ABSTRACT
Understanding the cancer risks in different transplant recipients helps early detection, evaluation, and treatment of post-transplant malignancies. Therefore, we performed a meta-analysis to determine the cancer risks at multiple sites for solid organ transplant recipients and their associations with tumor mutation burden (TMB), which reflects the immunogenicity. A comprehensive search of PubMed, Web of Science, EMBASE, Medline, and Cochrane Library was conducted. Random effects models were used to calculate the standardized incidence ratios (SIRs) versus the general population and determine the risks of different cancers. Linear regression (LR) was used to analyze the association between the SIRs and TMBs. Finally, seventy-two articles met our criteria, involving 2,105,122 solid organ transplant recipients. Compared with the general population, solid organ transplant recipients displayed a 2.68-fold cancer risk (SIR 2.68; 2.48–2.89; P <.001), renal transplant recipients displayed a 2.56-fold cancer risk (SIR 2.56; 2.31–2.84; P <.001), liver transplant recipients displayed a 2.45-fold cancer risk (SIR 2.45; 2.22–2.70; P <.001), heart and/or lung transplant recipients displayed a 3.72-fold cancer risk (SIR 3.72; 3.04–4.54; P <.001). The correlation coefficients between SIRs and TMBs were 0.68, 0.64, 0.59, 0.79 in solid organ recipients, renal recipients, liver recipients, heart and/or lung recipients, respectively. In conclusion, our study demonstrated that solid organ transplant recipients displayed a higher risk of some site-specific cancers, providing individualized guidance for clinicians to early detect, evaluate, and treat cancer among solid organ transplantation recipients. In addition, the increased cancer risk of solid organ transplant recipients is associated with TMB, suggesting that iatrogenic immunosuppression may contribute to the increased cancer risk in transplant recipients. (PROSPERO ID CRD42020160409).
Introduction
Solid organ transplantation is a life-saving option for patients with some end-stage diseases. In recent decades, the overall survival of solid organ transplant recipients has been remarkably improved with the use of immunosuppressive drugs.Citation1,Citation2 Nevertheless, cancer risk among solid organ transplant recipients is 2- to 5-fold higher compared with the general population.Citation3 Though great efficacy in the prolongation of survival in solid organ transplant recipients has been demonstrated, post-transplant immunosuppression therapy is considered to be an important inducement of de novo malignancies after solid organ transplantation.Citation4–6
Large population-based cohort studies can systematically evaluate cancer incidence in solid organ transplant recipients.Citation7,Citation8 However, the cancer risk might vary by region, population and transplantation category (such as lung or renal transplantation). Previous systematic analyses were limited to renal transplant patients, and relevant study on the field of liver, heart, or lung transplantation has not been reported yet.
TMB is defined as the total number of somatic gene coding errors, base substitution, gene insertion or deletion errors detected in every million bases using sequencing technology.Citation9 The diversity of TMB and cancer type reflects the different immunogenicity, which is closely related to the ability of the immune system to recognize tumors cells. To some extent, TMB may be associated with post-transplant site-specific cancer risks, but their causality is unclear.
Understanding the cancer risk profile in different solid organ transplant recipients helps early detection, evaluation and treatment of post-transplant malignancies. The aim of our study is to determine whether cancer risks in the post-transplant population would increase, to compare the associations among recipients with different characteristics, and to explore the potential association between TMBs and the corresponding SIRs of post-transplant malignancies to better understand the role of immune system in solid organ transplant recipients, by a comprehensive analysis.
Methods
Search strategy and selection criteria
Cochrane Library (Issue 12, 2019), PubMed (update to January 2020), Web of Science (update to January 2020), EMBASE (from 1980 to January 2020), and Medline (from 1949 to January 2020) databases were searched to identify relevant studies. The following keywords and their MeSH terms were used: solid organ transplantation, lung transplantation, kidney transplantation, and liver transplantation, combined with cancer risk or cancer incidence. We also searched the references from relevant articles. The authors were contacted for supplemental data when important information was missing. We evaluated all searched results according to the PRISMA statement.Citation10 The protocol was registered in the Prospective Register of Systematic Reviews (PROSPERO ID CRD42020160409).
Studies were included in our analysis if they met the following criteria:(1) population-based cohort studies on solid recipients, (2) included at least one type of site-specific organ transplantation (3) reported at least one site-specific cancer risk in solid organ transplant recipients. (4) published or accepted in English that could be retrieved from the network databases mentioned above as of January 2020. Studies were excluded for the following reasons:(1) sampling of non-solid organ transplantations, (2) SIRs and 95% CIs could not be obtained or estimated from the article, (3) studies that could not be retrieved from the network databases mentioned above, (4) lack of available data with appropriate statistics.
Data extraction and quality assessment
Three authors (Z.H., F.G., R.W.) extracted the necessary data independently and any disagreements were resolved after discussion by three investigators. The clinical characteristics and demographics of patients, first author’s name, year of publication, country, type of transplant, mean or median age, sample size, and duration of follow-up were recorded. The number of solid organ transplant cases, number of all cancers, SIRs of all cancers after solid organ transplantation, survival and other adverse events were extracted as outcome data.
In 2017, Chalmers et al.Citation9 measured the distribution of TMB across a diverse cohort study of 100,000 cancer cases through a targeted CGP assay, and validated the association between TMB and somatic alterations in over 100 tumor types. Relevant Median TMBs of malignancies were extracted from the study of Chalmers et al. (see Additional file 3: Table S1: Summary of TMB properties by disease) .Citation9 If there were no available Median TMB value given a certain malignancy, its TMB value is calculated by averaging the TMBs of its subtypes mentioned in this study (leukemia, non-Hodgkin’s lymphoma, the cancer of pancreas, ovary, small intestine, brain and central nervous system, colorectum, skin and lung). The extracted Median TMB values and their natural logarithm forms were listed in Supplementary Table S1 and Supplementary Table S2.
