ABSTRACT
Locally advanced upper urinary tract urothelial carcinoma (UTUC) exhibits high recurrence and metastasis rates even after radical nephroureterectomy. Adjuvant immunotherapy can be a reasonable option, and a simple, low-cost, and effective biomarker is further needed. Stromal tumor-infiltrating lymphocytes (sTILs) has been demonstrated as a prognostic and predictive biomarker in various tumor types, but not yet in locally advanced UTUC. In this multicenter, real-world and retrospective study, we tried to investigate the prognostic role of sTIL and its correlation with the PD-L1/PD-1/CD8 axis by reviewing the clinicopathologic variables of 398 locally advanced UTUC patients at four high-volume Chinese medical centers. sTIL density was evaluated with standardized methodology on H&E sections, and patients were stratified by the cutoff of sTIL (50%). Results showed that high sTIL indicated improved survival (CSS, p = .022; RFS, p = .015; DFS, p = .004), and was an independent predictor of better CSS (HR, 0.577; 95% CI, 0.391–0.851; p = .006), RFS (HR, 0.613; 95% CI 0.406–0.925; p = .020) and DFS (HR, 0.609; 95% CI, 0.447–0.829; p = .002). A strongly positive correlation between sTIL density and the expression level of PD-1/PD-L1/CD8 axis was observed. We also found that aristolochic acid (AA) exposure was associated with increased sTIL and elevated PD-L1 expression, indicating that AA-related UTUC might be a distinct subgroup with unique tumor microenvironment characteristics. Our results show that sTIL can be an easily acquired biomarker for prognostic stratification in locally advanced UTUC.
Introduction
Upper urinary tract urothelial carcinoma (UTUC) is a relatively rare disease, comprising only 5% to 10% of all urothelial carcinomas (UCs).Citation1 However, the disease has a relatively high incidence in China, which may be related to the aristolochic acid (AA) consumption.Citation2 For localized nonmetastatic UTUC, radical nephroureterectomy (RNU) with bladder cuff excision is the standard choice of treatment.Citation3 However, recurrence and progression are frequently reported in advanced stages. The 5‐year cancer‐specific survival rate of patients has reached 73% after RNU in overall populations, whereas those of patients with advanced-stage and/or lymph node metastasis have markedly decreased (pT3, 54%; pT4, 12%; nodal involvement, 35%).Citation4
For advanced UTUC, platinum-based chemotherapy might be the first-choice adjuvant treatment, but its application after RNU is limited by impaired renal function.Citation5 Current results on the application of adjuvant chemotherapy for UTUC remain controversial.Citation6-8 Neoadjuvant chemotherapy exhibits potential, providing improved survival outcomes and resulting in substantial tumor downstaging;Citation9-11 however, its clinical application is limited by the absence of definite preoperative pathologic information. Immunotherapy has recently emerged as a potential treatment for various malignancies, and PD-1/PD-L1 inhibitors have been approved as the first- or second-line treatment for UC by FDA.Citation12-15 However, the majority of cases remain unresponsive to these agents, and the adverse events and financial issues need to be considered.
Stromal tumor-infiltrating lymphocytes (sTILs), which can be assessed simply, practically, and at a low cost by using hematoxylin-eosin (H&E) staining, has been established as a unique biomarker with independent favorable prognostic and predictive values in various tumor types.Citation16 The presence of sTILs in UC has also drawn interest in recent decades. However, previous reports have mainly focused on the urothelial bladder cancer (UBC), and studies on locally advanced UTUC have rarely been reported. To elucidate the prognostic role of sTILs, and its relationship with the well-established biomarkers in tumor microenvironment in locally advanced UTUC, as well as to show the characteristic of sTIL in AA-related UTUC, we conducted this multicenter real-world study (TSU-02 study) in a large clinical cohort and presented the results of our exploratory analysis.
