ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is an immune resistant tumor. We recently demonstrated that inhibiting CDK1/2/5 by dinaciclib not only blocks immune checkpoint expression, but also triggers histone-dependent immunogenic cell death. This dual mechanism turns immunologically “cold” tumor microenvironment into a “hot” one, improves overall survival rates in mouse PDAC models.
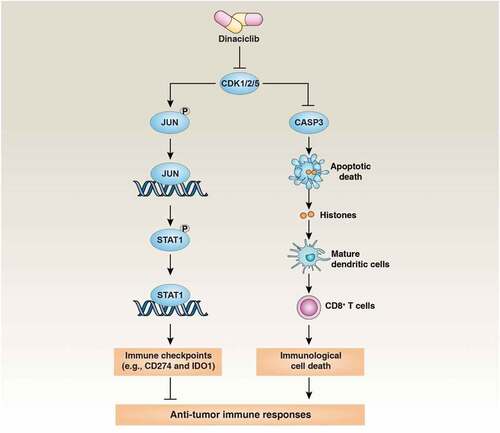
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive gastrointestinal cancer with an overall five-year survival rate of 9% in 2020. Globally, it is the seventh leading cause of cancer-related deaths. Despite the increasing understanding of its genetic background and pathophysiology, PDAC is still ineffective against most currently available treatments, including immune checkpoint inhibitors, which has improved the survival rate of many other cancers.Citation1 Although the underlying mechanism may be complex, the main reason challenging current immunotherapy is that PDAC has an immunosuppressive microenvironment and low immunogenicity.Citation2 In particular, the pancreatic tumor microenvironment shows increased stromal response and infiltration of M2 type anti-inflammatory macrophages, while total T-cell infiltration is reduced, which is the characteristic of the so-called “cold tumors”.Citation3 Accordingly, in recent years, a lot of efforts have focused on how to convert a “cold” into a “hot” tumor microenvironment using different ways.Citation2 Our recent drug screening and preclinical animal studies have demonstrated that the cyclin-dependent kinase (CDK) inhibitor dinaciclib not only inhibits the expression of immune checkpoints, but also induces immunogenic cell death (ICD), thereby exerting a powerful anti-PDAC effect ().Citation4
Inhibiting JUN-dependent immune checkpoint expression
Immune checkpoints are inhibitory modulators of the immune system and are essential for maintaining self-tolerance, preventing autoimmunity, and controlling the duration and extent of immune response to minimize collateral tissue damage. They are also expressed by certain types of immune system cells (e.g., T-cells and macrophages) and cancer cells in the tumor microenvironment, resulting in impaired anti-tumor immunity. Most immune checkpoints are membrane receptors or ligands (e.g., programmed cell death 1 [PDCD1/PD-1] and programmed death ligand 1 [CD274/PD-L1]), and some are cytoplasmic proteins or enzymes (e.g., indoleamine 2,3-dioxygenase 1 [IDO1]). Different tumor cells can express different immune checkpoints to prevent tumor killing mediated by cytotoxic T-cells. In particular, the combined use of IDO1 inhibitors produces promising responses in patients with metastatic PDAC (NCT02077881). Although the mechanism may change significantly on different cells, interferon gamma (IFNG/IFNγ)-mediated activation of the transcription factor signal transducer and activator of transcription 1 (STAT1) plays a central role in the up-regulation of immune checkpoint expression, thereby driving adaptive immune resistance.Citation5
To identify new inhibitors to block IFNG-induced immune checkpoint expression, we screened a protein kinase inhibitor library containing 429 small-molecule compounds in a human PDAC cell line CFPAC1 (harboring hotspot KRAS, TP53, and SMAD4 mutations) by western blot technology .Citation4 After three dose-dependent (10 μM, 1 μM, and 100 nM) screenings, we determined that dinaciclib ranked the strongest inhibitor to completely block IFNG-induced IDO1 and CD274 protein expression in CFPAC1 cells. Dinaciclib used at 100 nM also limited IFNG-induced the mRNA or protein expression of IDO1 and CD274 in 24 human and mouse cancer cell lines as well as primary cancer cells from patients with PDAC or ovarian cancer. Subsequent RNAi experiments confirmed that the accompanying CDK1/2/5 depletion mimics the effect of dinaciclib on blocking the upregulation of IDO1 and CD274 induced by IFNG. Moreover, combined with RNAi and site mutation technology, we proved that CDK1/2/5-mediated phosphorylation of JUN is necessary for transcriptional upregulation of STAT1, resulting in subsequent STAT1-dependent immune checkpoint expression. These findings reveal a new CDK1/2/5-dependent signal transduction mechanism responsible for immune checkpoint expression, although it is not yet known how IFNG causes CDK1/2/5 activation.
Inducing histone-mediated immunogenic cell death
Most anticancer agents or approaches, including chemotherapy and radiation therapy, can trigger anti-tumor immune response, which mainly depends on the antigenicity of cancer cells and their ability to produce adjuvant signals. In particular, ICD is accompanied by the various types of cell death (e.g., apoptosis, necroptosis, pyroptosis, and ferroptosis) and can endow dying cancer cells with a powerful adjuvant by exposing or releasing various damage-associated molecular patterns (DAMPs), which leads to the recruitment and activation of antigen presentation cells (e.g., dendritic cells) and the subsequent cytotoxic T-cell responses against tumors.Citation6 Identifying DAMPs in differential cell death modalities seems to be the key to understanding the diversity of ICD in tumor therapy.