The methodological quality of the selected studies was evaluated using criteria by the Newcastle Ottawa Scale (NOS) (Supplementary Table S3), which includes selection (4 items), comparability (1 item), and outcome (3 items).Citation11 Any disagreement was resolved by consensus.
Statistical analysis
We examined the cancer risks in solid organ transplant (all solid organs, kidney, liver, heart and/or lung) recipients based on the SIRs and their 95% CIs published in each study. A random-effects model was adopted to synthesize SIRs and 95% CIs for solid organ transplant recipients versus the general population .Citation12,Citation13 The synthesized SIRs were classified into eight modules by anatomical site or histology: overall cancer, digestive system, integumentary system, reproductive and urinary organs, respiratory system, hematological malignancies, head and neck cancers, and other malignancies. A heat map was generated to better observe the site-specific cancer risks’ spectrum of different transplantation types. We used the Cochran’s Q test and the I2 statistic to examine the heterogeneity across studies;Citation14 significant statistical heterogeneity was considered when an I2 statistic >50%.Citation14 Additionally, to explore potential associations among the included studies with different characteristics, a subgroup analysis was conducted according to the region (Europe, Asia, North America, Oceania) and age (<40 years, between 40 and 50 years, > 50 years).Sensitivity analysis was conducted by consecutive exclusion of each study. The Begg’s testCitation15 and Egger’s testCitation16 were performed to analyze the publication biases statistically.
In addition, we used LR method to analyze the association and calculated the correlation coefficients between TMBs and pooled SIRs of site-specific malignancies. Because both TMBs and SIRs were not normally distributed, we took the natural logarithm of each to perform the analyses. Statistical analyses and linear regression were conducted using the STATA 15.0 software (STATA Corp, College Station, TX, USA). GraphPad Prism 7® (GraphPad Software, Inc., La Jolla, USA) was used to generate the heat map. All the P-values were 2-tailed; statistical significance was set as P-value <0.05.
Role of the Funding Source
The funders had no role in the study design, data collection, data synthesis and analysis, writing of the manuscript, or the decision to submit the article for publication.
Results
Systematic search and study characteristics
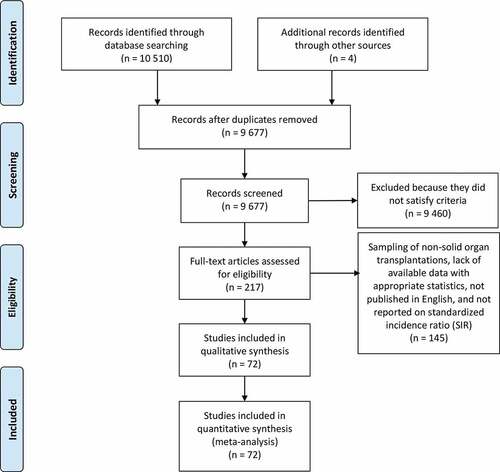
A total of 10,514 studies were identified through the database search and were screened on title and abstract. The full-texts of 217 articles were examined and 72 of themCitation7,Citation8,Citation17–75 met the inclusion criteria of the analyses.
All 72 studies were prospective cohort studies. Supplementary Table S3 provides details of the included studies, which involved a total of 2,105,122 solid organ transplant recipients and reported 45 types of site-specific cancer. Of them, 52 studiesCitation7,Citation8,Citation17–62,Citation75 provided SIRs of multiple cancers of solid organ transplantations (11 for multiple organs,Citation7,Citation8,Citation17–25 19 for kidney,Citation26–44 16 for liver,Citation45–55,Citation75 7 for heart and/or lungCitation60–62,Citation76–79). The other 20 studiesCitation63–74,Citation80–87 provided SIRs of several or single cancer risks of solid organ transplantations ().
Cancer risks in solid organ transplant recipients
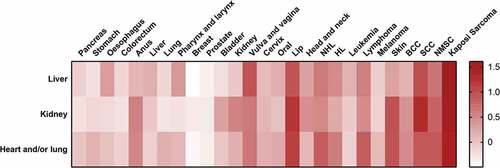
Compared with the general population, solid organ transplant recipients displayed a 2.68-fold cancer risk (SIR 2.68; 2.48–2.89; P < .001). Among them, renal transplant recipients displayed a 2.56-fold cancer risk (SIR 2.56; 2.31–2.84; P < .001), liver transplant recipients displayed a 2.45-fold cancer risk (SIR 2.45; 2.22–2.70; P < .001), heart and/or lung transplant recipients displayed a 3.72-fold cancer risk (SIR 3.72; 3.04–4.54; P < .001). SIRs of each site-specific malignancy were listed in . Comparison of common site-specific cancer risks from different transplant categories became more intuitionistic by generating a heat map ().
Table 1. SIRs of all-cancer and cancer types by anatomical site or histology among solid organ transplant recipients
Figure 2. Heat map of the comparation of common cancer risks. Each SIR value was treated as follows: (1) all-cancer risk of solid organ transplantation was taken as reference (ref = 1); (2) natural logarithm was taken
Associations between TMB and cancer incidence
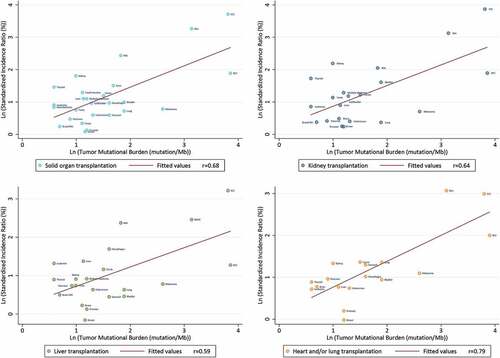
In solid organ transplant recipients, we observed a significant correlation between TMBs and SIRs (P < .001). The correlation coefficients between SIRs and TMBs were 0.68, 0.64, 0.59, 0.79 in solid organ transplant recipients, renal recipients, liver recipients and heart and/or lung recipients, respectively, suggesting that the corresponding 46%, 41%, 35%, and 63% of the differences in SIRs across cancer types might be explained by the TMBs ().