Materials and methods
Study design
The TSU study aims to evaluate the characteristics of the tumor microenvironment for patients with UTUC. The TSU-02 study mainly focuses on the prognostic and potential predictive roles of sTILs. We retrospectively reviewed charts from 1545 UTUC patients who underwent RNU from January 2006 to December 2017 in four high-volume Chinese medical centers, including Peking University First Hospital (PUFH), Sun Yat-sen Memorial Hospital (SYMH), Peking University Third Hospital (PUTH), and Fujian Provincial Hospital (FPH). Patients aged 18 years and older with a histologically confirmed diagnosis of locally advanced UTUC defined as ≥pT3 and/or pN+ were enrolled. Patients with distant metastasis at diagnosis and a history of other malignant tumors, as well as those received neoadjuvant chemotherapy, were excluded. The cases without available clinicopathological data, follow-up data or available H&E slides were also excluded. The establishment of clinical cohorts is illustrated in . A total of 398 patients were ultimately enrolled.
Figure 1. Study design and clinical cohorts. A total of 398 patients meeting the inclusion/exclusion criteria were included. Upper tract urothelial carcinoma = UTUC, Radical nephroureterectomy = RNU, Cancer-specific survival = CSS, Recurrence-free survival = RFS, Disease-free survival = DFS
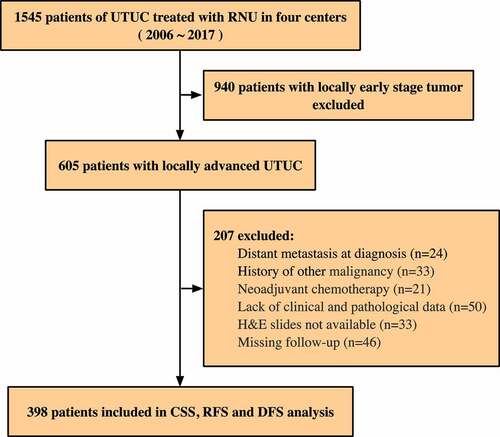
Data including age, gender, AA exposure history, and oncologic variables were retrospectively collected. Tumor staging and grading were reviewed by experienced pathologists blinded to clinicopathological data and patient outcomes, and then evaluated in accordance with the 7th AJCC classification and the 2004 WHO classification. The study was approved by an independent ethics committee in each participating institution, conducted in accordance with the Declaration of Helsinki, and registered in the Chinese clinical trial registry (ChiCTR2000030308). Informed consent was obtained from all individual participants.
Outcomes
The primary endpoint of this study was cancer-specific survival (CSS), and the secondary endpoints were recurrence-free survival (RFS) and disease-free survival (DFS). CSS was defined as the duration from the date of surgery to the last follow-up or death due to UTUC, RFS was defined as the duration from surgery to the first documented urothelial cancer relapse, and DFS was defined as the duration from surgery to any urothelial cancer relapse or death from any cause, whichever occurred first.
Histological assessment of sTIL
The H&E-stained slides were evaluated by two independent pathologists blinded to all clinicopathological and survival data in each institution, under the guidelines of the international TIL working group.Citation16,Citation17 The slides used for assessment were expected to present the most invasive part (i.e., the slides used in routine pathology to determine the T status). When facing more than one slide from the most invasive part, the section with the highest sTIL density determined the final assessment. Briefly, sTIL was evaluated within the borders of the invasive tumor, including both central tumor as well as invasive margin, and quantified as a percentage of tumoral stromal area occupied by mononuclear inflammatory cells. A full assessment of average sTILs across the whole slide (not hotspots) was conducted. The results were reported by increments of 10% initially: a score of 10% indicates a sTIL percentage between 0% and 10%; a score of 20% indicates a percentage between 10% and 20%; going on up through 100%. Then a binary classification was used to categorize them into low-sTIL (sTIL density ≤ 50%) and high-sTIL (sTIL density > 50%) (Figure S1).
