ABSTRACT
Integration of immune checkpoint inhibitors (ICIs) has improved the efficacy of treatment regimens for various cancers. The array of potential side effects keeps evolving and includes neurological complications. An increased risk of seizures and status epilepticus (SE) has been discussed and appears likely. In this report, we present clinical data from brain metastases patients undergoing ICI treatment revealing, for what we believe is the first time, SE as a serious adverse effect of ICI treatment. In our cohort of 3202 patients with brain metastases, we observed an increasing incidence of SE since the approval of ICIs in 2014 (16 patients in 2008–2013 vs. 36 patients in 2014–2019). Almost half of the patients treated in 2014–2019 received ICIs during the course of their disease, and in more than 80% of cases last dose of ICIs was given less than 30 days before SE. These findings suggest that ICIs may lead to an increased rate of SE in patients with brain metastases. Additional mechanistic research and prospective trials are necessary to elucidate the pathomechanism causing SE in patients treated with ICIs.
Introduction
Status epilepticus (SE) is a medical emergency associated with a high mortality rate. About 7% of SE cases are caused by brain tumors or metastases.Citation1,Citation2 Several studies indicate that SE related to brain tumors is associated with higher mortality than non-tumor-related SE.Citation1,Citation3 New therapeutic options for brain tumors and metastases improve tumor-related outcomes. However, neurological side effects should be closely monitored.
The introduction of immune checkpoint inhibitors (ICIs) is a milestone in tumor therapy and has improved the efficacy of treatment regimens across various cancers. ICIs enhance anti-tumor activity of the immune system by inhibiting immune control points: programmed death-1 (PD-1: pembrolizumab, nivolumab and cemiplimab), programmed death ligand-1 (PD-L1: atezolizumab, durvalumab and avelumab) and cytotoxic T-lymphocyte antigen-4 (CTLA-4: ipilimumab).Citation4 Several phase III trials have demonstrated efficacy in tumor treatment with an overall good level of tolerability; however, neurological immune-related adverse events may also occur.Citation5,Citation6 With increased use, rare but potentially serious adverse events will start occurring more frequently. In the past, a greater risk of seizures and SE has been proposed during treatment with ICIs. This side effect may be caused by an increased activation of the immune system or an increased inflammatory reaction.Citation7,Citation8
An association between therapy with ICIs and an increased rate of SE has yet to be shown. In this report, we present clinical data from patients with brain metastases undergoing ICI treatment that reveals SE as a serious adverse effect of ICI treatment.
Materials and methods
We investigated whether therapy with ICIs increases the risk for SE in patients with brain metastases and which characteristics were shown by patients belonging to this group. Therefore, we reviewed the medical records of all adult patients (age ≥18 years) treated for brain metastases using the hospital information system between January 2008 and December 2019. The University Hospital Frankfurt offers a full range of neurological care services with expertise in neuro-oncology, epileptology and intensive care medicine. The detailed evaluation of all SE patients is part of a study on SE outcomes.Citation9
This retrospective cohort study was approved by the Institutional Review Board of the University Cancer Center (UCT) and the Ethical Committee at the University Hospital Frankfurt (project-number: SNO-13-2019). Informed consent was not required due to the deidentified nature of the data. For secured diagnosis of SE, patients needed at least one EEG classified as SE by a physician specialized in EEG diagnostics or an unequivocal clinical event. The definition and classification of SE was adopted based on the latest definitions proposed by the International League Against Epilepsy.Citation10
Data on demographics, clinical diagnosis, tumor etiology and treatment, semiology and history of seizures or SE, use of anticonvulsants, total length of stay in hospital, and mortality were systematically collected in all patients. GraphPad Prism 8.0.2 and R version 3.5.2 was used for all statistical analyses. Chi-square test, Fisher’s exact test and Gehan-Breslow-Wilcoxon test were used to analyze the collected data. P values were two-sided at a 5%-level of statistical significance.
