ABSTRACT
Interleukin-9 (IL-9) is a T cell cytokine that is associated with inflammation and allergy, but the expression level of IL-9 in gastric cancer and its clinical significance are less well established. Our study aims to uncover the critical role of IL-9 in the progression of gastric cancer. Here, a total of 453 patients with gastric cancer undergoing curative resection were enrolled for immunohistochemical analyses, and Kaplan-Meier analysis was conducted to compare overall survival of patients in different subgroups. We further investigated the correlation between IL-9 expression and functional status of intratumoral CD8+ T cells by means of Flow cytometry. Moreover, in vitro study was preformed to further explore the influence of IL-9 on anti-tumor immunity. Results indicated that gastric cancer patients with high IL-9 expression showed improved overall survival and gained more benefit from 5-fluorouracil-based adjuvant chemotherapy (ACT). High IL-9 expression was associated with increased numbers and elevated function of intratumoral CD8+ T cells. In vitro study revealed that recombinant human IL-9 (rhIL-9) exhibit anti-tumor activity via enhancing the function of intratumoral CD8+ T cells. Moreover, we found rhIL-9 could augment the efficacy of Pembrolizumab in gastric cancer. In summary, these results suggest that IL-9 expression could act as an independent predictor for overall survival and ACT response and enhancing IL-9 signaling might represent an important therapeutic strategy in gastric cancer.
Introduction
Gastric cancer is one of the most common causes of cancer-related death around the world. especially in Eastern Asia.Citation1, Citation2 Owing to nonspecific symptoms in early stage, most patients are diagnosed at an advanced stage and showed a high rate of recurrence despite receiving standard radical gastrectomy. For these patients, a 5-fluorouracil-based adjuvant chemotherapy (ACT) regimen is commonly used as a first-line choice to reduce postoperative recurrent rate and prolong overall survival.Citation3,Citation4 However, large variations in clinical outcomes have been reported in patients with the same stage and similar treatment regimens. Hence, exploring the potential individual benefit of adjuvant chemotherapy is drawing more and more attention.
Recently, the clinical significance of tumor-infiltrating immune cells has been recognized in gastric cancer.Citation5–7 As the bridge of interaction among immune cells, cytokines also play a critical role in tumor progression by orchestrating the entire immune microenvironment. Interleukin-9 (IL-9) is a newly identified cytokine in the late 1980s which was firstly described as a member of T cell growth factor.Citation8,Citation9 However, subsequent researches showed that IL-9 had pleiotropic functions with multiple target cells including T cells, mast cells, B cells as well as nonimmune cells.Citation8 So far, the main role of IL-9 has been described in the immune responses against parasites and pathogenesis of allergic diseases.Citation10 However, the function of IL-9 in cancer biology is still dim and needs much more exploration. IL-9 was described as a pro-tumorigenic cytokine by promoting tumor cell growth in hepatocellular and breast cancer.Citation11,Citation12 While in melanoma, IL-9 exhibits anti-tumor activity by provoking CD8+ T cell-mediated antitumor immunity.Citation13 These data revealed that IL-9 seemed to have pleiotropic effect depended on different tumor types. While in gastric cancer, the impact of IL-9 on anti-tumor immunity and its association with patients’ prognosis remains unexplored.
Thus, in this study, we evaluated the intratumoral IL-9 expression in gastric cancer and further explored its prognostic value for overall survival and influence on anti-tumor immunity. We found that IL-9 expression exhibited outstanding prognostic value and predictive ability for overall survival benefit from ACT. Further analysis showed that IL-9 enhanced the anti-tumor immunity by intratumoral CD8+ T cells in gastric cancer. Moreover, we found that recombined human interleukin-9 (rhIL-9) could augment response to programmed cell death protein-1 (PD-1) blockade. All these results revealed that IL-9 expression could serve as a biomarker for prognosis and adjuvant response in gastric cancer patients and exogenous IL-9 might exert anti-tumor efficacy.