In addition, we evaluated both the number of TILs infiltrated into the tumor cell nests and stromal TILs in 100 randomly selected cases, and found that the number of intratumoral TILs was positively correlated with the number of sTILs, but had generally lower density (Figure S2). The same viewpoint has been proved by other studies.Citation17,Citation18 Therefore, we mainly focused on stromal TILs in current study.
Immunohistochemical staining and evaluation
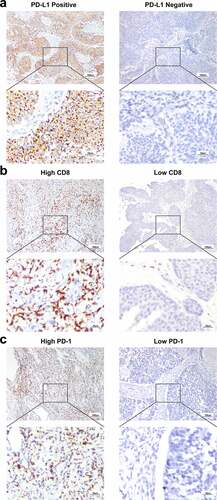
Immunohistochemical (IHC) staining was performed on 4 μm serial sections of formalin-fixed, paraffin-embedded tumor tissues in SYMH cohort as previously described,Citation19 using primary antibodies against PD-L1 (1:400, clone SP142, Spring Bioscience), PD-1 (1:100, clone EH33, Cell Signaling Technology), and CD8 (1:400, clone D8A8Y, Cell Signaling Technology). For the evaluation of IHC staining, all stained slides were scanned using a high-resolution digital slide scanner with up to ×400 magnification (Aperio Digital Pathology, Leica, Germany). PD-L1 expression was evaluated on tumor cells (TCs) and a 1% or greater PD-L1+ TC staining percentage was considered positiveCitation20,Citation21 (). The number of CD8+ lymphocytes and PD-1+ lymphocytes were counted manually, and averaged in 5 representative high-power fields (×400 magnification, 0.07 mm2 per field) (). Two independent pathologists blinded to clinicopathological data and patient outcomes conducted the evaluation.
Figure 2. Expression of PD-L1/PD-1/CD8 in locally advanced UTUC. (a) Representative images of immunohistochemical detection of PD-L1 (brown) in tumor cells (TCs). (b, c) Representative images of immunohistochemical detection of CD8+ lymphocytes and PD-1+ lymphocytes (brown). (scale bar, 100 μm for upper rows, 25 μm for lower rows). Programed death-1 = PD-1, Programed death-ligand 1 = PD-L1
Statistical analysis
The SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. The Kaplan-Meier survival analysis was used to assess the correlations between sTIL categories with CSS, RFS, and DFS by using the Log-rank test. Univariate and multivariate Cox regression analysis were performed to evaluate the prognostic significance of each variable. We analyzed correlations between sTIL with pathological parameters by using Pearson’s chi-squared test and Pearson’s correlation test. To minimize the effect of inclusion bias, patients with AA exposure and non/possible AA exposure were propensity score-matched (PSM) at a 1:1 ratio, based on age, gender, multifocality, pathologic T stage, pathologic N stage and tumor grade. The two cohorts were matched using a caliper width of 0.2 of the standard deviation. For all analyses, the two-sided p < .05 was considered to indicate statistical significance.
Results
After the exclusion criteria were applied, 398 patients with locally advanced UTUC were included for final analysis. The patients consisted of 215 males and 183 females with a mean age of 65.5 years (range 20–92 years). Overall, 236 patients (59.3%) had low sTIL(≤50%) and 162 patients (40.7%) had high sTIL(>50%). The detailed clinicopathological parameters are listed in .
Table 1. Clinical and pathological characteristics of 398 patients
Association of sTILs with patient survival in UTUC
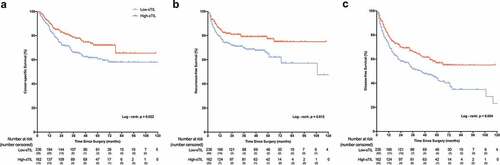
During a median follow-up of 55 months (interquartile range 32–71 months), 143 patients (35.9%) died and 106 patients (26.7%) exhibited disease recurrence. Kaplan-Meier analysis demonstrated that patients with high sTIL had significantly better cancer-specific survival than that of patients with low sTIL (p = .022) (). At the end of the 1-, 3- and 5-year follow-ups, the CSS rates were 90.4%, 78.7%, and 72.0%, respectively, in the high-sTIL group and 85.1%, 65.9% and 60.6%, respectively, in the low- sTIL group. A significantly better survival outcomes in RFS (p = .015) and DFS (p = .004) for patients with high-sTIL were also observed ().