Sensitivity analysis
The results of sensitivity analyses were listed in Supplementary Figure S1-S4. It indicated that the omission of any single study did not result in a significant difference in the pooled results, even though there was inevitably a mild amount of overlapping of the included population. The variable findings may be attributed to the limited included cohorts.
Subgroup analyses
The forest plots of subgroup analyses were presented in Supplementary Figure S5. We conducted the subgroup analyses by region (Europe, Asia, North America, Oceania) and age (<40 years, between 40 and 50, > 50 years). First, we found that the overall cancer risk did not show significant differences in different regions. (SIR 2.45; 2.22–2.70; P < .001) Second, we noticed that solid organ transplant recipients over 50 had a 3-fold cancer risk (SIR 3.01; 2.41–3.75; P < .001) while solid organ transplant recipients between 40 and 50 and under 40 had a 2.6-fold risk (SIR 2.61; 3.04–4.54; P < .001) and a 2-fold risk (SIR 2.00; 1.63–2.47; P < .001), respectively.
Publication bias
Significant heterogeneity was observed in the pooled analyses. With the limited information, we were unable to detect any source leading to substantial heterogeneity. Furthermore, the Egger’s and Begg’s test results showed no evidence of significant publication bias for all cancers analyzed in solid organ transplant recipients, renal transplant recipients, liver transplant recipients and heart and/or lung transplant recipients (Supplementary Table S4).
Discussion
This study showed a risk spectrum of overall cancer and site-specific cancers in solid organ transplant recipients compared with the general population. Subgroup analyses showed that age could contribute to the elevated overall post-transplant cancer risk. When stratified by region, the overall post-transplant cancer risk did not show a significant difference. When it comes to the correlation between the cancer risk and TMBs, the correlation coefficient was 0.68, suggesting that the increased incidence of cancer was associated with immunosuppression.
Several mechanisms could explain the increased cancer risk in solid organ transplant recipients. Both viral and non-viral factors are involved in the progression of post-transplant malignancies. Infections with the hepatitis C and hepatitis B virus are considered risk factors for liver cancer, while EBV infection may be associated with an increased risk of non-Hodgkin’s lymphoma.Citation88,Citation89 HPV infection may be related to squamous cell carcinoma.Citation5 However, compared with people with HIV or AIDS, solid organ transplant recipients demonstrated higher HPV-related cancer risks, while EBV-related cancer risks were lower than HIV-infected.Citation5 The difference between the above two immune deficiencies remains to be explored. These infectious factors support our findings of the elevated site-specific cancer risks.
Long-term use of post-transplant immunosuppressive therapy is related to the increased incidence of cancer. Immunosuppression therapy is possibly related to the direct damage of cells and cell repair systems .Citation90,Citation91 Generally, the immunosuppressive drugs act by depleting T lymphocytes, leading to the decreased acute rejection rates, which results in the increased graft survival.Citation92 In the meantime, they also have the ability to reduce immune surveillance, which facilitates the survival and proliferation of atypic cells.Citation93 In addition, the significant elevation of skin-related malignancies (e.g. BCC and SCC) and cervical cancer in transplant recipients may be related to the increased susceptibility to human papillomavirus.Citation94 Compared with 11% to 32% in normal skin, up to 90% of SCCs in solid organ transplant recipients contain human papillomavirus DNA.Citation95 Immunosuppressive drugs have also shown the possibility to increase the risk of ultraviolet-related carcinogenic effects.Citation96,Citation97
Among solid organ transplant recipients, heart and/or lung transplant recipients were found to have the highest lung cancer risk. The risk factors include post-transplant chronic immunosuppression and previous smoking status.Citation98 Meanwhile, due to the etiologic factors of end-stage pulmonary diseases such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis, these patients are at increased lung cancer risk compared with the general population. Also, the native lung disease or undetected cancerous cells in the donor’s lung predispose to an increased incidence of lung cancer in the allograft after transplantation.Citation98–100 Moreover, a higher intensity and a longer duration of immunosuppressive therapy in heart and/or lung transplant recipients contribute to the elevation of the risks, which results in a higher inhibitory effect on the immune system, leading to a further decline in its ability to monitor and clear pathogens and cancer cells, and ultimately an increase in lung cancer risk.Citation8,Citation101
We also found that liver cancer risk was most elevated in liver transplant recipients among solid organ transplant recipients. Liver cancer is the most common complication in end-stage liver disease patients, and liver transplantation can be an ideal therapy for patients with localized liver cancer .Citation102 Possible reasons to explain the elevated liver cancer risk include the relapse of infection of HBV and HCV, diabetes mellitus, and the delate recognition of liver cancer in the donor liver.Citation56 Due to the significant organ specificity, we speculate the elevated liver cancer risk may also be related to the chronic rejection reaction after liver transplantation. Also, the incidence of ulcerative colitis after liver transplantation was increased, which could result in the elevated colorectal cancer risk in post-liver transplant recipientsCitation55 .Citation103
Among solid organ transplant recipients, the kidney recipients were found to have the highest renal cancer risk. Guba et al.Citation6 found that some nephrotoxic effects or direct carcinogenic effects in immunosuppressive drugs may lead to a higher renal cancer risk. Some early post-transplant renal cancer cases were the results of malignant conversion from benign cysts developing in pre-transplant donor kidneys.Citation104 Another potential reason could be the aging donor population, some of whom may have unrecognized kidney cancer before transplantation, which could contribute to the facilitation of renal cancer.Citation18 These mechanisms support our finding of the elevated 9-fold renal cancer risk we found in renal transplant recipients. In summary, lung cancer risk, liver cancer risk and renal cancer risk were mostly elevated in heart and/or lung recipients, liver recipients and kidney recipients, respectively.