Materials and methods
Patients and specimens
We enrolled 453 gastric cancer patients who received total or partial gastrectomy by the same surgical team in Zhongshan Hospital, Fudan University (Shanghai, China) during August 2007 and December 2008 for survival analysis. Initially, 496 patients were recruited in this study. 28 patients were excluded owing to lack of chemotherapy information, clinicopathological data, or survival outcomes for complete analysis. In this study, we utilized the tissue microarrays (TMAs) constructed by Shanghai Outdo Biotech Co, Itd, and 7 dots were detached after immunohistochemistry. Additionally, we excluded 8 patients of TNM stage IV. Finally, 453 patients were included in our study and we randomly divided them into two independent data sets, the Discovery set (n = 207) and the Validation set (n = 246). There were totally of 260 patients (57.4%) received fluorouracil-based ACT according to practical needs and patients’ will after their total or partial gastrectomy. Clinicopathological characteristics and their association with intratumoral IL-9 expression were shown in . Additionally, fresh gastric cancer samples from 44 patients who underwent gastrectomy in Zhongshan from August 2018 to October 2020 were obtained. Written informed consents of all patients in this study were provided and the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University had approved our study.
Table 1. Relationship between intratumoral IL-9 expression expression and baseline characteristics of patients
Assay methods
The protocol of immunohistochemistry (IHC) and flow cytometry (FCM) were listed in Supplementary Methods, and antibodies involved were summarized in Supplementary Table S1. TMA slides were scanned on Leica DM6000 B (Leica Microsystems, Wetzlar, Germany). In our study, two pathologists (Dr. Peipei Zhang and Dr. Lingli Chen) who were blinded to the clinical data evaluated all samples separately. Both of them scored independently according to the intensity of cellular staining and the proportion of stained cells.Citation14 The mean score of their evaluation was adopted. Variations in IL-9 IHC score, exceeding 10, were reevaluated separately by both pathologists to acquire consensus. In brief, the IHC cellular staining intensity was stratified as 0 (negative staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown) and 3 (strong staining, brown), while the proportion was defined as the percentage of positive cells (0–100%). The staining intensity and proportion were multiplied to generate an overall IL-9 IHC score ranging from 0 to 300. The density of immune cells was recorded as the average number of cells with positive staining marker per high power field (HPF). Determined by X-tile software using minimum p-value method in the discovery set, the cutoff point was set as 20 to define the intratumoral IL-9 high/low expression. In fresh tumor samples, intratumoral IL-9 expression was assessed by mean fluorescence intensity (MFI) with flow cytometry. The cutoff value for the classification of IL-9 high/low subgroup in the 44 fresh tumor samples was the median value of MFI. All flow cytometry data were obtained using BD FACS Celesta and analyzed by FlowJo v10.0 (Treestar). The gating strategy of flow cytometry is provided in Supplementary Figure 1.
Elisa (Enzyme linked immunosorbent assay)
IFN-γ, GZMB, and PRF1 were measured by ELISA in tumor tissue supernatant. ELISA kit used was the human IFN-γ, GZMB and PRF1 kits obtained from RUIXIN BIOTECH (RUIXIN BIOTECH, Cat.# 10296, Cat.#16262, Cat.#10448).
In vitro therapeutic assay
The fresh tumor tissue was dissociated into single-cell suspensions as above. The digests were cultured in assay medium (RPMI-1640 with 100 U/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum) with anti-CD3 antibody (0.5 μg/ml, OKT3; Biolegend) and anti-CD28 antibody (2 μg/ml, clone 28.2; eBioscience). In CD8+ T cells-deprived system, CD8+ T cells were depleted from single cell suspensions by negative selection using CD8 MicroBeads (Life Technologies). Recombinant human IL-9 (0.3 ng/ml, R&D Systems), anti-IL-9 antibody (5 μg/mL, R&D Systems) or PBS were added to the culture system to study the effect of IL-9 on anti-tumor immunity. Pembrolizumab (10ug/ml; Pembrolizumab, Selleck) were used to explore the synergistic effect of PD-1 blockade and rhIL-9. After 24 hours of incubation, we collected cells for flow cytometry as indicated above.
Statistical analysis
Pearson χ2 test or Student’s t test was respectively applied to compare categorical variables and continuous variables. Overall survival of the two patient data sets was calculated by Kaplan–Meier method and analyzed by log rank test. Cox proportional hazard regression model was used to perform multivariate analyses. Spearman correlation analysis was conducted to assess the correlation between IL-9 expression and other immune cells infiltration. All analyses were performed using IBM SPSS Statistics v20.0, MedCalc 15.6.1 and GraphPad Prism v6.02. All statistical tests were two–tailed and only P < .05 were considered as statistically significant.