Figure 3. Kaplan-Meier curves on patient survival by sTIL density. sTIL can predict (a) cancer-specific survival (p= .022), (b) recurrence-free survival (p= .015), and (c) disease-free survival (p = .004). P values were calculated by the log-rank test. Vertical tick marks represent censored subjects. Stromal tumor-infiltrating lymphocyte = sTIL
Univariate analysis identified that sTIL (low or high), tumor size (>3 or ≤3 cm), T stage (1–2 or 3–4), and tumor grade (G1-2 or G3) were significantly associated with CSS. After adjusting for clinicopathological variables, sTIL (HR, 0.577; 95% CI, 0.391–0.851; p = .006), tumor size (HR, 1.521; 95% CI, 1.025–2.256; p = .037), T stage (HR, 9.458; 95% CI, 1.283–69.717; p = .027), and tumor grade (HR, 2.393; 95% CI 1.434–3.995; p = .001) were independent predictors of CSS in multivariate analysis ().
Table 2. Univariate and multivariate Cox regression analysis of factors associated with cancer-specific survival
Univariate analysis and multivariate analysis confirmed that sTIL (HR, 0.613; 95% CI, 0.406–0.925; p = .020) and T stage (HR, 3.255; 95% CI, 1.320–8.028; p = .010) were independent predictors of RFS (). In addition, sTIL, tumor size, T stage and tumor grade were significantly associated with DFS in univariate analysis. Multivariate analysis further proved the independent prognostic roles for DFS of sTIL (HR, 0.609; 95% CI, 0.447–0.829; p = .002), T stage (HR, 3.126; 95% CI, 1.406–6.951; p = .005) and tumor grade (HR, 1.543; 95% CI, 1.078–2.211; p = .018) ().
Table 3. Univariate and multivariate Cox regression analysis of factors associated with recurrence-free survival
Table 4. Univariate and multivariate Cox regression analysis of factors associated with disease-free survival
Correlations between sTIL and PD-L1/PD-1/CD8 axis
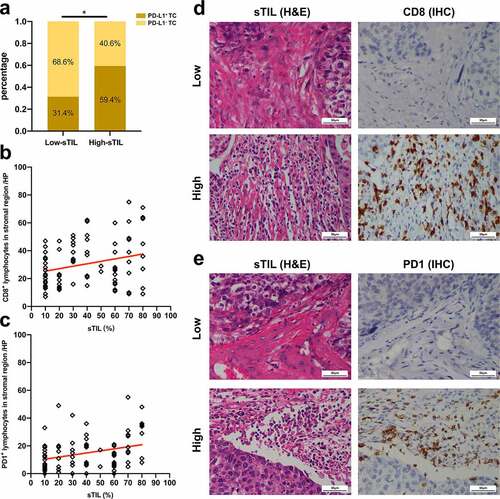
A significant correlation was determined between the PD-L1+ TC and sTIL, with high-sTIL showing more intense PD-L1+ TCs than low-sTIL (p = .012) (). Moreover, we found that sTIL density was positively correlated with the number of CD8+ lymphocytes (p = .017, Pearson’s correlation test) () and PD-1+ lymphocytes (p = .005, Pearson’s correlation test) ().
