ABSTRACT
Drug-induced ferroptosis, an iron-dependent regulatory necrosis, has been proposed for the therapy of pancreatic ductal adenocarcinoma. However, genetically engineered mouse models have revealed that high-iron diets or deletion of pancreatic GPX4 (a key repressor of ferroptosis) accelerate the development of mutant Kras-driven PDAC by activating the STING1/TMEM173-dependent DNA sensor pathway.
Abbreviations ADM: acinar-to-ductal metaplasia; CGAS: cyclic GMP-AMP synthase; DAMP: damage-associated molecular pattern; GPX4: glutathione peroxidase 4; GEMM: genetically engineered mouse models; PDAC: pancreatic ductal adenocarcinoma; PanIN: pancreatic intraepithelial neoplasia, SLC7A11: solute carrier family 7 member 11; STING1: cGAMP-stimulator of interferon response cGAMP interactor 1; TME: tumor microenvironment; 8-OHG: 8-hydroxy-2ʹ-deoxyguanosine
Ferroptosis is an iron-dependent necrotic-like process in which cells use excessive lipid peroxidation signals to trigger plasma membrane damage and release of intracellular contents.Citation1 The induction of ferroptosis can be divided into two categories: biological versus chemical. In particular, chemical inhibition of the extrinsic cystine/glutamate antiporter system xc− or the intrinsic glutathione peroxidase 4 (GPX4) is the classical method to trigger ferroptosis. In recent years, this type of regulated cell death has attracted great attention in oncology, because the process can suppress the growth of many types of tumors and improve the efficacy of chemotherapy, radiotherapy, or immunotherapy. For example, selective and conditional depletion of pancreatic solute carrier family 7 member 11 (Slc7a11, a structural component of system xc−) inhibits pancreatic tumorigenesis in mice.Citation2 However, there is also accumulating evidence that abnormal ferroptotic response may play oncogenic roles in tumor progression by reprogramming of the tumor microenvironment (TME).Citation3 Our recent preclinical animal studies (using pancreas-specific Gpx4 knockout mice) and clinical retrospective analyses document that ferroptotic damage promotes pancreatic tumorigenesisCitation4 (), raising new concerns about the harmful impact of ferroptosis in tumor biology.
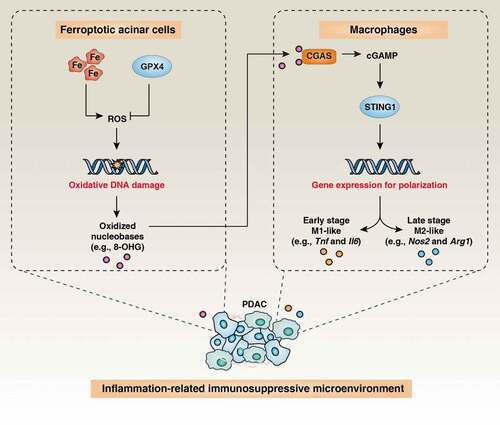
Figure 1. Ferroptotic damage promotes Kras-driven pancreatic tumorigenesis by macrophage polarization. The induction of ferroptotic damage by high-iron diets or Gpx4 depletion in pancreatic acinar cells promotes the release of nuclear DNA containing 8-hydroxy-2ʹ-deoxyguanosine (8-OHG) into the cytosol and thus activates the STING1-dependent DNA sensor pathway, resulting in macrophage infiltration and polarization during Kras-driven PDAC in mice
Ferroptosis mediates experimental pancreatitis
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal malignant tumors, mainly driven by integrated signals involved in gene mutations (e.g., universal Kras mutation) and the inflammatory microenvironment. While the cell of origin of PDAC has been controversial, acinar-to-ductal metaplasia (ADM) of the pancreas is an early initiation event of pancreatic tumorigenesis. Pancreatitis is a sterile inflammation of the pancreas caused by the death of acinar cells, which account for about 99% of all secretory cells in the pancreas. Epidemiological studies have found that both acute and chronic pancreatitis is associated with an increased risk of developing PDAC. Mouse studies further confirm that experimental pancreatitis induced by cerulein (an analogue of cholecystokinin) or high-fat diets accelerates mutant Kras-induced formation of ADM, pancreatic intraepithelial neoplasia (PanIN), as well as stromal responses in the pancreas. Our animal study demonstrated that high-iron diets or the conditional knockout of Gpx4 in the pancreas (genotype: Pdx1-Cre;Gpx4−/-) promoted experimental pancreatitis in mice induced by the administration of cerulein or L-arginine (a conditionally essential amino acid).Citation4 In contrast, liproxstatin-1 (a ferroptosis inhibitor) reversed this type of pancreatic inflammatory damage,Citation4 suggesting a pathogenic role for ferroptosis in experimental pancreatitis. Since trypsin activity is considered to be the main trigger mechanism of acinar cell death, it appears interesting to determine whether the serine protease trypsin is a direct effector of ferroptosis.