Results
Intratumoral IL-9 expression and its association with patients’ clinicopathological characteristics in gastric cancer
By means of immunohistochemistry, we found a widely distribution of IL-9 in tumor tissues and the staining intensity of IL-9 varied greatly among different specimens. The representative pictures of IL-9 high and low expression are shown in Supplementary Figure 2. By flow cytometry, we assessed the surface markers expressed by tumor-infiltrating IL-9+ cells in freshly isolated clinical specimens. Firstly, we found that the majority of IL-9 was produced by CD45+ immune cells in gastric cancer tissues. Further analysis showed that nearly half of CD45+ IL-9+ cells expressed CD3 and CD4, which indicated that CD4+ Th cells might be one of the main producer of IL-9 in gastric cancer (Supplementary Figure 3). We further assessed the association between IL-9 expression and clinicopathologic characteristics, but no significant association was observed ().
Intratumoral IL-9 expression indicates better prognosis in gastric cancer patients
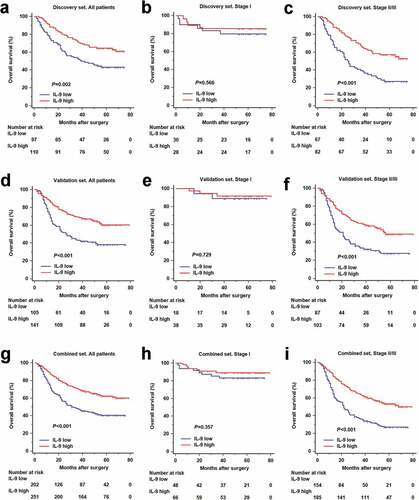
In order to investigate the prognostic value of IL-9 expression, we performed Kaplan-Meier survival analysis according IL-9 expression level. Patients with high IL-9 expression had an obvious better overall survival in the discovery (P = .002, ), validation (P < .001, ) and combined set (P < .001, ). We further classified patients into an early-stage (TNM stage I) disease subgroup or an advanced-stage (TNM stage II and III) disease subgroup. IL-9 high expression could still stratify patients’ survival in advanced-stage disease group (P < .001, P < .001 and P < .001, respectively; , f and i). . However, overall survival didn’t show significant difference between IL-9 low and high expression subgroup in stage I patients (P = .566, P = .729 and P = .357, respectively; , e and h). Overall, intratumoral IL-9 expression was associated with overall survival in the patients with gastric cancer, especially for advanced-stage disease.
Figure 1. Interleukin-9 high expression predicts better overall survival in gastric cancer. (a, d and g). Kaplan-Meier analyses comparing OS of all patients according to IL-9 expression in Discovery set (n = 207, P = .002), Validation set (n = 246, P < .001) and Combined set (n = 453, P < .001). (b, e and h). Kaplan-Meier analyses comparing OS of stage I patients according to IL-9 expression in Discovery set (n = 58, P = .566), Validation set (n = 56, P = .729) and Combined set (n = 114, P = .357). (c, f and i). Kaplan-Meier analyses comparing OS of stage II/III patients according to IL-9 expression in Discovery set (n = 149, P < .001), Validation set (n = 190, P < 0.001) and Combined set (n = 339, P < 0.001). P-value was calculated by log-rank t test. OS = overall survival
Intratumoral IL-9 expression acts as an independent prognostic factor
Multivariate cox regression model including IL-9 expression, adjuvant chemotherapy, TNM classification, localization, size, differentiation, Lauren classification, sex, and age were constructed to demonstrate the independent prognostic value of IL-9 expression. In multivariate cox regression model, IL-9 high expression could still indicate longer overall survival patients in discovery (HR 0.495, 95%CI 0.321–0.764, P = .001), validation (HR 0.561, 95%CI 0.382–0.823, P = .003) and combined set (HR 0.553, 95%CI 0.418–0.732, P < .001) (Table S2). These results indicated that intratumoral IL-9 expression could act as an independent prognostic factor.