Figure 4. Correlations between sTIL and PD-L1/PD-1/CD8 axis. (a) Correlation between sTIL and PD-L1+ TCs. (Pearson’s chi-squared test, p= .012). (b) Correlation between sTIL and CD8+ lymphocytes density. (Pearson’s correlation test, p = .017, r = 0.2604). (c) Correlation between sTIL and PD-1+ lymphocytes density. (Pearson’s correlation test, p = .005, r = 0.3039). (d) Representative images of CD8+ lymphocytes in low- or high-sTIL cases (scale bar, 50 μm). (e) Representative images of PD-1+ lymphocytes in low- or high- sTIL cases (scale bar, 50 μm). Programed death-1 = PD-1, Programed death-ligand 1 = PD-L1, Tumor cell = TC, Stromal tumor-infiltrating lymphocyte = sTIL, Immunohistochemistry = IHC, High-power field = HP
Correlations between sTIL and AA-related UTUC
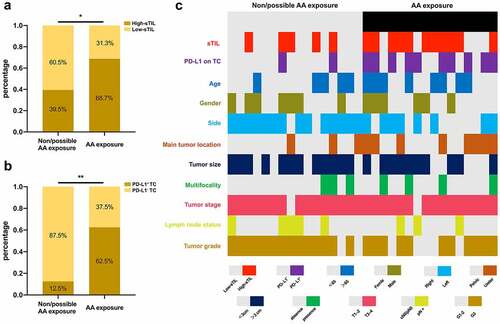
Among the patients, 16 were found to have a history of confirmed AA exposure. We found that patients with AA exposure were more likely to have high sTIL, compared with patients without AA exposure (p = .020, χ2 test) (). We further compared PD-L1 expression in 32 samples after PSM at a 1:1 ratio. Patients with AA exposure showed significantly higher PD-L1 expression in TCs (p = .003, χ2 test) (). The correlation between AA exposure and sTIL was further verified after PSM (p = .034, χ2 test). The distributions of sTIL, PD-L1 expression, and clinicopathological parameters of these 32 patients are presented in .
Figure 5. Correlations between sTIL and AA-related UTUC. (a) Correlation between AA exposure and sTIL density (Pearson’s chi-squared test, p= .020). (b) Correlation between AA exposure and PD-L1 expression in TCs after PSM. (Pearson’s chi-squared test, p= .003). (c) Heat map of clinicopathologic factors in patients with or without AA exposure after PSM. Aristolochic acid = AA, Programed death-ligand 1 = PD-L1, Tumor cell = TC, Stromal tumor-infiltrating lymphocyte = sTIL, Propensity score matching = PSM
Discussion
UTUC, particularly the advanced type, is an aggressive disease with high recurrence and progression. However, current prognostic factors such as TNM stage, tumor grade, and molecular biomarkers have their own limitations.Citation3 To evaluate whether the sTIL could potentially be used as a biomarker for clinical applications in advanced UTUC, we conducted a multicenter real-world study encompassing a large cohort of 398 RNU-treated locally advanced UTUC with a long-term median follow-up. We found that sTIL was positively correlated with higher survival and had strong correlation with several well-established immunotherapy biomarkers. We also found that AA-related UTUC exhibited distinct features with enhanced sTIL and PD-L1 expression, indicating its distinct tumor microenvironment characteristics. To the best of our knowledge, this study is the first to focus on the prognostic role of sTIL and its relationship with the immunotherapy biomarkers in locally advanced UTUC.