Ferroptosis facilitates Kras-driven pancreatic tumorigenesis
In the past decade, a variety of genetically engineered mouse models (GEMMs) of PDAC have been developed. Such models incorporate KRAS mutations and other changes in tumor suppressor genes (e.g., mutation of tumor protein p53 [Tp53] or deletion of cyclin-dependent kinase inhibitor 2A [Cdkn2a]). These models have different characteristics, and none of them perfectly mimics the clinical pathology of PDAC. Among these GEMMs, two basic models, including the Pdx1-Cre;KrasG12D/+ mice (termed KC) and Pdx1-Cre;KrasG12D/+;Tp53R172H/+ mice (termed KPC), are widely used to study the signals, mechanisms, and therapeutic modulation of PDAC. Compared with KC, KPC shows faster histopathological progress, especially poor vascularity, fibrosis, local invasion, and metastatic dissemination. We observed that, in KC mice with additional pancreatic Gpx4 depletion (genotype: Pdx1-Cre;KrasG12D/+;Gpx4−/-) or a high-iron diet, injections of the ferroptosis inhibitor lipoxstatin-1 protected against Kras-driven animal death as well as pancreatic pathology (e.g., PanIN) and molecular changes.Citation4 In contrast, depletion of pancreatic Slc7a11 in KPC mice (that in contrast to KC mice also lack mutant TP53) yields a different phenotype, suggesting that induction of ferroptosis limits mutant Kras/Tp53-induced pancreatic tumorigenesis.Citation2 Regardless of the non-ferroptosis regulating function of SLC7A11 (e.g., in amino acid metabolism), these GEMM studies indicate that Tp53 may switch the oncogene-like function of ferroptotic damage (observed in KC mice) to a tumor-suppressive function (observed in KPC mice). Indeed, TP53 plays a dual role in ferroptosis, depending on both transcriptional and non-transcriptional functions of TP53.
Ferroptotic damage reprograms macrophages for pancreatic tumorigenesis
PDAC has a unique TME, which forms a dynamic network of mutual supports between cancer and non-cancer cells, resulting in immune escape. Macrophages are an essential part of the pancreatic TME and can switch from an M1-like to an M2-like phenotype to sustain the growth of pancreatic tumors. Consistently, we observed the increased polarization of tumor-associated macrophages (TAMs) in KC mice with Gpx4 depletion or a high-iron diet.Citation4 These ferroptotic PDAC mice had higher mRNA expression of markers of M1-like macrophages (e.g., tumor necrosis factor [Tnf] and interleukin 6 [Il6]) at 3 months and of M2-like macrophages (e.g., nitric oxide synthase 2 [Nos2/iNos] and arginase-1 [Arg1]) at 6 months. This ferroptotic macrophage M1→M2 polarization was reversed by the ferroptosis inhibitor lipoxstatin-1.Citation4 Importantly, the depletion of TAMs using clodronate liposomes blocked the ferroptotic damage-accelerated pancreatic tumorigenesis in KC mice.Citation4 Further mechanistic studies have shown that the release of 8-hydroxy-2ʹ-deoxyguanosine (8-OHG) produced by ferroptosis-associated oxidative DNA damage promoted Kras-driven pancreatic tumorigenesis by activating the cyclic GMP-AMP synthase (CGAS)-cGAMP-stimulator of interferon response cGAMP interactor 1 (STING1/STING/TMEM1173) pathway in macrophages.Citation4 Consequently, administration of anti-8-OHG antibodies or the depletion of Sting1 prevented pancreatic tumorigenesis accelerated by Gpx4 depletion or high-iron diet-induced.Citation4 Furthermore, bioinformatics analyses of the cancer genome atlas (TCGA) analysis correlated the mortality of PDAC patients with low mRNA expression of GPX4 combined with high mRNA expression of STING1 in the tumors.Citation4 These findings establish a direct role for the chronic activation of the cytosolic DNA sensor pathway in driving ferroptosis-related PDAC.