Results
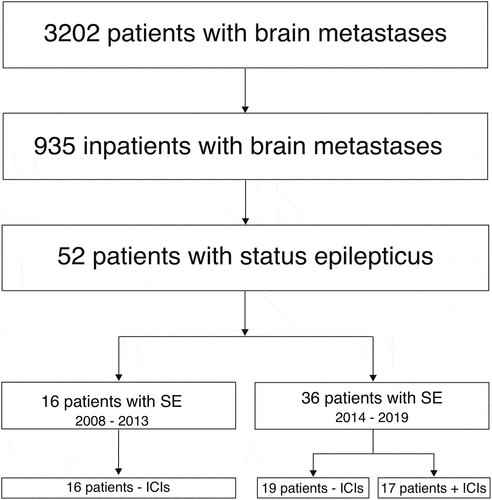
During the observation period, 3202 patients were identified that suffered from brain metastases, and 935 of these patients presented with neurological symptoms to the department of neurology. Among this population, we could confirm 52 cases of SE (CONSORT flow diagram ).
Figure 1. CONSORT flow diagram of study inclusion (“+ ICI” with and “- ICI” without checkpoint inhibitor treatment)
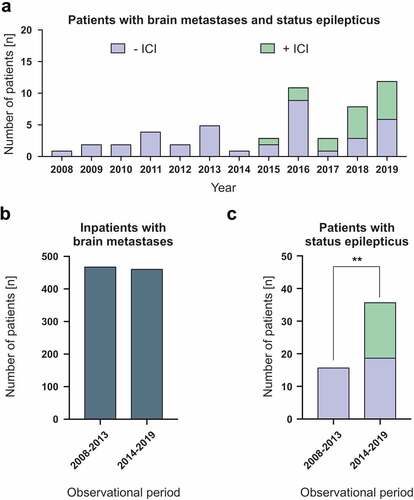
We observed an increasing incidence of SE since the approval of ICIs in 2014 (). The overall number of inpatients with brain metastases at our center had remained fairly constant over a six-year evaluation period before (471 patients in 2008–2013; ) and after (464 patients in 2014–2019; ) the introduction of ICIs. However, while the frequency of brain metastases patients with SE without ICI treatment has increased only slightly (16 patients in 2008–2013 vs. 19 patients in 2014–2019; ), the overall frequency of SE at our center has increased significantly since the approval and regular employment of ICIs in 2014 (36 patients in 2014–2019; p < 0.01; ).
Figure 2. Increasing number of patients with status epilepticus in conjunction with immune checkpoint inhibitor therapy
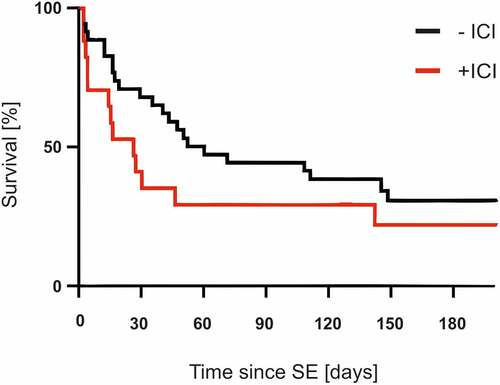
The mean age of patients suffering from SE associated with ICI treatment was 57.7 years (n = 17, median: 58 years; range: 40–71 years; female: 41.2%, n = 7). Patients’ characteristics are shown in and . Primary cancer entities included melanoma (n = 13), lung cancer (n = 3), and bladder cancer (n = 1). Although most patients in the checkpoint-group suffered from metastasized melanoma, the main primary cancer in the non-ICI-group was lung cancer. Most patients (n = 10) received anti-PD-1/PD-L1 monotherapy with nivolumab, ipilimumab or pembrolizumab, whereas seven patients were treated with a combination of ipilimumab and nivolumab or pembrolizumab. The last dose of ICIs was given less than 30 days before onset of SE in 82% of patients [n = 14; median: 23.5 days, range: 2–325 days (interquartile range: 5.5–29.5 days)]. In total, 76.4% (n = 13) of the ICI patients had refractory SE. For comparison, only 17.1% (p = .01) of the patients in the non-ICI group had refractory SE. During the course of status therapy, 88.2% (n = 15) of the patients stopped ICI therapy. Beyond the discontinuation of ICI therapy, 82.4% of the patients were treated with steroids due to its anti-inflammatory and anti-edematous effect. Besides being administered a benzodiazepine (88.2%), 70.2% of patients received levetiracetam as the most common anticonvulsant. An average of 3.2 anticonvulsants were needed to terminate the SE. Comparing mortality across both cohorts, the Kaplan-Meier curve suggests a trend toward higher mortality in patients treated with ICIs, although this difference was not significant. The 30-day mortality rates were 64.7% in SE patients with ICI treatment and 34.3% in SE patients without ICI treatment (p = .09; median survival time with ICI therapy 26 days vs. 60 days without ICI therapy; ).