The presence of increased tumor-infiltrating lymphocytes (TILs) has been established as a prognostic factor in various tumors, including melanoma, breast cancer, gastrointestinal tract carcinoma, ovarian carcinoma and urothelial cancer.Citation16,Citation18,Citation22-24 Previous studies on TILs in UC mainly focused on UBC, and various studies presented different results. Some studies reported that high TILs correlated with improved outcomes,Citation25,Citation26 others considered TILs predicted a poor survival,Citation19,Citation27 and even some studies found that stromal TIL was not a prognostic indicator.Citation28 Similarly, the role of TILs in UTUC remains inconclusive. WangCitation29 reported that high CD4+ T cells and CD8+ T cells could predict improved survival. Conversely, NukuiCitation30 found that low stromal TIL combined with low PD-L1 expression by TC predicted increased survival. The variations in the results might be attributable to the distinct characteristics of the included populations, the relatively small size of the study samples, heterogeneity of urothelial carcinoma, and the complexity of the underlying immune regulatory pathway. In the current study, we used a large study sample from multi-centers to provide a higher level of evidence. The present study indicated that sTIL was an independent prognostic factor and high sTIL predicted improved outcomes in locally advanced UTUC. Adding this easily acquired and robust indicator into the clinical practice may make beneficial supplement to the current pathological system for UTUC.
The results of both PD-L1 and PD-1 inhibitor immunotherapy were encouraging in locally advanced or metastatic urothelial cancer, and higher response rate was observed in UTUC compared with UBC (39% vs.17%).Citation12,Citation13 However, the response rates were still far from satisfactory, and the majority of patients did not respond to checkpoint inhibitors. Previous reports indicated higher objective response rates in PD-L1 high urothelial tumors,Citation13-15 leading to the approval of PD-L1 expression by FDA as a biomarker for frontline drug use. However, in the cases of insufficient TILs, PD-1/PD-L1 blockade is not likely to cause specific T-cell proliferation and increased effector function, even with high PD-L1 expression.Citation31 Several studies demonstrated the predictive value of CD8+ T cells,Citation14,Citation32,Citation33 as well as the TIME (tumor immunity in the microenvironment) classification, which can be a valuable indicator to guide immunotherapy on the basis of PD-L1 expression and the TILs density.Citation34,Citation35 The current study found that sTILs were strongly correlated with PD-L1 expression in TCs, as well as with CD8+ lymphocyte density and PD-l+ lymphocyte density. Due to the strong correlation between sTIL and PD-L1/PD-1/CD8 axis, we speculate that sTIL might become a potential biomarker to predict clinical response to immunotherapy in the future, although comparative analysis between the response and non-response cohorts must be performed before a conclusion can be obtained.
Exposure to AA is widespread in East Asia and has led to the relatively high prevalence of UTUC.Citation2 Distinct from non-AA UTUC, AA-related UTUC exhibits unique clinicopathologic features, such as prevalence in females, multifocality, and lower T stage, as demonstrated in both the present study and our previous reports.Citation36,Citation37 AA-related UTUC with unique underlying pathogenesis may have distinct profiles of prognosis and therapeutic strategy. As previously reported, increased gene mutational burden and neoantigen load were observed in patients with AA exposure,Citation38,Citation39 which can be recognized by T cells and generate antibodies. AA UTUC may then be hypothesized as a good candidate for immune checkpoint blockade therapy. In the current study, patients with AA exposure were more likely to have increased sTIL and higher PD-L1+ expression. Patients with AA-related locally advanced UTUC might be a distinct population with potentially increased sensitivity to immunotherapy. In addition, the renal function of patients with AA-related UTUC was found to be poorer than that of patients with other types of UTUC and was less likely to receive chemotherapy,Citation36 rendering immunotherapy a reasonable option. We suggest the use of AA-related factors in future trials studying immune checkpoint inhibitors for UTUC to further explore its distinct treatment strategy.
This study has several limitations. First, this is a retrospective study, and selection bias may exist. Second, although we conducted a multicenter cohort, the entire population is confined to the Chinese population, disregarding the effect of racial differences on the outcomes for patients with UTUC.Citation40 In addition, this finding does not include more advanced ways to characterize UTUC, such as gene expression signatures and mutation-based subtyping, which might result in more refined subtypes with different immunobiology.