TMB is a promising biomarker for predicting the response to immune checkpoint inhibitors (ICIs) of tumors.Citation9,Citation105 In a clinical trial, TMB was more remarkably associated with response rate than the expression of PD-L1 by immunohistochemistry.Citation106 In 2017, Mark Yarchoan et al.Citation105 plotted the objective response rate for anti–PD-1/PD-L1 therapy against the corresponding median TMB values across multiple cancer types, which highlighted the strong relationship between TMB and the activity of anti–PD-1 treatments across site-specific cancers. To some extent, TMB reflects the immunogenicity of the tumor. The higher the TMB of a specific cancer is, the more kinds of abnormal proteins it produces. These proteins are recognized as antigens, leading to a higher possibility of being recognized by the immune system, which makes them the targets of activated immune cells.Citation9 Therefore, when the immune system is normal, malignancies with a high TMB are less likely to grow. Immunosuppressive drugs would lower immune surveillance of the immune system, leading to increased survival of high-TMB malignancies, which may eventually lead to an increase of overall and site-specific cancer incidence. In this study, linear regression was used to analyze the association between TMBs and corresponding cancer incidences. shows that the occurrence of cancers in multiple sites is possibly immunosuppression-related. Heart and/or lung transplantation has the highest correlation coefficients (r = 0.79), which may be related to a higher intensity and a longer duration of immunosuppressive therapy.Citation8,Citation101 In comparison, renal transplantation and liver transplantation had relatively lower correlation coefficients (r = 0.64 and 0.59, respectively), which could be attributed to the lower intensity and shorter duration of immunosuppressive therapy.Citation17 In conclusion, the high correlation between the cancers’ SIRs and their TMBs in solid organ transplantation supported that iatrogenic immunosuppression-generated site-specific cancer risk was elevated in transplant recipients.
Three strengths of our study should be highlighted. First, to our knowledge, this is the first and the most comprehensive quantitative summary estimating the cancer risks after multiple types of solid organ transplantation, and exploring the relationship between the corresponding SIRs and their TMBs. Second, previous meta-analyses were limited to a specific organ, specific malignancy, single region, or small sample size. Our study initially collected large-sample data and assessed the cancer risks of solid organ transplantation from various areas of the world. The collected global data and our findings could provide clinicians and researchers with ideas to prevent and treat cancer in solid organ transplant recipients. Third, this is also the first study to associate TMBs with site-specific cancer risks, which could provide a way to explore the cancer risk of solid organ transplantation at the perspective of immunology.
We acknowledge some limitations in regards to our comprehensive analysis. First, significant heterogeneity between studies was observed, which may be due to the following reasons: (1) various transplant types were included in one overall analysis (2) differences between oncological characteristics of included malignancies (3) no detailed information on the smoking status,Citation107 body mass index,Citation108 alcohol useCitation109 and immunosuppressive drugsCitation110 were available to perform an adjustment for these potential confounders; (4) although all studies used the general population as references, the matching criteria for studies in different countries may be different. Second, as there were no pre-transplant disease data for solid organ transplant recipients, we could not rule out their effects on solid organ transplant recipients’ cancer risk. Third, due to the inclusion of research publications, publication bias is inevitable.
Conclusion
This comprehensive analysis showed that solid organ transplant recipients displayed a higher cancer risk, and different malignancies presented different risks. Such associations provided guidance for clinicians to prevent specific types of post-transplant malignancies. In addition, the increased cancer risk of solid organ transplant recipients is significantly associated with TMB, suggesting that iatrogenic immunosuppression may lead to an increased cancer risk in transplant recipients.
Author contributions
HZY, GF, XX and WRC designed this study. LCC, CB, LJF and XS searched the literature. HZY, GF and WRC extracted data. HZY, CY, LHR, LWY, PHX and WXR analysed data. HZY and GF wrote the first draft of the manuscript. LCC, HDX, LR and ZR revised the primary version and performed English editing. LWH and HJX revised the final version of the manuscript. All authors contributed to revisions of the manuscript. HZY is the guarantor. All authors approved the final manuscript
Abbreviations
| BCC | = | Basal Cell Carcinoma |
| CGP | = | Comprehensive Genomic Profiling |
| CI | = | Confidence Interval |
| EBV | = | Epstein-Barr virus |
| HPV | = | Human papillomavirus |
| LR | = | Linear Regression |
| MeSH | = | Medical Subject Headings |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta‐Analysis |
| SCC | = | Squamous Cell Carcinoma |
| SIR | = | Standardized Incidence Ratio |
| TMB | = | Tumor Mutational Burden |
Conflict of interest
All the authors declare no conflicts of interest.
Ethical statement
This study was approved by the ethics committee of The First Affiliated Hospital of Guangzhou Medical University. Considering that the study was a retrospective analysis, informed consent of all patients was waived by the ethics committee.
Highlights
We summarized the spectrum of overall and site-specific cancer risks in patients with different types of solid organ transplantation, and showed the relationship between TMB and risks of post-transplant cancers.
Supplemental Material
Download ()Acknowledgments
The authors thank Ms. Lindsey Hamblin for helping to edit the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009;87(6):785–10.doi: 10.1097/TP.0b013e3181952623.
- Charlton M, Levitsky J, Aqel B, OʼGrady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102(5):727–743. doi:10.1097/TP.0000000000002147.
- Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125(8):1747–1754. doi:10.1002/ijc.24439.
- Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605–615. doi:10.1586/14737140.8.4.605.
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi:10.1016/S0140-6736(07)61050-2.
- Guba M, Graeb C, Jauch K-W, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77(12):1777–1782. doi:10.1097/01.TP.0000120181.89206.54.
- Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population-based study. Int J Cancer. 2013;132(6):1429–1438. doi:10.1002/ijc.27765.
- Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. doi:10.1001/jama.2011.1592.
- Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017 Apr 19;9(1):34. doi:10.1186/s13073-017-0424-2.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009 Jul;21(339):b2535. doi:10.1136/bmj.b2535.
- Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. 2001.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Ades A, Lu G, Higgins JJMDM. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560.