Histones and their post-translational modifications play a key role in chromatin remodeling and epigenetic regulation. In addition to nuclear functions, histones also act as DAMPs when released into the extracellular space during infection, sterile inflammation, and oxidative stresses .Citation7 We further provided multiple evidences that indicate that extracellular histones are key DAMPs responsible for IFNG/dinaciclib-induced ICD. First, we observed that IFNG/dinaciclib induced DNA damage and caspase-3-dependent apoptosis, but did not cause other types of cell deaths, which resulted in the release of histones (H3 and H4) and high mobility group box 1 (HMGB1, a classic nuclear DAMP). Second, the antibody-mediated neutralization of H3 and H4, but not that of HMGB1, reduced dendritic cell migration and maturation and subsequent T-cell cross priming in response to the supernatants from IFNG/dinaciclib-treated PDAC cells. Third, the preventive tumor vaccination model validated IFNG/dinaciclib-treated KPC cells (a cell line derived from pancreatic tumors of K-RasG12D;Tp53R172H;Pdx1-Cre mice) were immunogenic in immunocompetent C57BL/6 J mice, and this process was inhibited by the administration of anti-H3 and H4 neutralizing antibodies. Fourth, exposing calreticulin on the surface of dying cells acted as an “eat-me” signal, further enhancing histone-related ICD. Collectively, these findings indicate that extracellular histones play a new role in mediating the anti-tumor immunity of ICD, although in this case its receptor has not been identified.
New drug combination shows promise for pancreatic cancer
Finally, we evaluated the potential utility of dinaciclib in combination with IFNG in the treatment of PDAC in three different preclinical mouse models (subcutaneous, orthotopic, and transgenic models). Compared with IFNG or dinaciclib alone, the administration of IFNG/dinaciclib not only blocked the growth of KPC or PANC02 tumors in subcutaneous models, but also did not cause significant toxicity in C57BL/6 mice. After orthogonally implanting syngeneic KPC cells into the pancreas, IFNG/dinaciclib prolonged the animal survival of C57BL/6 mice. Intracellular HMGB1 is a potential tumor suppressor of PDAC and the conditional depletion of pancreatic Hmgb1 (Pdx1-Cre;K-RasG12D/+;hmgb1−/-, termed KCH mice) can significantly promote the initiation and progression of Kras-driven PDAC in mice.Citation8 Using this KCH model, we further observed that IFNG/dinaciclib prolonged animal survival and inhibited the development of Kras-driven PDAC. In these three different preclinical mouse models of PDAC, the use of IFNG/dinaciclib increased the induction of apoptosis and infiltration of CD8+ T cells (instead of CD4+ T, B, and NK cells), whereas inhibited the expression of IDO1 and CD274 in tumor tissues. Importantly, the IFN/dinaciclib-mediated tumor suppression in subcutaneous or orthotopic models was diminished by the administration of anti-CD8–depleting antibodies. Together, these animal studies establish that the anti-cancer activity of IFNG/dinaciclib requires CD8+ T cells.
Conclusion and outlook
In summary, we reported that the CDK1/2/5 targeted drug dinaciclib has a powerful immunostimulatory effect by suppressing the expression of immune checkpoints and simultaneously inducing immunogenic cell death ().Citation4 The properties of dinaciclib are different from previously reported CDK4/6 inhibitors that stabilize the CD274 protein in certain cancers (breast cancer, colon cancer or melanoma),Citation9 although CDK4/6 inhibition can enhance chemotherapy and anticancer activity of immune checkpoint inhibitors in preclinical models.Citation9 These different mechanisms mediated by different CDK family members also indicate that either the down-regulation or the up-regulation of immune checkpoints can be used as a combination therapy strategy in clinical trials. Further analysis of the immunomodulatory effects of targeted anticancer agents, including CDK inhibitors, is not only important for understanding the activity and side effects of drugs, but also may provide new clues for the development of potential immunochemotherapy approaches.Citation10
Abbreviations
| CDK | = | Cyclin-dependent kinase |
| CD274/PD-L1 | = | Programmed death ligand 1 |
| HMGB1 | = | High mobility group box 1 |
| IDO1 | = | Indoleamine 2,3-dioxygenase 1 |
| ICD | = | Immunogenic cell death |
| IFNG/IFNγ | = | Interferon gamma |
| PDAC | = | Pancreatic ductal adenocarcinoma |
| PDCD1/PD-1 | = | Programmed cell death 1 |
| STAT1 | = | Signal transducer and activator of transcription 1 |
Disclosure of potential conflicts of interest
The authors declare no conflicts of interests.
References
- Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–3. doi:10.1016/j.ctrv.2019.06.005.
- Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156:2056–2072. doi:10.1053/j.gastro.2018.12.038.
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–540. doi:10.1038/s41571-020-0363-5.
- Huang J, Chen P, Liu K, Liu J, Zhou B, Wu R, Peng Q, Liu Z-X, Li C, Kroemer G, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2020:gutjnl-2019-320441. doi:10.1136/gutjnl-2019-320441
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi:10.1016/j.ccell.2015.03.001.
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337.
- Chen R, Kang R, Fan XG, Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi:10.1038/cddis.2014.337.
- Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, et al. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017;27:916–932. doi:10.1038/cr.2017.51.
- Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, Tan Y, Ci Y, Wu F, Dai X, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi:10.1038/nature25015.
- Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–741.