In summary, we evaluated the role of sTIL in a quite large clinical cohort for this rare and aggressive disease, locally advanced UTUC. We found that high sTIL is independently correlated with improved survival and there are strong correlations between sTIL and the PD-L1/PD-1/CD8 axis, suggesting that it can be an easily acquired biomarker for prognostic stratification and might have potential role in immunotherapy response prediction. Furthermore, we presume that AA-related UTUC patients with distinct tumor microenvironment characteristics might be good candidates for immunotherapy while further tries are needed. Our findings may make good supplements to the current pathological evaluation system and helpdistinguish the optimal treatment strategy for locally advanced UTUC.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Supplemental Material
Download ()Acknowledgments
We thank the entire staff of the Urology Departments and Pathology Department in these four medical centers.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–8. doi:10.3322/caac.21551.
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342(23):1686–1692. doi:10.1056/NEJM200006083422301.
- Roupret M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P, Hugh Mostafid A, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2020. doi:10.1016/j.eururo.2020.05.042.
- Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115(6):1224–1233. doi:10.1002/cncr.24135.
- Kaag MG, O’Malley RL, O’Malley P, Godoy G, Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–587. doi:10.1016/j.eururo.2010.06.029.
- Seisen T, Krasnow RE, Bellmunt J, Rouprêt M, Leow JJ, Lipsitz SR, Vetterlein MW, Preston MA, Hanna N, Kibel AS, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35(8):852–860. doi:10.1200/JCO.2016.69.4141.
- Kim TS, Oh JH, Rhew HY. The efficacy of adjuvant chemotherapy for locally advanced upper tract urothelial cell carcinoma. J Cancer. 2013;4:686–690. doi:10.7150/jca.7326.
- Necchi A, Lo Vullo S, Mariani L, Moschini M, Hendricksen K, Rink M, Sosnowski R, Dobruch J, Raman JD, Wood CG, Margulis V. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. 2018;121:252–259.
- Porten S, Siefker-Radtke AO, Xiao L, Margulis V, Kamat AM, Wood CG, Jonasch E, Dinney CPN, Matin SF. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120(12):1794–1799. doi:10.1002/cncr.28655.
- Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, Dinney CPN, Millikan R, Siefker-Radtke AO. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116(13):3127–3134. doi:10.1002/cncr.25050.
- Kim DK, Lee JY, Kim JW, Hah YS, Cho KS. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2019;135:59–65. doi:10.1016/j.critrevonc.2019.01.019.
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi:10.1016/S0140-6736(16)32455-2.
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi:10.1016/S1470-2045(17)30616-2.
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi:10.1016/S0140-6736(16)00561-4.
- Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–3125. doi:10.1200/JCO.2016.67.9761.
- Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, Van De Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–335.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi:10.1093/annonc/mdu450.
- Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/S1470-2045(17)30904-X.
- Wang B, Pan W, Yang M, Yang W, He W, Chen X, Bi J, Jiang N, Huang J, Lin T, et al. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019;110(2):489–498. doi:10.1111/cas.13887.
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm M-O, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi:10.1016/S1470-2045(17)30065-7.
- Takada K, Toyokawa G, Okamoto T, Shimokawa M, Kozuma Y, Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, et al. A comprehensive analysis of programmed cell death ligand-1 expression with the clone SP142 antibody in non-small-cell lung cancer patients. Clin Lung Cancer. 2017;18:572–82 e1. doi:10.1016/j.cllc.2017.02.004.
- Fuchs TL, Sioson L, Sheen A, Jafari-Nejad K, Renaud CJ, Andrici J, Ahadi M, Chou A, Gill AJ. Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) system is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients. Am J Surg Pathol. 2020;44(4):536–544. doi:10.1097/PAS.0000000000001409.
- Fu Q, Chen N, Ge C, Li R, Li Z, Zeng B, Li C, Wang Y, Xue Y, Song X, et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019;8:1593806.