Intratumoral IL-9 expression associates with response to adjuvant chemotherapy
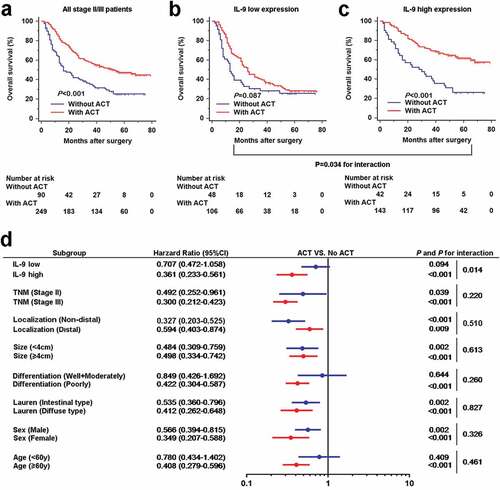
Gastric cancer patients with Stage II/III who received postoperative ACT showed better overall survival compared with those who received surgery only (), which had been identified by the MAGIC and CLASSIC trails.Citation15,Citation16 To evaluate whether intratumoral IL-9 expression has an impact on response to adjuvant chemotherapy, we investigated the association between IL-9 expression and overall survival among stage II/III patients who either did or did not receive adjuvant chemotherapy. The results presented that treatment with ACT was associated with better OS only in patients with high intratumoral IL-9 expression (P < .001, ), but not in patients with low intratumoral IL-9 expression (P = .087, ). A test for the interaction between IL-9 expression and the treatment revealed that the benefit observed in IL-9 high subgroup was significantly superior to that observed in IL-9 low subgroup (P for interaction = 0.034). Multivariate analysis suggested that association between IL-9 expression and benefit from adjuvant chemotherapy was not confounded by risk factors that are known to affect the survival rates among patients with stage II and stage III disease (). All these results demonstrated that patients with high intratumoral IL-9 expression might benefit more from adjuvant chemotherapy.
Figure 2. Interleukin-9 high expression is associated with better benefit from adjuvant chemotherapy in stage II/III gastric cancer patients. (a-c). Kaplan-Meier analyses comparing OS according to treatment in all stage II/III patients (left panel), IL-9 low expression subgroup (middle panel) and IL-9 high expression subgroup (right panel). Subgroup interaction analysis showed IL-9 expression could distinguish the benefit from ACT (P = .034). (d). Multivariate analysis adjusted for TNM stage, localization, tumor size, differentiation, Lauren classification Sex, age and IL-9 expression. Interaction p-value for association between clinical variable and adjuvant chemotherapy benefit are shown
IL-9 high expression was associated with an anti-tumor microenvironment
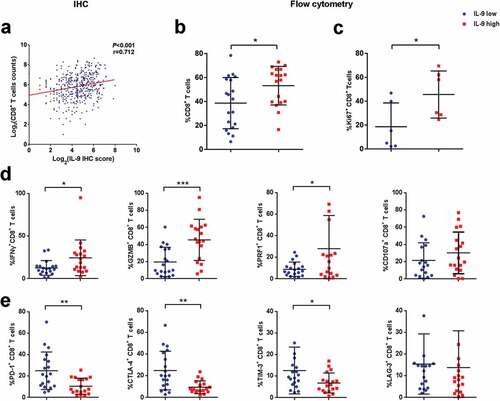
In order to figure out a possible mechanism for the different OS between IL-9 high and low expression patients, we decided to look into the differences of immune cell infiltration between two groups of patients. We found the number of intratumoral CD8+ T cells, NK cells, CD4+ T cells, macrophages and B cells were significantly higher in patients with IL-9 high expression (Supplementary Figure 4). Then, we focused on CD8+ T cells owing to their central role in anti-tumor immunity.Citation17 Positive correlation was found between intratumoral IL-9 expression and number of CD8+ T cells by immunohistochemistry (). In order to detect the infiltration and exact functional state of CD8+ T cells in IL-9 high/low tumors, flow cytometry analysis were conducted with 44 additional patients and showed higher proportion of CD8+ T cells among CD45+ leukocytes (). Then, we compared the functional state of CD8+ T cells between two groups of patients. It was found that the percentage of CD8+ T cells expressing proliferative marker Ki-67 and effector molecules including interferon gamma (IFN-γ), granzyme B (GZMB) and perforin (PRF1) increased significantly in IL-9 high tumors, while CD107a didn't show significant difference ( and d). Consistently, IL-9 expression was positively correlated with intratumoral expression of effector molecules including INF-γ, GZMB, and PRF1 by immunohistochemistry (Supplementary Figure 5). Furthermore, we found a decreased expression of immune checkpoint receptors including PD-1, CTLA-4, and TIM-3 (), which was reported to be associated with T cell dysfunction in tumor tissues. Together, we found elevated intratumoral IL-9 expression correlated with an enhanced anti-tumor immunity in gastric cancer.
