5. Kaplan-Meier curves illustrating differences in 5-year distant recurrence-free interval (DRFi) according to CD169, CD68 and PDL1 expression in metastatic lymph node (MLN) and primary tumors (PT) of breast cancer patients. P value by log-rank test. (a) Combined individual expression of CD169 and PDL1 in metastatic lymph node (left) and primary tumor (right). Solid lines indicate PDL1− tumors, and dashed lines PDL1+ tumors, with (red) or without (blue) CD169 expression respectively (3-df test). (b) CD169 expression (-/+) in metastatic lymph node (CD169 MLN) in patients with PDL1 positive primary tumor (PDL1+ PT) (left) and PDL1 negative primary tumor (PDL1− PT) (right). (c) CD68 expression (-/+) in metastatic lymph node (CD68 MLN) in patients with PDL1 positive primary tumor (PDL1+ PT) (left) and PDL1 negative primary tumor (PDL1− PT) (right)
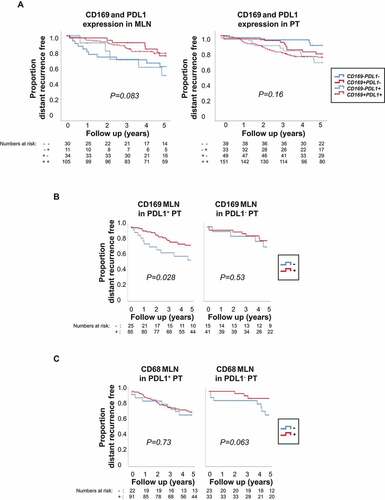
We next investigated the effect of PDL1+ primary tumors on lymph node macrophages. Since primary tumors have the capacity to modify draining lymph nodesCitation40 and PDL1 expression is induced by IFNs and proinflammatory cytokines that can be produced at higher levels in breast tumor subtypes like TNBC,Citation12,Citation36 we investigated whether PDL1+ primary tumors would affect the prognostic importance of metastatic lymph node macrophages to a higher extent than PDL1− primary tumors would. Interestingly, when stratifying for PDL1 expressing primary tumors (), we saw that patients with CD169+ macrophages in metastatic lymph node seemed to have a better prognosis only when primary tumors were PDL1+ () left; HR = 0.45, 95% CI: (0.22–0.94), P = 0.033). This trend was not observed in patients with PDL1− tumors () right; HR = 0.68, 95% CI: (0.20–2.31), P = 0.53). When the same division was used to analyze CD68+ macrophages in PDL1+ tumors, no effect was seen () left; HR = 0.86, 95% CI: (0.37–1.99), P = 0.73). However, patients with CD68+ macrophages in metastatic lymph node and PDL1− tumor did show a trend toward better prognosis () right; HR = 0.30, 95% CI: (0.08–1.16), P = 0.080).
Finally, we analyzed CD169 and PDL1 co-expression. Patients with co-expression of CD169 and PDL1 on either the same cell or nearby cells (CD169+PDL1+) in metastatic lymph nodes had slightly better prognosis (Supplementary Fig. 1B left; HR = 0.60, 95% CI: (0.32–1.12), P = 0.11) and in primary tumors a slightly worse prognosis compared to all other patients, but the evidence was weak (Supplementary Fig. 1B right; HR = 1.42, 95% CI: (0.85–2.36), P = 0.18).