- Begg CB, Mazumdar MJB. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994. p. 1088–1101.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634.
- Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami H-O, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89(7):1221–1227. doi:10.1038/sj.bjc.6601219.
- Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–1896. doi:10.1111/j.1600-6143.2010.03181.x.
- Hortlund M, Arroyo Mühr LS, Storm H, Engholm G, Dillner J, Bzhalava D. Cancer risks after solid organ transplantation and after long-term dialysis. Int J Cancer. 2017;140(5):1091–1101. doi:10.1002/ijc.30531.
- Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of De Novo cancer incidence in australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13(1):174–183. doi:10.1111/j.1600-6143.2012.04302.x.
- O'Neill JP, Sexton DJ, O'Leary E, O'Kelly P, Murray S, Deady S, Daly F, Williams Y, Dean B, Fitzgerald C, et al. Post-transplant malignancy in solid organ transplant recipients in Ireland, the Irish transplant cancer group. Clin Transplant. 2019;33(10):e13669–e13669.
- Park B, Yoon J, Choi D, Kim HJ, Jung YK, Kwon OJ, Lee KG. De novo cancer incidence after kidney and liver transplantation: results from a nationwide population based data. Sci Rep. 2019;9(1):17202. doi:10.1038/s41598-019-53163-9.
- Serraino D, Piselli P, Busnach G, Burra P, Citterio F, Arbustini E, Baccarani U, De Juli E, Pozzetto U, Bellelli S, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. Eur J Cancer. 2007;43(14):2117–2123. doi:10.1016/j.ejca.2007.07.015.
- Tsai H-I, Lee C-W, Kuo C-F, See L-C, Liu F-C, Chiou M-J, Yu H-P. De novo malignancy in organ transplant recipients in Taiwan: a nationwide cohort population study. Oncotarget. 2017;8(22):36685–36695. doi:10.18632/oncotarget.13124.
- Wareham NE, Li Q, Sengeløv H, Da Cunha-Bang C, Gustafsson F, Heilmann C, Perch M, Rasmussen A, Sørensen SS, Mocroft A, et al. Risk of de novo or secondary cancer after solid organ or allogeneic haematopoietic stem cell transplantation. J Cancer Res Clin Oncol. 2019;145(12):3125–3135. doi:10.1007/s00432-019-03039-2.
- Agraharkar ML, Cinclair RD, Kuo Y-F, Daller JA, Shahinian VB. Risk of malignancy with long-term immunosuppression in renal transplant recipients. Kidney Int. 2004;66(1):383–389. doi:10.1111/j.1523-1755.2004.00741.x.
- Birkeland SA, L⊘kkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355(9218):1886–1887. doi:10.1016/S0140-6736(00)02298-4.
- Buxeda A, Redondo-Pachón D, Pérez-Sáez MJ, Bartolomé Á, Mir M, Pascual-Dapena A, Sans A, Duran X, Crespo M, Pascual J, et al. Gender differences in cancer risk after kidney transplantation. Oncotarget. 2019;10(33):3114–3128. doi:10.18632/oncotarget.26859.
- Cheung CY, Lam MF, Chu KH, Chow KM, Tsang KY, Yuen SK, Wong PN, Chan SK, Leung KT, Chan CK, et al. Malignancies after kidney transplantation: Hong Kong renal registry. Am J Transplant. 2012;12(11):3039–3046. doi:10.1111/j.1600-6143.2012.04209.x.
- Heo J, Noh OK, Oh Y-T, Chun M, Kim L. Cancer risk after renal transplantation in South Korea: a nationwide population-based study. BMC Nephrol. 2018;19(1):311. doi:10.1186/s12882-018-1110-3.
- Hoshida Y, Tsukuma H, Yasunaga Y, Xu N, Fujita MQ, Satoh T, Ichikawa Y, Kurihara K, Imanishi M, Matsuno T, et al. Cancer risk after renal transplantation in Japan. Int J Cancer. 1997;71(4):517–520. doi:10.1002/(SICI)1097-0215(19970516)71:4<517::AID-IJC3>3.0.CO;2-X.
- Kessler M, Jay N, Molle R, Guillemin F. Excess risk of cancer in renal transplant patients. Transpl Int. 2006;19(11):908–914. doi:10.1111/j.1432-2277.2006.00383.x.
- Kim JH, Kim S-O, Han DJ, Park S-K. Post-transplant malignancy: a burdensome complication in renal allograft recipients in Korea. Clin Transplant. 2014;28(4):434–442. doi:10.1111/ctr.12328.
- Kyllönen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13(Suppl 1):S394–S398. doi:10.1111/j.1432-2277.2000.tb02068.x.
- Li W-H, Chen Y-J, Tseng W-C, Lin M-W, Chen T-J, Chu S-Y, Hwang C-Y, Chen -C-C, Lee -D-D, Chang Y-T, et al. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrol Dial Transplant. 2012;27(2):833–839. doi:10.1093/ndt/gfr277.
- Mazzucotelli V, Piselli P, Verdirosi D, Cimaglia C, Cancarini G, Serraino D, Sandrini S. De novo cancer in patients on dialysis and after renal transplantation: north-western Italy, 1997–2012. J Nephrol. 2017;30(6):851–857. doi:10.1007/s40620-017-0385-y.
- Opelz G, Unterrainer C, Süsal C, Döhler B. Immunosuppression with mammalian target of rapamycin inhibitor and incidence of post-transplant cancer in kidney transplant recipients. Nephrol Dial Transplant. 2016;31(8):1360–1367. doi:10.1093/ndt/gfw088.
- Piselli P, Serraino D, Segoloni GP, Sandrini S, Piredda GB, Scolari MP, Rigotti P, Busnach G, Messa P, Donati D, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidney transplant recipients, Italy 1997-2009. Eur J Cancer. 2013;49(2):336–344. doi:10.1016/j.ejca.2012.09.013.