Figure 3. Interleukin-9 expression positively correlates with the numbers and functional status of CD8+ T cells in gastric cancer. (a). Scatter diagram showing the positive correlation between IL-9 expression and CD8+ T cells infiltration by means of immunohistochemistry. (b). Proportion of CD8+ T cells among CD45+ leukocytes in IL-9 high and low expression subgroup by flow cytometry (n = 37). (c). Expression of proliferation marker Ki-67 on CD8+ T cells in IL-9 high and low expression subgroup (n = 12). (d). Expression of effector molecules (IFN-γ, GZMB, PRF1 and CD107a; n = 37,37,37,35, respectively) on CD8+ T cells in IL-9 high and low expression subgroup. (e). Expression of co-inhibitory receptors (PD-1, CTLA-4, TIM-3 and LAG-3; n = 37,37,37,37, respectively) on CD8+ T cells in IL-9 high and low expression subgroup. Each symbol represents an individual patient. IFN-γ, interferon-γ; GZMB, granzyme B; PRF1, perforin-1; PD-1, programmed cell death-1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; LAG-3, lymphocyte-activation gene-3. *P < .05, **P < .01 and *** P < .001
Recombined human IL-9 enhanced anti-tumor immune response by CD8+ T cells in vitro
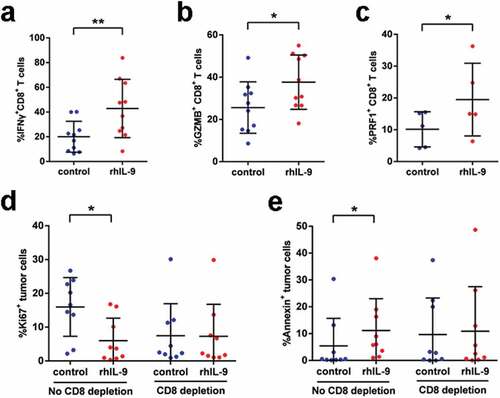
With the positive correlation between IL-9 expression and anti-tumor immunity by CD8+ T cells, we detected the expression of IL-9 receptor (IL-9 R) on CD8+ T cells in gastric cancer tissues and found that about 59.8% CD8+ T cells showed positive expression of IL-9 R (Supplementary Figure 6). Furthermore, in vitro experiments were conducted to validate the impact of IL-9 on intratumoral CD8+ T cells. Flow cytometry analysis indicated that adding recombined human IL-9 (rhIL-9) significantly increased the expression of effector molecules including IFN-γ, GZMB and PRF1 on CD8+ T cells (), which was validated by Elisa (Supplementary Figure 7). We also detected the proliferative and apoptotic status of tumor cells to evaluate the anti-tumor effect of rhIL-9. It was shown that rhIL-9 caused decreased frequency of Ki67+ but increased frequency of Annexin V+ tumor cells ( and e) Interestingly, the anti-tumor activity of rhIL-9 was abolished when CD8+ T cells were depleted from single-cell suspension ( and e), which indicated that the anti-tumor activity of IL-9 might be mediated by CD8+ T cells. Taken together, these findings confirmed that IL-9 enhanced the anti-tumor immunity by CD8+ T cells in gastric cancer.
