ABSTRACT
This study aims to identify the density of TILs in ductal carcinoma in situ (DCIS) in terms of prognostic significance with recurrence and the benefit of whole breast irradiation (WBI). The clinicopathological data of DCIS patients from Jan 2009 to Dec 2016 who received breast-conserving surgery (BCS) were retrospectively reviewed. Cox regression analysis was used to confirm independent prognostic factors of ipsilateral breast tumor recurrence (IBTR). Kaplan–Meier method was utilized to analyze IBTR and values of WBI. Touching-tumor-infiltrating lymphocytes (TILs) were defined by TILs touching or within one lymphocyte cell thickness from the malignant ducts’ basement membrane. In total, 129 patients were enrolled in this analysis with 98 patients who received WBI. After a median follow-up of 53.0 months, there were 16 IBTR events with five invasive IBTRs. Univariate and multivariate analyses showed that touching-TILs >5 were an independent prognostic factor for higher IBTR (HR = 6.17, 95%CI 1.95–19.56, p < .01). The whole cohort was classified into two subgroups: dense group (>5 touching-TILs per duct) and sparse group (≤5 touching-TILs per duct). Dense touching-TILs were associated with unfavorable biologic characteristics. The 5-y rate of IBTR between dense and sparse group was 29.0% versus 4.5% (p < .01). For the sparse group, WBI significantly reduced the rate of 5-y-IBTR risk from 13.2% to 1.7% (p = .02), but there was no benefit of WBI in the dense group. Touching-TILs density was heterogeneous in patients with DCIS. Sparse touching-TILs were associated with better prognosis and benefit from WBI. Dense touching-TILs not only were associated with a higher risk of IBTR but also lack of benefit from WBI.
Introduction
With the increasing use of screening mammography, the incidence of ductal carcinoma in situ (DCIS) has increased to 20% of all newly diagnosed breast cancers.Citation1 The main goal in the treatment of DCIS is to prevent local recurrence (LR), as up to 50% of LR after breast-conserving surgery (BCS) is in the form of invasive carcinoma.Citation2 Hence, in addition to BCS, most patients with DCIS will be treated with whole-breast irradiation (WBI) which significantly reduced the rate of LR by 50%.Citation3 However, with the excellent survival prognosis of DCIS tumors and the limited survival benefit from WBI, controversy persists regarding whether DCIS is being over-diagnosed or over-treated.Citation4 To date, novel biomarkers are required to stratify DCIS into those that are more likely to remain indolent or to become invasive, and thus to tailor personalized local treatment.
Tumor-immune microenvironment, specifically the presence of tumor-infiltrating lymphocytes (TILs), has been found to play an important role in breast cancer development and prognosis.Citation5 In previous studies on invasive breast cancers (IBCs), the density of TILs was significantly higher in triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2)–positive tumors compared with luminal tumors.Citation6,Citation7 The study conducted by the German Breast Cancer Group demonstrated that high stromal TILs were associated with a higher pCR rate and higher survival benefit among TNBC and HER2-positive patients receiving neoadjuvant chemotherapy (NAC).Citation8 The analysis from the study population of the BIG 02–98 trial has also revealed that each 10% increment in stromal TILs was associated with a 15% reduced risk of relapse in TNBC patients, while no significant association was observed in the luminal IBC population.Citation9 The density of TILs was found to be increased in the process of the tumor progression from DCIS to IBC.Citation10,Citation11 The role of the immune response in DCIS is an area of interest; however, the association between density of TILs and DCIS recurrence has not been well established due to a variety of controversial data.Citation12,Citation13 Assessment of stromal TILs, as detailed by the International Immuno-Oncology Biomarker Working Group, did not demonstrate an association with DCIS recurrence.Citation14–16 A more recent methodology of TILs assessment, which looked at TILs touching DCIS ducts (touching-TILs), was found to be an independent prognostic variable for LR in DCIS.Citation17
In this investigation, we assessed the density of touching-TILs in DCIS patients to identify its association with traditional clinicopathologic characteristics and further evaluated its predictive value for risk of LR and the benefit of radiotherapy.
Materials and methods
Study cohort
Consecutive patients with pathologically confirmed DCIS, including pure DCIS and DCIS with microinvasion, who received BCS in our institution between January 2009 and December 2016 were enrolled in this retrospective study. Patients with positive or unclear surgical margin, mixed DCIS and lobular carcinoma-in-situ, simultaneous contralateral breast cancer, pathological positive lymph nodes, or prior or concurrent malignancy (except non-melanoma skin cancer) were excluded.
For all patients, clinicopathological data from pathology and medical reports including patient age, menopausal status, tumor size, surgical margin, presence of comedo necrosis, nuclear grade, Ki67 index, and the status of estrogen receptor (ER) and HER2 were recorded.