Discussion
In this study we observed that CD169+ macrophages presence near lymph node metastases of breast cancer patients was associated with smaller tumor size and, in univariable analyses, to improved prognosis after adjuvant tamoxifen. This is in contrast with CD68+ macrophages in lymph node metastases, which were not associated with prognosis, although these macrophages were associated with more aggressive tumor characteristics of the primary tumor (higher histological grade, high Ki67, and ER-negativity). One possible explanation to this difference in prognostic importance may be that patients with advanced tumors have a stronger tumor-derived effect on the draining lymph node follicles, resulting in loss of beneficial CD169+ subcapsular sinus macrophages specifically.Citation41 Another explanation could be that the CD169+ macrophages present in metastatic lymph nodes reorganize to other sites picking up tumor antigens for cross presentation.Citation16 Our findings in this study differ from another study published on CD169+ lymph node subcapsular sinus macrophages in breast cancer patients.Citation21 There, presence of CD169+ lymph node macrophages correlated to small tumor size, no lymph node metastasis, and low Ki67 in the primary tumor, but did not correlate with relapse-free or breast cancer-specific survival. The reason for this may be that in our study we evaluated CD169+ macrophages in direct contact with metastasis, while Shiota et al. only used cancer cell-free lymph nodes for analysis and did not analyze CD169 expression in the primary tumor samples, only CD8 expression.Citation21 To our knowledge, we here show for the first time that CD169+ macrophages located in direct vicinity of lymph node metastasis in breast cancer patients, correlate with improved prognosis. The evidence for a prognostic importance in our study was, however, not retained after adjustment for other clinicopathological features. In multivariable analysis, we found that the presence of CD169+ macrophages in lymph node metastases was not a strong independent risk factor for prognosis. The patients in the cohort used in this study had all received adjuvant tamoxifen, which also could have an impact on outcome of this study, and therefore further studies are needed to verify our results. On the other hand, this fact also excludes any treatment-related effect on the CD169+ macrophages other than tamoxifen.
We also compared the differences between CD169+ macrophages in metastatic lymph node and primary tumor. In many cases, although the correlation with clinicopathological biomarkers was weak, the location of CD169+ macrophages rendered opposite trends in metastatic lymph node and primary tumor. The same was noted for the 5-year DRFi analysis where CD169+ macrophages in metastatic lymph node correlated with better prognosis while CD169+ macrophages in primary tumor did not. At this stage, it is impossible to say whether the CD169+ macrophages in the metastatic lymph nodes are solely resident CD169+ macrophages or a blend of resident and monocyte-derived CD169+ macrophages. Our finding would, however, support that the CD169+ macrophages in these two different locations have different functions with regard to tumor cells, or adaptive immune cells, and that they most likely have different origin, although further evidence is needed to prove this. These findings could also give an explanation to a previous experimental study performed in mice, where depletion of all CD169+ macrophages, and not only lymph node resident, lead to a reduced breast tumor growth and less metastasis.Citation42 Interestingly, high expression of SIGLEC1 in primary breast tumors has formerly been associated with shorter recurrence-free survival in public datasets.Citation22
Around 30% of the primary tumors were PDL1+, and PDL1 expression in the primary tumor of the breast cancer patients in this cohort correlated with PD1 expression, TNBC primary tumor subtype classification, and hallmarks of TNBC; ER and PR negativity and high Ki67. This is in line with previous research that shows that PDL1 is associated with more aggressive basal subtypes of breast cancer.Citation43 We further saw that breast cancer patients with PDL1 expression in the primary tumor had worse prognosis than patients with PDL1 negative tumors. The same effect was not seen when PDL1 expression in the lymph node metastasis was examined. PDL1 expression on APCs, as compared to on malignant cells, is of more relevance for successful anti-PDL1 therapy.Citation44 Interestingly, a recent study showed that it was PDL1 expression on tumor-infiltrating lymphocytes (TILs) in tumors of TNBC patients, but not on the tumor cells themselves, that was associated with poor prognosis.Citation45 As mentioned before, in viral infections CD169+ macrophages have been shown to induce type I IFNs that promotes PDL1 expression.Citation12 That supports our findings in this study, where the presence of CD169+ macrophages both in primary tumor and in metastatic lymph node correlated with the presence of PDL1+ cells in the same location. In our hands, the PDL1-expressing nonmalignant cells could probably be of both lymphoid as well as myeloid origin, but the CD169+PDL1+ co-expressing cells are most likely macrophages (APCs) as judged by their morphology and CD169 expression.