Figure 4. Recombined human IL-9 enhances anti-tumor immune response by CD8+ T cells in vitro. (a, b and c). Effector molecule expression (GZMB, IFN-γ, PRF-1) on CD8+ T cells treated with rhIL-9 or isotype (n = 10,10,5, respectively). (d). Ki-67+ tumor cells frequencies of tumor cells in gastric cancer tissue samples from patients in absence or presence of CD8+ T cells after treated rhIL-9 (n = 9). (e). Frequencies of Annexin V+ tumor cells in gastric cancer tissue samples from patients in absence or presence of CD8+ T cells after treated rhIL-9 (n = 9). *P < .05, **P < .01 and *** P < .001
Recombined human IL-9 augmented responses to PD-1 blockade
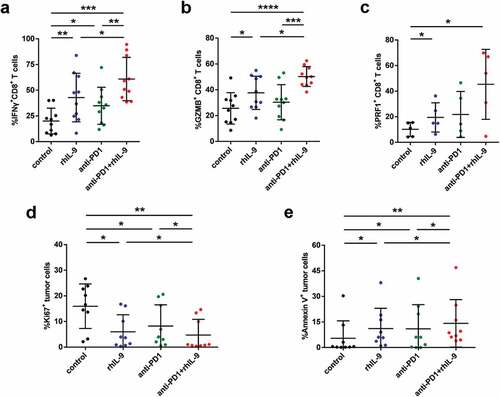
As immune checkpoint inhibitors (ICPI) such as Pembrolizumab exert therapeutic effect also by motivating cytotoxicity by CD8+ T cells, we asked whether rhIL-9 was synergistic with ICPI treatment. Thus, we performed a combined treatment utilizing rhIL-9 and Pembrolizumab, an anti-PD-1 antibody. It was shown that combination of rhIL-9 and Pembrolizumab led to further functional restoration of intratumoral CD8+ T cells ( and Supplementary Figure 7). Likewise, we uncovered the synergistic effect of rhIL-9 and Pembrolizumab on triggering the apoptosis and limiting proliferation of tumor cells ( and e). Taken together, these findings indicate that rhIL-9 may elicit a synergistic effect with Pembrolizumab to facilitate functional restoration of CD8+ T cells and the clearance of tumor cells.
Figure 5. Recombined human IL-9 augmented responses to PD-1 blockade by CD8+ T cells in gastric cancer. (a, b and c). Effector molecule expression (GZMB, IFN-γ, PRF-1) on CD8+ T cells treated with isotype, rhIL-9, Pembrolizumab or combination of rhIL-9 and Pembrolizumab (n = 10,10,5, respectively). (d). Ki-67+ tumor cells frequencies of tumor cells in gastric cancer tissue samples from patients after treated isotype, rhIL-9, Pembrolizumab or combination of rhIL-9 and Pembrolizumab (n = 10). (e). Annexin V+ tumor cells frequencies of tumor cells in gastric cancer tissue samples from patients after treated isotype, rhIL-9, Pembrolizumab or combination of rhIL-9 and Pembrolizumab (n = 10). *P < .05, **P < .01 and ***P < .001
Discussion
In this study, we explored the clinical significance and impact on anti-tumor immunity of intratumoral IL-9 in gastric cancer. It was shown that intratumoral IL-9 expression had strong prognostic value and indicated better adjuvant chemotherapy benefits in gastric cancer patients. In vitro experiments showed that rhIL-9 could enhance anti-tumor immunity by CD8+ T cells and raise the efficacy of PD-1 blockade on CD8+ T cells. These findings suggested that efforts to increase intratumoral IL-9 level might be new immunotherapeutic strategy in gastric cancer.
Prognostic assessment is crucial for formation of appropriate treatment choices. Recently, the association between immune contexture and clinical outcome has been drawing more and more attention owing to their critical role in tumorigenesis and progression.Citation18 Our previous studies have demonstrated the clinical significance of tumor-infiltrating immune cells including macrophages, neutrophils, mast cells and IL-17-producing cells in gastric cancer,Citation6,Citation7,Citation19,Citation20 here we concluded that the immune regulatory cytokine, IL-9 could also serve as an independent prognostic factor for overall survival in gastric cancer patients. Remarkably, further analysis showed IL-9 high expression also indicated better benefit from 5-fluorouracil-based adjuvant chemotherapy in gastric cancer patients with stage II/III disease. Although 5-fluorouracil-based ACT has been recommended as a first-line adjuvant therapy for stage II/III patients with gastric cancer, not everyone was responsive to chemotherapy and the criterion for selection of candidates is still controversial.Citation21 Thus, our findings provided more evidence for better selection of patients who should receive adjuvant chemotherapy.