Conclusion and outlook
Our mouse studies indicate that ferroptotic signaling drives macrophage-induced adaptive immune suppression in Kras-induced PDAC (). We provide the first evidence that 8-OHG functions as a damage-associated molecular pattern (DAMP) during ferroptotic cell death to trigger STING1-dependent macrophage polarization, supporting pancreatic cancer initiation and progression. Combined with previous studies using Slc7a11−/- mice,Citation2 our Gpx4−/- model argues for an ambiguous implication of ferroptosis in PDAC. These contradictory ferroptotic phenotypes may reflect the fact that cell death-related inflammatory responses act as a double-edged sword in tumor immunity. In addition to causing inflammation-related immunosuppression, Citation3 several ferroptosis agents (e.g., RSL3) can provoke immunogenic cell death to improve cytotoxic T cell responses against tumors.Citation5 Similar, in addition to the chronic activation of the STING1 pathway that mediates genomic instability-induced tumorigenesis and metastasis, Citation6 the robust activation of the STING1 pathway by reagents (e.g., MSA-2, DMXAA, ADU-S100, and zalcitabine) or radiation therapy is an approach to enhance antitumor immunity or direct killing cancer cells in mouse models or clinical trials.Citation7–9 Acute activation of STING1-mediated T cell apoptosis may weaken anti-tumor immunity,Citation10 further arguing the dual role of STING1 in tumor therapy. Thus, it will be important to identify key DAMP mediators and to decipher the molecular mechanisms that explain reprogramming from immune activation to tolerance during tumor progression. Of note, ferroptosis occurring in different cells of TME may also be relevant in shaping tumor immunity and its failure. Thus, the lack of Gpx4 in T or B cells leads to ferroptosis and impaired immune function in mice. It may be important to develop genetic and pharmacological approaches to induced or inhibit cell death pathways including ferroptosis in specific cell types rather than in all cells present in the TME to gain a clear picture and to progress toward therapeutic interventions.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
References
- Tang D, Kroemer G. Ferroptosis. Curr Biol. 2020;30:R1292–3. doi:10.1016/j.cub.2020.09.068.
- Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi:10.1126/science.aaw9872.
- Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi:10.1080/15548627.2020.1714209.
- Dai E, Han L, Liu J, Xie Y, Zeh H, Kang R, Bai L, Tang D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. 2020;11:1–11. doi:10.1038/s41467-020-20154-8.
- Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8: e001369. doi:10.1136/jitc–2020–001369
- Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–472. doi:10.1038/nature25432.
- Le Naour J, Zitvogel L, Galluzzi L, Vacchelli E, Kroemer G. Trial watch: STING agonists in cancer therapy. Oncoimmunology. 2020;9:1777624. doi:10.1080/2162402X.2020.1777624.
- Yamazaki T, Kirchmair A, Sato A, Buque A, Rybstein M, Petroni G, Bloy N, Finotello F, Stafford L, Navarro Manzano E, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21:1160–1171. doi:10.1038/s41590-020-0751-0.
- Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2020:1–13. doi:10.1080/15548627.2020.1739447.
- Long J, Yang C, Zheng Y, Loughran P, Guang F, Li Y, Liao H, Scott MJ, Tang D, Billiar TR, et al. Notch signaling protects CD4 T cells from STING-mediated apoptosis during acute systemic inflammation. Sci Adv. 2020;6:eabc5447. doi:10.1126/sciadv.abc5447