- Wouters MC, Komdeur FL, Workel HH, Klip HG, Plat A, Kooi NM, Wisman GBA, Mourits MJE, Arts HJG, Oonk MHM. Treatment regimen, surgical outcome, and T-cell differentiation influence prognostic benefit of tumor-infiltrating lymphocytes in high-grade serous ovarian cancer. Clin Cancer Res. 2016;22(3):714–724. doi:10.1158/1078-0432.CCR-15-1617.
- Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972.
- Otto W, Denzinger S, Wieland WF, Hartmann A. First analysis of immune cell infiltration in stage pT1 urothelial bladder carcinoma: CD3 positivity as a prognostic marker for cancer-specific survival. World J Urol. 2012;30(6):875–877. doi:10.1007/s00345-012-0974-2.
- Krpina K, Babarovic E, Jonjic N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015;467:443–448. doi:10.1007/s00428-015-1808-6.
- Rouanne M, Betari R, Radulescu C, Goubar A, Signolle N, Neuzillet Y, Allory Y, Marabelle A, Adam J, Lebret T. Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur J Cancer. 2019;108:111–119. doi:10.1016/j.ejca.2018.12.010.
- Wang L-A, Yang B, Rao W, Xiao H, Wang D, Jiang J. The correlation of BER protein, IRF3 with CD8+ T cell and their prognostic significance in upper tract urothelial carcinoma. Onco Targets Ther. 2019;12:7725–7735. doi:10.2147/OTT.S222422.
- Nukui A, Kamai T, Arai K, Kijima T, Kobayashi M, Narimatsu T, Kambara T, Yuki H, Betsunoh H, Abe H, et al. Association of cancer progression with elevated expression of programmed cell death protein 1 ligand 1 by upper tract urothelial carcinoma and increased tumor-infiltrating lymphocyte density. Cancer Immunol Immunother. 2020;69:689–702.
- Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210–219. doi:10.1016/j.intimp.2017.03.015.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature13954.
- Fumet JD, Richard C, Ledys F, Klopfenstein Q, Joubert P, Routy B, Truntzer C, Gagné A, Hamel M-A, Guimaraes CF, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119:950–960. doi:10.1038/s41416-018-0220-9.
- Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi:10.1158/0008-5472.CAN-15-0255.
- Hamada T, Soong TR, Masugi Y, Kosumi K, Nowak JA, da Silva A, Mu XJ, Twombly TS, Koh H, Yang J, et al. TIME (Tumor Immunity in the MicroEnvironment) classification based on tumor CD274 (PD-L1) expression status and tumor-infiltrating lymphocytes in colorectal carcinomas. Oncoimmunology. 2018;7:e1442999.
- Zhong W, Zhang L, Ma J, Shao S, Lin R, Li X, Xiong G, Fang D, Zhou L. Impact of aristolochic acid exposure on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Onco Targets Ther. 2017;10:5775–5782. doi:10.2147/OTT.S148641.
- Xiong G, Yao L, Hong P, Yang L, Ci W, Liu L, He Q, Gong K, Li X, Zhou L. Aristolochic acid containing herbs induce gender-related oncological differences in upper tract urothelial carcinoma patients. Cancer Manag Res. 2018;10:6627–6639. doi:10.2147/CMAR.S178554.
- Lu H, Liang Y, Guan B, Shi Y, Gong Y, Li J, Kong W, Liu J, Fang D, Liu L, et al. Aristolochic acid mutational signature defines the low-risk subtype in upper tract urothelial carcinoma. Theranostics. 2020;10(10):4323–4333. doi:10.7150/thno.43251.
- Hoang ML, Chen CH, Sidorenko VS, He J, Dickman KG, Yun BH, Moriya M, Niknafs N, Douville C, Karchin R, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci Transl Med. 2013;5:197ra02.
- Matsumoto K, Novara G, Gupta A, Margulis V, Walton TJ, Roscigno M, Ng C, Kikuchi E, Zigeuner R, Kassouf W, et al. Racial differences in the outcome of patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int. 2011;108:E304–9. doi:10.1111/j.1464-410X.2011.10188.x.