When we combined the individual scoring of PDL1 and CD169, we saw that CD169 expression was associated with the prognosis in the metastatic lymph node, while PDL1 expression affected the prognosis in the primary tumor negatively, although this was more pronounced in primary tumors lacking CD169+ TAMs. Interestingly, though, patients with CD169+ macrophages in metastatic lymph node seemed to have a better prognosis only when primary tumors were PDL1+. When assessing co-expression, CD169+PDL1+, on the same or nearby cells, we observed a similar pattern. In the metastatic lymph nodes, the prognostic effect of CD169 alone is stronger than that of CD169+PDL1+ co-expressing cells. This indicates that CD169+ macrophages, independent of PDL1 expression, are important for prognosis when present in metastatic lymph nodes, while in the primary tumors, a subpopulation of CD169+ macrophages co-expressing PDL1 may have a worse effect on tumor progression than CD169+ macrophages alone. Interestingly, the co-expression of CD169 and PDL1 in both primary tumor and metastatic lymph node did not seem to change the correlation to clinicopathological features that PDL1 expression alone had.
In conclusion, we observed that CD169+ macrophages have a positive effect on the prognosis when expressed in the metastatic lymph node, compared to no effect when expressed in the primary tumor, which further supports the theory that CD169+ macrophages differ in the properties between the two locations. This effect was not seen in patients with PDL1− primary tumors. We also observed that the expression of CD169 was correlated with expression of PDL1, both in metastatic lymph node and in the primary tumor. This merits further research since to our knowledge, the relationship between CD169 and PDL1 expression in breast cancer has not been explored, thus investigating the biological differences between lymph node and primary tumor CD169+ macrophages will be of importance in the near future.
Abbreviations
| APC | = | Antigen presenting cells |
| ER | = | Estrogen receptor |
| HER2 | = | Human epidermal growth factor receptor 2 |
| PDL1 | = | Programmed death-ligand 1 |
| IHC | = | Immunohistochemical |
| MLN | = | Metastatic lymph node |
| PR | = | Progesterone receptor |
| TAM | = | Tumor associated macrophages |
| TMA | = | Tissue microarray |
| PT | = | Primary tumor |
| TNBC | = | Triple negative breast cancer |
Author contributions
FBG was responsible for analyzing data and for writing the initial manuscript together with KL. NA and FBG were responsible for annotating the IHC together with KL. LR and MF were responsible for the clinical patient cohort. POB and FBG were responsible for statistical evaluations. KL was responsible for designing the study, for analyzing data and for writing the initial manuscript.
Disclosure of potential conflicts of interest
KL is a board member of Cantargia AB, a company developing IL1RAP inhibitors. This does not alter the Author’s adherence to all guidelines for publication. The authors otherwise declare no competing interest.
Supplemental Material
Download ()Acknowledgments
The authors thank Kristina Lövgren for professional technical skills in preparation of the TMA. The authors thank Kristina Ekström-Holka for professional technical skills in preparation of the IHC. This work was supported by grants from the Swedish Cancer Society; the Swedish Research Council; the Governmental Funding of Clinical Research within the National Health Service (ALF), the UMAS Cancer foundation, the Gunnar Nilsson’s Cancer Foundation, the Åke Wibergs foundation, the Percy Falks Foundation, and the Gyllenstiernska Krapperups foundation.
Data availability
All datasets generated in the course of the current study are presented in the main text and the Supplementary Information available online.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn H-J. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017 Aug 1;28(8):1700–13. doi:10.1093/annonc/mdx308.
- Sleeman JP. The lymph node pre-metastatic niche. J Mol Med (Berl). 2015 Nov;93(11):1173–1184.
- Martinez-Pomares L, Gordon S. Antigen presentation the macrophage way. Cell. 2007 Nov 16;131(4):641–643. doi:10.1016/j.cell.2007.10.046.
- Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005 Sep;2(9):e284. doi:10.1371/journal.pmed.0020284.
- Grotz TE, Jakub JW, Mansfield AS, Goldenstein R, Enninga EAL, Nevala WK, Leontovich AA, Markovic SN. Evidence of Th2 polarization of the sentinel lymph node (SLN) in melanoma. Oncoimmunology. 2015 Aug;4(8):e1026504. doi:10.1080/2162402X.2015.1026504.
- Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients [Research Support, Non-U.S. Gov’t]. BMC Cancer. 2012;12(1):306. doi:10.1186/1471-2407-12-306.
- Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions [Review]. Int J Dev Biol. 2011;55(7–9):861–867. doi:10.1387/ijdb.113371dl.
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity [Review]. Curr Opin Immunol. 2010 Apr;22(2):231–237. doi:10.1016/j.coi.2010.01.009.
- Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. Immunol Res. 2013 Dec;57(1–3):172–184.
- Asano K, Kikuchi K, Tanaka M. CD169 macrophages regulate immune responses toward particulate materials in the circulating fluid. J Biochem. 2018 Aug 1;164(2):77–85. doi:10.1093/jb/mvy050.
- Louie DAP, Liao S. Lymph node subcapsular sinus macrophages as the frontline of lymphatic immune defense. Front Immunol. 2019;10:347. doi:10.3389/fimmu.2019.00347.
- Shaabani N, Duhan V, Khairnar V, Gassa A, Ferrer-Tur R, Häussinger D, Recher M, Zelinskyy G, Liu J, Dittmer U. CD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis. 2016 Nov 3;7(11):e2446. doi:10.1038/cddis.2016.350.
- Muerkoster S, Rocha M, Crocker PR, Schirrmacher V, Umansky V. Sialoadhesin-positive host macrophages play an essential role in graft-versus-leukemia reactivity in mice. Blood. 1999 Jun 15;93(12):4375–4386. doi:10.1182/blood.V93.12.4375.
- Martinez-Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012 Feb;33(2):66–70. doi:10.1016/j.it.2011.11.001.
- Garcia Z, Lemaitre F, van Rooijen N, Albert ML, Levy Y, Schwartz O, Bousso P. Subcapsular sinus macrophages promote NK cell accumulation and activation in response to lymph-borne viral particles. Blood. 2012 Dec 6;120(24):4744–4750. doi:10.1182/blood-2012-02-408179.
- Asano K, Nabeyama A, Miyake Y, Qiu C-H, Kurita A, Tomura M, Kanagawa O, Fujii S-I, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011 Jan 28;34(1):85–95. doi:10.1016/j.immuni.2010.12.011.
- Ohnishi K, Yamaguchi M, Erdenebaatar C, Saito F, Tashiro H, Katabuchi H, Takeya M, Komohara Y. Prognostic significance of CD 169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016 Jun;107(6):846–852. doi:10.1111/cas.12929.
- Ohnishi K, Komohara Y, Saito Y, Miyamoto Y, Watanabe M, Baba H, Takeya M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013 Sep;104(9):1237–1244. doi:10.1111/cas.12212.
- Asano T, Ohnishi K, Shiota T, Motoshima T, Sugiyama Y, Yatsuda J, Kamba T, Ishizaka K, Komohara Y. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018 May;109(5):1723–1730. doi:10.1111/cas.13565.
- Stromvall K, Sundkvist K, Ljungberg B, Halin Bergström S, Bergh A. Reduced number of CD169 + macrophages in pre-metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with poor outcome in prostate cancer patients. Prostate. 2017 Nov;77(15):1468–1477. doi:10.1002/pros.23407.
- Shiota T, Miyasato Y, Ohnishi K, Yamamoto-Ibusuki M, Yamamoto Y, Iwase H, Takeya M, Komohara Y. The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PLoS One. 2016;11(11):e0166680. doi:10.1371/journal.pone.0166680.
- Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019 Apr 15;35(4):588–602.e10. doi:10.1016/j.ccell.2019.02.009.
- Pico de Coana Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015 Aug;21(8):482–491. doi:10.1016/j.molmed.2015.05.005.
- Swoboda A, Nanda R. Immune checkpoint blockade for breast cancer. Cancer Treat Res. 2018;173:155–165.
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018 Nov 29;379(22):2108–2121. doi:10.1056/NEJMoa1809615.
- Wu Y, Chen W, Xu ZP, Gu W. PD-L1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol. 2019;10:2022. doi:10.3389/fimmu.2019.02022.