Table 1. Characteristics of status epilepticus patients for the overall cohort and those with and without ICI treatment
Table 2. Characteristics of status epilepticus patient for the overall cohort and for the time periods 2008–2013 and 2014–2019
Figure 3. Survival of patients with an without immune checkpoint therapy after the status epilepticus
Discussion
Our results demonstrate a significantly elevated risk of SE in patients with brain metastases under ICI treatment. Although we cannot decipher the underlying mechanism at the current time, there is a notable link between the use of ICIs and the rising incidence of SE. Several mechanisms can be considered: (i) Increased immune cell infiltration may generate an inflammatory microenvironment with increased perilesional edema and/or release of proconvulsive cytokines.Citation7,Citation8,Citation11 Furthermore, (ii) epileptic seizures and SE may be caused by autoimmune encephalitis mediated by neuronal autoantibodies.Citation12,Citation13 This would offer an explanation for the significantly increased rate of refractory status epilepticus in the checkpoint group and as well as for the comparatively poor response of ICI-associated SE to standard therapy with anti-seizure drugs. However, as observed in our cohort, ICI-associated SE responds relatively well to treatment with steroids or the discontinuation of ICI therapy. This is of particular importance since the occurrence of SE was associated with increased mortality in the group of patients treated with ICIs. Although this effect was not statistically significant, probably due to the small sample size, a connection can nevertheless be assumed here. It is important to consider inherent limitations associated with a noncontrolled study design and retrospective review format. The SE cases reported here occurred in a heterogeneous population of varying ages and comorbidities and showed different severity levels of SE. Patients were treated with different strategies that might influence the outcome of SE as shown in several studies.Citation14–16 Individual cases may therefore respond differently to treatments and have contrasting prognoses.
Conclusion
Our results need to be interpreted with caution given the small sample size and the retrospective study design from a single center. However, since ICIs are increasingly employed, a further rise in the occurrence of SE in patients with brain metastases can be expected. We therefore recommend vigilance for SE in patients treated with ICIs, as this is a potentially life-limiting side effect of an otherwise effective therapy. Future mechanistic studies and prospective trials are needed to elucidate the mechanism of SE associated with ICIs.
Authors’ contributions
HU, LMW and AS developed the idea for this study. HU, LMW, and AS collected the data. HU, FR, JPS and AS analyzed the data. HU, FR and AS interpreted the data. HU and MWR designed the figures. HU, LMW, MWR, JPS and AS wrote the manuscript. All authors read and approved the final manuscript.
List of Abbreviations
| ICIs | = | immune checkpoint inhibitors |
| SE | = | status epilepticus |
| UCT | = | University Cancer Center |
Ethical publication guidelines
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of potential conflicts of interest
HU and LMW have nothing to disclose.
MWR reports research grants from UCB, outside the submitted work.