We showed that IL-9 exhibited anti-tumor role in gastric cancer, which was consistent with studies on melanomaCitation13 and colon cancer,Citation22 but contradictory with those on breast cancer,Citation12 non-small cell lung cancerCitation23 and hepatocellular cancer.Citation11 Clearly, IL-9 has a complicated dual function depending on tumor type, tumor microenvironment, or other experimental conditions. For example, it was found that IL-9 was involved in proliferation, cell growth, and migration process by triggering JAK/STAT signaling of tumor cells.Citation24 However, multiple studies demonstrated that IL-9 also had anti-tumoral functions by provoking robust anti-tumor immunity.Citation25 Our study discovered that IL-9 enhanced the cytotoxicity by CD8+ T cells in gastric cancer, which gave possible explanations for the better prognosis and chemotherapy sensitivity of patients with high IL-9 expression. But indeed, multiple cell types like mast cells and dendritic cells might also be target cells by IL-9 in tumor microenvironment.Citation26,Citation27
Immune checkpoint inhibitors have made a clinically significant impact on the treatment of several different types of cancer, of which PD-1 inhibitors were the most successful immunotherapy drugs. However, PD-1 inhibition is not capable of reversing all immunosuppressive mechanisms, and a large proportion of patients do not respond adequately to anti-PD-1 immunotherapies.Citation28 Thus, synergistic combinations of checkpoint with other strategies have been proposed to further enhance the antitumor efficacy of anti-PD-1 individual treatments.Citation29 A previous study revealed that peripheral blood Th9 cells, which produce IL-9, play an important role in the successful treatment of melanoma patients with PD-1 blockade.Citation30 While in our study, we further discovered that rhIL-9 and Pembrolizumab had a synergistic role in reactivated intratumoral CD8+ T cells. These results provided the preclinical rational for combination rhIL-9 and PD-1 blockade to restore anti-tumor immune response.
Several mechanisms involved in IL-9-mediated tumor suppression has been discovered. Purwar R et al found that IL-9 could activate the antitumorigenic effect of innate immune cells like mast cells.Citation25 Moreover, IL-9 was shown to involve CCL20-mediated leukocyte recruitment into the tumor and the regulation of dendritic cell function in a CCR6-dependent manner.Citation13 Apart from the stimulation of the innate immune system, IL-9 could also activate adaptive immune cells. For example, IL-9 is responsible for the expansion and IFN-γ production of CD8+ T cells.Citation31 However, there are also studies demonstrated that IL-9 also had pro-tumoral functions in several hematopoietic malignancies as well as solid tumors like hepatocellular cancer and lung cancer. Most studies indicated that IL-9 could drive JAK/STAT3 signaling activity and thus trigger tumor cell proliferation and migration.Citation11,Citation24 All this evidence suggested that, as a pleiotropic cytokine, IL-9 is not limited to any one function and has been shown to induce various physiological responses in tumor.
A mainly limitation of our study was the lack of in vivo validation for our findings because no appropriate mouse-derived cells line for gastric cancer could be acquired for building immune-competent mice model. Furthermore, as the intervention experiments were based on bulks of cells dissociated from tumor tissues, it remains to be explored whether the effect of rhIL-9 on CD8+ T cells was direct or indirect.
In summary, we reported for the first time that intratumoral IL-9 expression could serve as a prognostic and predictive indicator in gastric cancer. Enhancing IL-9 signaling might be an effective therapeutic approach in gastric cancer, either alone or in combination with immune checkpoint inhibitors.
Authors’ contributions
H. Fang, R. Li and Y. Gu for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Fei, K. Jin, Y. Chen, Y. Cao, X. Liu, K. Lv, J. Wang, K. Yu, C. Lin, H. Liu, H. Li and H. He for technical and material support; W. Zhang, H. Zhang, and Z. Shen for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download ()Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–9. doi:10.1016/S0140-6736(16)30354-3.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New Engl J Med. 2007;357(18):1810–1820. doi:10.1056/NEJMoa072252.
- Cho JH, Lim JY, Cho JY, Katoh M. Comparison of capecitabine and oxaliplatin with S-1 as adjuvant chemotherapy in stage III gastric cancer after D2 gastrectomy. PLoS One. 2017;12(10):e0186362–e. doi:10.1371/journal.pone.0186362.
- Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267:504–513. doi:10.1097/SLA.0000000000002116.
- Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–750. doi:10.1007/s10120-014-0422-7.
- Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267(2):311–318. doi:10.1097/SLA.0000000000002058.
- Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi:10.4049/jimmunol.1003049.
- Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi:10.1038/nri2848.
- Rojas-Zuleta WG, Sanchez E. IL-9: function, sources, and detection. Methods Mol Biol (Clifton, NJ). 2017;1585:21–35.
- Tan H, Wang S, Zhao L. A tumour-promoting role of Th9 cells in hepatocellular carcinoma through CCL20 and STAT3 pathways. Clin Exp Pharmacol Physiol. 2017;44:213–221. doi:10.1111/1440-1681.12689.