- Heeren AM, de Boer E, Bleeker MC, Musters RJP, Buist MR, Kenter GG, de Gruijl TD, Jordanova ES. Nodal metastasis in cervical cancer occurs in clearly delineated fields of immune suppression in the pelvic lymph catchment area. Oncotarget. 2015 Oct 20;6(32):32484–32493. doi:10.18632/oncotarget.5398.
- Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, Jordanova ES, de Gruijl TD. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015 Jan;3(1):48–58. doi:10.1158/2326-6066.CIR-14-0149.
- Ryden L, Jonsson PE, Chebil G, Dufmats M, Fernö M, Jirström K, Källström A-C, Landberg G, Stål O, Thorstenson S, et al. Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer. 2005 Jan;41(2):256–264. doi:10.1016/j.ejca.2004.06.030.
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer.. J Natl Cancer Inst. 1996 Nov 6;88(21):1543–1549. doi:10.1093/jnci/88.21.1543.
- Chebil G, Bendahl PO, Idvall I, Fernö M. Comparison of immunohistochemical and biochemical assay of steroid receptors in primary breast cancer–clinical associations and reasons for discrepancies. Acta Oncol. 2003;42(7):719–725. doi:10.1080/02841860310004724.
- Bergenfelz C, Leandersson K. The generation and identity of human myeloid-derived suppressor cells. Front Oncol. 2020;10:109. doi:10.3389/fonc.2020.00109.
- Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA et al. Evidence for a role of the PD-1: PD-L1pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013 Mar 15;73(6):1733–1741. doi:10.1158/0008-5472.CAN-12-2384.
- Strand C, Bak M, Borgquist S, Chebil G, Falck A-K, Fjällskog M-L, Grabau D, Hedenfalk I, Jirström K, Klintman M, et al. The combination of Ki67, histological grade and estrogen receptor status identifies a low-risk group among 1,854 chemo-naive women with N0/N1 primary breast cancer. Springerplus. 2013 Dec;2(1):111. doi:10.1186/2193-1801-2-111.
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A et al. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007 Apr 1;13(7):1987–1994. doi:10.1158/1078-0432.CCR-06-1823.
- Mehmeti M, Allaoui R, Bergenfelz C, Saal LH, Ethier SP, Johansson ME, Jirström K, Leandersson K. Expression of functional toll like receptor 4 in estrogen receptor/progesterone receptor-negative breast cancer. Breast Cancer Res. 2015 Sep 22;17(1):130. doi:10.1186/s13058-015-0640-x.
- Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018 Jan;2(1):6–10.
- Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, Wang J, Yuan F, Sun L, Yu Q, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014 Apr;63(4):395–406. doi:10.1007/s00262-014-1519-x.
- Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013 Jun;139(3):667–676.
- Jones D, Pereira ER, Padera TP. Growth and immune evasion of lymph node metastasis. Front Oncol. 2018;8:36. doi:10.3389/fonc.2018.00036.
- Chang AY, Bhattacharya N, Mu J, Setiadi AF, Carcamo-Cavazos V, Lee GH, Simons DL, Yadegarynia S, Hemati K, Kapelner A, et al. Spatial organization of dendritic cells within tumor draining lymph nodes impacts clinical outcome in breast cancer patients. J Transl Med. 2013 Oct;2(11):242. doi:10.1186/1479-5876-11-242.
- Jing W, Guo X, Wang G, Bi Y, Han L, Zhu Q, Qiu C, Tanaka M, Zhao Y. Breast cancer cells promote CD169+ macrophage-associated immunosuppression through JAK2-mediated PD-L1 upregulation on macrophages. Int Immunopharmacol. 2020 Jan;78:106012. doi:10.1016/j.intimp.2019.106012.
- Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015 Mar 10;6(7):5449–5464. doi:10.18632/oncotarget.3216.
- Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014 Nov 27;515(7528):563–567. doi:10.1038/nature14011.
- Wang X, Liu Y. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res Pract. 2020 Mar;216(3):152802. doi:10.1016/j.prp.2019.152802.