- Ramsey-Goldman R, Brar A, Richardson C, Salifu MO, Clarke A, Bernatsky S, Stefanov DG, Jindal RM. Standardised incidence ratios (SIRs) for cancer after renal transplant in systemic lupus erythematosus (SLE) and non-SLE recipients. Lupus Sci Med. 2016;3(1):e000156–e000156. doi:10.1136/lupus-2016-000156.
- Schrem H, Schneider V, Kurok M, Goldis A, Dreier M, Kaltenborn A, Gwinner W, Barthold M, Liebeneiner J, Winny M. Independent pre-transplant recipient cancer risk factors after kidney transplantation and the utility of G-Chart analysis for clinical process control. PLoS One. 2016;11(7):e0158732–e0158732. doi:10.1371/journal.pone.0158732.
- Teo SH, Lee KG, Lim GH, Koo SX, Ramirez ME, Chow KY, Kee T. Incidence, risk factors and outcomes of malignancies after kidney transplantation in Singapore: a 12-year experience. Singapore Med J. 2019;60(5):253–259. doi:10.11622/smedj.2018122.
- Tessari G, Naldi L, Boschiero L, Minetti E, Sandrini S, Nacchia F, Valerio F, Rugiu C, Sassi F, Gotti E, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant. 2013;13(1):214–221. doi:10.1111/j.1600-6143.2012.04294.x.
- Vajdic CM, McDonald SP, McCredie MRE, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–2831. doi:10.1001/jama.296.23.2823.
- Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941–948. doi:10.1111/j.1600-6143.2007.01736.x.
- Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–1436.
- Baccarani U, Piselli P, Serraino D, Adani GL, Lorenzin D, Gambato M, Buda A, Zanus G, Vitale A, De Paoli A, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. 2010;42(1):55–60. doi:10.1016/j.dld.2009.04.017.
- Chatrath H, Berman K, Vuppalanchi R, Slaven J, Kwo P, Tector AJ, Chalasani N, Ghabril M. De novo malignancy post-liver transplantation: a single center, population controlled study. Clin Transplant. 2013 Jul-Aug;27(4):582–590. doi:10.1111/ctr.12171.
- Ettorre GM, Piselli P, Galatioto L, Rendina M, Nudo F, Sforza D, Miglioresi L, Fantola G, Cimaglia C, Vennarecci G, et al. De novo malignancies following liver transplantation: results from a multicentric study in central and southern Italy, 1990–2008. Transplant Proc. 2013;45(7):2729–2732. doi:10.1016/j.transproceed.2013.07.050.
- Heo J, Noh OK, Oh Y-T, Chun M, Kim L. Second primary cancer after liver transplantation in hepatocellular carcinoma: a nationwide population-based study. Hepatol Int. 2017;11(6):523–528. doi:10.1007/s12072-017-9824-z.
- Hsiao C-Y, Lee P-H, Ho C-M, Wu Y-M, Ho M-C, Hu R-H. Post-transplant malignancy in liver transplantation: a single center experience. Medicine (Baltimore). 2014;93(28):e310–e310. doi:10.1097/MD.0000000000000310.
- Jiang Y, Villeneuve PJ, Fenton SSA, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14(11):1588–1597. doi:10.1002/lt.21554.
- Kaneko J, Sugawara Y, Tamura S, Aoki T, Sakamoto Y, Hasegawa K, Yamashiki N, Kokudo N. De novo malignancies after adult-to-adult living-donor liver transplantation with a malignancy surveillance program: comparison with a Japanese population-based study. Transplantation. 2013;95(9):1142–1147. doi:10.1097/TP.0b013e318288ca83.
- Maggi U, Consonni D, Manini MA, Gatti S, Cuccaro F, Donato F, Conte G, Bertazzi PA, Rossi G. Early and late de novo tumors after liver transplantation in adults: the late onset of bladder tumors in men. PLoS One. 2013;8(6):e65238–e65238. doi:10.1371/journal.pone.0065238.
- Nordin A, Åberg F, Pukkala E, Pedersen CR, Storm HH, Rasmussen A, Bennet W, Olausson M, Wilczek H, Ericzon B-G, et al. Decreasing incidence of cancer after liver transplantation-A Nordic population-based study over 3 decades. Am J Transplant. 2018;18(4):952–963. doi:10.1111/ajt.14507.
- Oo YH, Gunson BK, Lancashire RJ, Cheng KK, Neuberger JM. Incidence of cancers following orthotopic liver transplantation in a single center: comparison with national cancer incidence rates for England and Wales. Transplantation. 2005;80(6):759–764. doi:10.1097/01.TP.0000173775.16579.18.
- Schrem H, Kurok M, Kaltenborn A, Vogel A, Walter U, Zachau L, Manns MP, Klempnauer J, Kleine M. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19(11):1252–1261. doi:10.1002/lt.23722.
- Sérée O, Altieri M, Guillaume E, De Mil R, Lobbedez T, Robinson P, Segol P, Salamé E, Abergel A, Boillot O. Longterm risk of solid organ De Novo malignancies after liver transplantation: A French national study on 11,226 patients. Liver Transpl. 2018;24(10):1425–1436. doi:10.1002/lt.25310.
- Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, Emre S, Fishbein TM, Guy SR, Schwartz ME, et al. Long-term medical complications in patients surviving ???5 years after liver transplant. Transplantation. 2000;69(5):781–789. doi:10.1097/00007890-200003150-00018.
- Taborelli M, Piselli P, Ettorre GM, Lauro A, Galatioto L, Baccarani U, Rendina M, Shalaby S, Petrara R, Nudo F, et al. Risk of virus and non-virus related malignancies following immunosuppression in a cohort of liver transplant recipients. Italy, 1985–2014. Int J Cancer. 2018;143(7):1588–1594.
- Jäämaa-Holmberg S, Salmela B, Lemström K, Pukkala E, Lommi J. Cancer incidence and mortality after heart transplantation – A population-based national cohort study. Acta Oncol. 2019;58(6):859–863. doi:10.1080/0284186X.2019.1580385.