- Hsieh T-H, Hsu C-Y, Tsai C-F, Chiu -C-C, Liang -S-S, Wang T-N, Kuo P-L, Long C-Y, Tsai E-M. A novel cell-penetrating peptide suppresses breast tumorigenesis by inhibiting β-catenin/LEF-1 signaling. Sci Rep. 2016;6:19156. doi:10.1038/srep19156.
- Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122(11):4160–4171. doi:10.1172/JCI65459.
- Shou ZX, Jin X, Zhao ZS. Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann Surg. 2012;256:1014–1022. doi:10.1097/SLA.0b013e3182592f56.
- Noh SH, Park SR, Yang H-K, Chung HC, Chung I-J, Kim S-W, Kim -H-H, Choi J-H, Kim H-K, Yu W, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi:10.1016/S1470-2045(14)70473-5.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531.
- Speiser DE, Ho P-C, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599. doi:10.1038/nri.2016.80.
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi:10.1038/nrc3245.
- Lin C, Liu H, Zhang H, Cao Y, Li R, Wu S, Li H, He H, Xu J, Sun Y, et al. Tryptase expression as a prognostic marker in patients with resected gastric cancer. BJS. 2017;104:1037–1044. doi:10.1002/bjs.10546.
- Wang JT, Li H, Zhang H, Chen YF, Cao YF, Li RC, Lin C, Wei YC, Xiang XN, Fang HJ, et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol. 2018;30:266–273. doi:10.1093/annonc/mdy505.
- Jiang Y, Xie J, Han Z, Liu W, Xi S, Huang L, Huang W, Lin T, Zhao L, Hu Y, et al. Immunomarker support vector machine classifier for prediction of gastric cancer survival and adjuvant chemotherapeutic benefit. Clin Cancer Res. 2018;24(22):5574. doi:10.1158/1078-0432.CCR-18-0848.
- Wang J, Sun M, Zhao H, Huang Y, Li D, Mao D, Zhang Z, Zhu X, Dong X, Zhao X, et al. IL-9 exerts antitumor effects in colon cancer and transforms the tumor microenvironment in vivo. Technol Cancer Res Treat. 2019;18:1533033819857737. doi:10.1177/1533033819857737.
- He J, Wang L, Zhang C, Shen W, Zhang Y, Liu T, Hu H, Xie X, Luo F. Interleukin-9 promotes tumorigenesis through augmenting angiogenesis in non-small cell lung cancer. Int Immunopharmacol. 2019;75:105766. doi:10.1016/j.intimp.2019.105766.
- Ye Z-J, Zhou Q, Yin W, Yuan M-L, Yang W-B, Xiong X-Z, Zhang J-C, Shi H-Z. Differentiation and immune regulation of IL-9−producing CD4 + T cells in malignant pleural effusion. Am J Respir Crit Care Med. 2012;186(11):1168–1179. doi:10.1164/rccm.201207-1307OC.
- Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–1253. doi:10.1038/nm.2856.
- Abdul-Wahid A, Cydzik M, Prodeus A, Alwash M, Stanojcic M, Thompson M, Huang EHB, Shively JE, Gray-Owen SD, Gariépy J, et al. Induction of antigen-specific TH9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int J Cancer. 2016;139:841–853. doi:10.1002/ijc.30121.
- Park J, Li H, Zhang M, Lu Y, Hong B, Zheng Y, He J, Yang J, Qian J, Yi Q, et al. Murine Th9 cells promote the survival of myeloid dendritic cells in cancer immunotherapy. Cancer Immunol Immunother. 2014;63(8):835–845. doi:10.1007/s00262-014-1557-4.
- Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–726. doi:10.1016/S1470-2045(16)00175-3.
- Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JLP, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457. doi:10.1038/nrc3973.
- Nonomura Y, Otsuka A, Nakashima C, Seidel JA, Kitoh A, Dainichi T, Nakajima S, Sawada Y, Matsushita S, Aoki M, et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology. 2016;5:e1248327–e. doi:10.1080/2162402X.2016.1248327.
- Wang C, Lu Y, Chen L, Gao T, Yang Q, Zhu C, Chen Y. Th9 cells are subjected to PD-1/PD-L1-mediated inhibition and are capable of promoting CD8 T cell expansion through IL-9R in colorectal cancer. Int Immunopharmacol. 2020;78:106019. doi:10.1016/j.intimp.2019.106019.