FR reports personal fees from Eisai, personal fees from GW-Pharma, personal fees from Desitin Pharma, personal fees and other from Novartis, personal fees from Medtronic, personal fees from Cerbomed, personal fees from Shire, grants from European Union, grants from German Minister for Education and Research (BMBF), grants from LOEWE Programm of the state of Hesse, grants from Deutsche Forschungsgemeinschaft (DFG), grants from Detlev-Wrobel-Fonds for Epilepsy Research, outside the submitted work.
JPS reports personal fees from Abbvie, personal fees from Medac, personal fees from Novocure, personal fees from Roche, personal fees from USB, outside the submitted work.
AS reports personal fees and grants from Bayer HealthCare, Boehringer Ingelheim, Desitin Arzneimittel, Eisai, LivaNova, Pfizer, Sage Therapeutics, UCB Pharma, and Zogenix, outside the submitted work.
Additional information
Funding
References
- Arik Y, Leijten FS, Seute T, Robe PA, Snijders TJ. Prognosis and therapy of tumor-related versus non-tumor-related status epilepticus: a systematic review and meta-analysis. BMC Neurol. 2014;14:152. doi:10.1186/1471-2377-14-152.
- Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14:615–6. doi:10.1016/S1474-4422(15)00042-3.
- Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611–615. doi:10.1136/jnnp.2005.080887.
- Hoos A. Development of immuno-oncology drugs – from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–247. doi:10.1038/nrd.2015.35.
- Dalakas MC. Neurological complications of immune checkpoint inhibitors: what happens when you ‘take the brakes off’ the immune system. Ther Adv Neurol Disord. 2018;11:1756286418799864. doi:10.1177/1756286418799864.
- Wick W, Hertenstein A, Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17:e529–e541. doi:10.1016/S1470-2045(16)30571-X.
- Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev Neurother. 2015;15:1081–1092. doi:10.1586/14737175.2015.1079130.
- Berghoff AS, Preusser M. The inflammatory microenvironment in brain metastases: potential treatment target? Chin Clin Oncol. 2015;4:21.
- Kortland LM, Alfter A, Bahr O, Carl B, Dodel R, Freiman TM, Hubert K, Jahnke K, Knake S, von Podewils F, et al. Costs and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia. 2016;57:2056–2066. doi:10.1111/epi.13584.
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH. A definition and classification of status epilepticus – report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–1523. doi:10.1111/epi.13121.
- Tran TT, Mahajan A, Chiang VL, Goldberg SB, Nguyen DX, Jilaveanu LB, Kluger HM. Perilesional edema in brain metastases: potential causes and implications for treatment with immune therapy. J Immunother Cancer. 2019;7:200. doi:10.1186/s40425-019-0684-z
- Schneider S, Potthast S, Komminoth P, Schwegler G, Bohm SPD-1. Checkpoint inhibitor associated autoimmune encephalitis. Case Rep Oncol. 2017;10:473–478. doi:10.1159/000477162.
- Lyons S, Joyce R, Moynagh P, O’Donnell L, Blazkova S, Counihan TJ. Autoimmune encephalitis associated with Ma2 antibodies and immune checkpoint inhibitor therapy. Pract Neurol. 2020;20:256–259. doi:10.1136/practneurol-2019-002464.
- Alvarez V, Januel JM, Burnand B, Rossetti AO. Role of comorbidities in outcome prediction after status epilepticus. Epilepsia. 2012;53:e89–92. doi:10.1111/j.1528-1167.2012.03451.x.
- Sutter R, Semmlack S, Kaplan PW, Opic P, Marsch S, Ruegg S. Prolonged status epilepticus: early recognition and prediction of full recovery in a 12-year cohort. Epilepsia. 2019;60:42–52. doi:10.1111/epi.14603.
- Kellinghaus C, Rossetti AO, Trinka E, Lang N, May TW, Unterberger I, Rüegg S, Sutter R, Strzelczyk A, Tilz C, et al. Factors predicting cessation of status epilepticus in clinical practice: data from a prospective observational registry (SENSE). Ann Neurol. 2019;85:421–432. doi: 10.1002/ana.25416.