- Jiang Y, Villeneuve PJ, Wielgosz A, Schaubel DE, Fenton SSA, Mao Y. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant. 2010;10(3):637–645. doi:10.1111/j.1600-6143.2009.02973.x.
- Kellerman L, Neugut A, Burke B, Mancini D. Comparison of the incidence of de novo solid malignancies after heart transplantation to that in the general population. Am J Cardiol. 2009;103(4):562–566. doi:10.1016/j.amjcard.2008.10.026.
- Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sørensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol. 2010;90(5):474–479. doi:10.2340/00015555-0919.
- Koshiol J, Pawlish K, Goodman MT, McGlynn KA, Engels EA. Risk of hepatobiliary cancer after solid organ transplant in the United States. Clin Gastroenterol Hepatol. 2014;12(9):1541–9.e3. doi:10.1016/j.cgh.2013.12.018.
- Krynitz B, Olsson H, Lundh Rozell B, Lindelöf B, Edgren G, Smedby KE. Risk of basal cell carcinoma in Swedish organ transplant recipients: a population-based study. Br J Dermatol. 2016;174(1):95–103. doi:10.1111/bjd.14153.
- Mahale P, Shiels MS, Lynch CF, Engels EA. Incidence and outcomes of primary central nervous system lymphoma in solid organ transplant recipients. Am J Transplant. 2018;18(2):453–461. doi:10.1111/ajt.14465.
- Park MJ, Roh J-L, Choi S-H, Nam SY, Kim SY, Lee YS. De novo head and neck cancer arising in solid organ transplantation recipients: the Asan medical center experience. Auris Nasus Larynx. 2018;45(4):838–845. doi:10.1016/j.anl.2017.11.006.
- Quinlan SC, Landgren O, Morton LM, Engels EA. Hodgkin lymphoma among US solid organ transplant recipients. Transplantation. 2010;90(9):1011–1015. doi:10.1097/TP.0b013e3181f5c3a6.
- Rizvi SMH, Aagnes B, Holdaas H, Gude E, Boberg KM, Bjørtuft Ø, Helsing P, Leivestad T, Møller B, Gjersvik P. Long-term change in the risk of skin cancer after organ transplantation: a population-based nationwide cohort study. JAMA Dermatol. 2017;153(12):1270–1277. doi:10.1001/jamadermatol.2017.2984.
- Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, Paddock L, Yanik EL, Lynch CF, Kasiske BL, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135(11):2657–2665. doi:10.1038/jid.2015.312.
- Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF, Engels EA. Risk of colorectal cancer after solid organ transplantation in the United States. Am J Transplant. 2016;16(3):960–967. doi:10.1111/ajt.13549.
- Kalinova L, Majek O, Stehlik D, Krejci K, Bachleda P. Skin cancer incidence in renal transplant recipients - a single center study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(3):257–260. doi:10.5507/bp.2010.039.
- Karami S, Yanik EL, Moore LE, Pfeiffer RM, Copeland G, Gonsalves L, Hernandez BY, Lynch CF, Pawlish K, Engels EA. Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant. 2016;16(12):3479–3489. doi:10.1111/ajt.13862.
- Mäkitie AA, Lundberg M, Salmela K, Kyllönen L, Pukkala E. Head and neck cancer in renal transplant patients in Finland. Acta Otolaryngol. 2008;128(11):1255–1258. doi:10.1080/00016480801901725.
- Medani S, O'Kelly P, O'Brien KM, Mohan P, Magee C, Conlon P. Bladder cancer in renal allograft recipients: risk factors and outcomes. Transplant Proc 2014;46(10):3466-3473.
- Ekstrom M, Riise GC, Tanash HA. Risk of cancer after lung transplantation for COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2841–2847. doi:10.2147/COPD.S147065.
- Fink AK, Yanik EL, Marshall BC, Wilschanski M, Lynch CF, Austin AA, Copeland G, Safaeian M, Engels EA. Cancer risk among lung transplant recipients with cystic fibrosis. J Cystic Fibrosis**. 2017 Jan;16(1):91–97. doi:10.1016/j.jcf.2016.07.011.
- Magruder JT, Crawford TC, Grimm JC, Kim B, Shah AS, Bush EL, Higgins RS, Merlo CA. Risk factors for de novo malignancy following lung transplantation. Am J Transplant. 2017 Jan;17(1):227–238. doi:10.1111/ajt.13925.
- Ohman J, Rexius H, Mjornstedt L, Gonzalez H, Holmberg E, Dellgren G, Hasséus B. Oral and lip cancer in solid organ transplant patients–a cohort study from a Swedish transplant centre. Oral Oncol. 2015 Feb;51(2):146–150. doi:10.1016/j.oraloncology.2014.11.007.
- Medani S, O’Kelly P, O’Brien KM, Mohan P, Magee C, Conlon P. Bladder cancer in renal allograft recipients: risk factors and outcomes. Transplant Proc. 2014;46(10):3466–3473. doi:10.1016/j.transproceed.2014.06.075.
- Vajdic CM, van Leeuwen MT, McDonald SP, McCredie MRE, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Increased incidence of squamous cell carcinoma of eye after kidney transplantation. J Natl Cancer Inst. 2007;99(17):1340–1342. doi:10.1093/jnci/djm085.
- Vajdic CM, van Leeuwen MT, Turner JJ, McDonald AM, Webster AC, McDonald SP, Chapman JR, Kaldor JM, Grulich AE. No excess risk of follicular lymphoma in kidney transplant and HIV-related immunodeficiency. Int J Cancer. 2010;127(11):2732–2735. doi:10.1002/ijc.25272.
- Verran DJ, Mulhearn MH, Dilworth PJ, Balderson GA, Munn S, Chen JW, Fink MA, Crawford MD, McCaughan GW. Nature and outcomes of the increased incidence of colorectal malignancy after liver transplantation in Australasia. Med J Aust. 2013;199(9):610–612. doi:10.5694/mja13.10102.
- Tessari G, Naldi L, Boschiero L, Cordiano C, Piaserico S, Fortina AB, Cerimele D, La Parola IL, Capuano M, Gotti E, et al. Incidence and clinical predictors of Kaposi’s sarcoma among 1721 Italian solid organ transplant recipients: a multicenter study. Eur J Dermatol. 2006 Sep-Oct;16(5):553–557.
- Triplette M, Crothers K, Mahale P, Yanik EL, Valapour M, Lynch CF, Schabath MB, Castenson D, Engels EA. Risk of lung cancer in lung transplant recipients in the United States. Am J Transplant. 2019 May;19(5):1478–1490. doi:10.1111/ajt.15181.
- Laprise C, Cahoon EK, Lynch CF, Kahn AR, Copeland G, Gonsalves L, Madeleine MM, Pfeiffer RM, Engels EA. Risk of lip cancer after solid organ transplantation in the United States. Am J Transplant. 2019 Jan;19(1):227–237. doi:10.1111/ajt.15052.
- Morton LM, Gibson TM, Clarke CA, Lynch CF, Anderson LA, Pfeiffer R, Landgren O, Weisenburger DD, Engels EA. Risk of myeloid neoplasms after solid organ transplantation. Leukemia. 2014 Dec;28(12):2317–2323. doi:10.1038/leu.2014.132.
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi:10.1016/S1470-2045(09)70096-8.
- Mayer V, Ebbesen P. Persistent viral infections in human carcinogenesis. Eur J Cancer Prev. 1994;3(1):5–14. doi:10.1097/00008469-199401000-00002.
- Buzzeo BD, Heisey DM, Messing EM; Buzzeo BD, Heisey DM, Messing EM. Bladder cancer in renal transplant recipients. Urology. 1997;50(4):525–528. doi:10.1016/S0090-4295(97)00305-1.
- Yan L, Chen P, Chen EZ, Gu A, Jiang Z-Y. Risk of bladder cancer in renal transplant recipients: a meta-analysis. Br J Cancer. 2014;110(7):1871–1877. doi:10.1038/bjc.2014.44.
- Holt CD. Overview of immunosuppressive therapy in solid organ transplantation. Anesthesiol Clin. 2017;35(3):365–380. doi:10.1016/j.anclin.2017.04.001.
- Sanches MM, Travassos AR, Soares-de-Almeida L. The relationship between immunodepression and the development of skin cancer [A Relação Entre a Imunodepressão e o Desenvolvimento de Cancro Cutâneo]. Acta Med Port. 2017;30:69–72.
- Chin-Hong PV, Reid GE, Practice A. Human papillomavirus infection in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13590–e13590. doi:10.1111/ctr.13590.
- Mittal A, Colegio OR. Skin cancers in organ transplant recipients. American J Transplantation. 2017;17(10):2509–2530. doi:10.1111/ajt.14382.
- Brem R, Li F, Karran P. Reactive oxygen species generated by thiopurine/UVA cause irreparable transcription-blocking DNA lesions. Nucleic Acids Res. 2009 Apr;37(6):1951–1961. doi:10.1093/nar/gkp070.
- Kelly GE, Meikle W, Sheil AG. Effects of immunosuppressive therapy on the induction of skin tumors by ultraviolet irradiation in hairless mice. Transplantation. 1987 Sep;44(3):429–434. doi:10.1097/00007890-198709000-00021.
- Brand T, Haithcock B. Lung cancer and lung transplantation. Thorac Surg Clin. 2018 Feb;28(1):15–18. doi:10.1016/j.thorsurg.2017.09.003.
- Chambers DC, Cherikh WS, Goldfarb SB, Hayes D, Kucheryavaya AY, Toll AE, Khush KK, Levvey BJ, Meiser B, Rossano JW, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183. doi:10.1016/j.healun.2018.07.020.
- ISHLT adult heart transplantation statistics. 2018. [cited 2020 Jan 20]. Available from: https://ishltregistries.org/registries/slides.asp.
- de Fijter JW. Cancer and mTOR inhibitors in transplant recipients. Transplantation. 2017;101:45–55.
- Oweira H, Schmidt J, Helbling D, Petrausch U, Schöb O, Mehrabi A, Giryes A, Elhadedy H, Abdel-Rahman O. Impact of liver transplantation on the risk of second malignant tumors among hepatocellular carcinoma patients. Expert Rev Gastroenterol Hepatol. 2017;11(9):865–869. doi:10.1080/17474124.2017.1355235.
- Rompianesi G, Ravikumar R, Jose S, Allison M, Athale A, Creamer F, Gunson B, Manas D, Monaco A, Mirza D, et al. Incidence and outcome of colorectal cancer in liver transplant recipients: a national, multicentre analysis on 8115 patients. Liver Int. 2019;39(2):353–360.
- Heinz-Peer G, Schoder M, Rand T, Mayer G, Mostbeck GH. Prevalence of acquired cystic kidney disease and tumors in native kidneys of renal transplant recipients: a prospective US study. Radiology. 1995;195(3):667–671. doi:10.1148/radiology.195.3.7753991.
- Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017 Dec 21;377(25):2500–2501. doi:10.1056/NEJMc1713444.
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016 May 7;387(10031):1909–1920. doi:10.1016/S0140-6736(16)00561-4.
- Ordóñez-Mena JM, Schöttker B, Mons U, Jenab M, Freisling H, Bueno-de-Mesquita B, O'Doherty MG, Scott A, Kee F, Stricker BH, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016;14:62.
- Freisling H, Arnold M, Soerjomataram I, O’Doherty MG, Ordóñez-Mena JM, Bamia C, Kampman E, Leitzmann M, Romieu I, Kee F, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116(11):1486–1497. doi:10.1038/bjc.2017.106.
- Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580–593. doi:10.1038/bjc.2014.579.
- Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397(6719):530–534. doi:10.1038/17401.