Immunohistochemical staining
The 4-μm-thick paraffin sections were immunohistochemically stained, which was performed with an automatic staining device (Dako EnVision™ FLEX+, Denmark) and the EDTA (Ethylene Diamine Tetra-acetic Acid, pH 9.0, buffer, x50) was used for antigen retrieval. The commercially available antibodies (Dako FLEX RTU) including P120, E-cadherin, P63, calponin, and collagen type IV were used. The membranous staining of P120 catenin and E-cadherin was routinely used to differentiate DCIS from lobular carcinoma in situ (LCIS).Citation18 The P63 and calponin staining was used to show the existence of the myoepithelium.Citation19The collagen type IV staining highlighted the basement membrane (BM) of the DCIS ducts, which was essential for the diagnosis of DCIS.Citation20 In our cohort, all the malignant cells in DCIS tumors, including the microinvasion tumors, underwent membranous staining of E-cadherin and P120, and the epitheliums were positive for P63 or calponin. The immunohistochemical results were interpreted by two pathologists (WCF and ZSF).
Pathology assessment
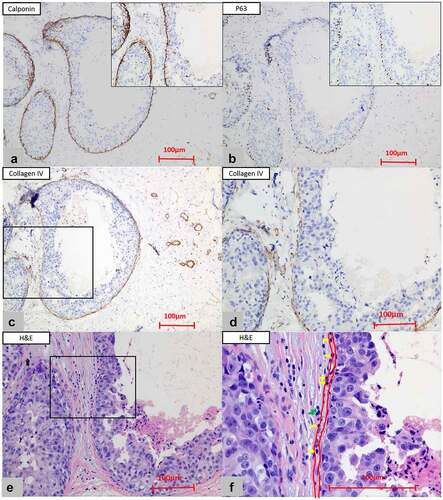
In all cases, the hematoxylin and eosin (H&E)–stained slides from surgically excised specimens of each patient were completely and independently reviewed by two pathologists (WCF and ZSF). TILs scoring carried out by the first observer (WCF) was considered in the final statistical analysis. There were 29.5% of patients (38/129) who underwent diagnostic biopsy before definitive surgery. All of the enrolled patients were pathologically confirmed to have DCIS after reviewing the specimens of definitive surgery. After comparison, we found that the TILs in the specimens of biopsy were not always consistent with the TILs in the specimens of definitive surgery, which might be explained by the limited tissue of the biopsy. Moreover, the post-biopsy wound healing/inflammation response was frequently presented as the infiltration of inflammatory cells including macrophages and lymphocytes in the stroma which may further interfere with the concordance of TILs assessment. Thus, for patients who underwent diagnostic biopsy before definitive surgery, the TILs were assessed on the sections of definitive surgery excluding the region with the presence of the complicating post-biopsy wound healing/inflammation response. The H&E-stained full-face sections (4 μm thick) were scanned with a slide scanner (KF-PRO-005) and viewed by the “K-ViEWER Software Program, version 2.5.2.0.” The TILs were counted manually (eyeballing) through the high-solution digital images. The International Working Group Recommendations for TILs assessment were applied to our case series to assess TILs which were identified as all recognizable mononuclear inflammatory cells including lymphocytes and plasma cells (polymorphonuclear cells such as neutrophils were excluded).Citation21 Prior to the present study, variant methods were utilized to evaluate the distribution of TILs in DCIS, such as the percentage of stromal TILs (sTIL), hotspot-TILs, and touching-TILs. In our cohort, we adopted the methodology described by TossCitation17 and only scored the number of touching-TILs. Touching-TILs were defined as the recognizable mononuclear inflammatory cells (including lymphocytes and plasma cells) that were either touching or within one lymphocyte cell thickness from the BM of the DCIS duct (identified by the P63, calponin, and collagen type IV staining). In this study, touching-TILs were counted for up to 20 ducts. For 24 cases with less than 20 malignant ducts, we evaluated all the available ducts which were confirmed as DCIS by immunohistochemistry. For cases with more than 20 malignant ducts, we thus divided the section fields into four relatively average quadrants by the K-ViEWER Software program and selected five ducts in each quadrant to score TILs in order to keep the counting more representative, especially in cases with heterogeneously distributed TILs. We divided total touching-TILs by the number of ducts counted, and the density of touching-TILs was identified as the mean number of TILs per DCIS duct. The detailed methods followed to assess TILs are illustrated in .
Figure 1. The assessment of touching-TILs. (a) The immunohistochemistry of the calponin (x10 and x20) and (b) P63 (x10 and x20) staining were used to show the existence of the myoepithelium. (c) The positive staining of collagen type IV (x10) highlighted the basement membrane (BM). (d) Inset closer view of C for collagen type IV staining (x20). (e) Touching-TILs were defined as lymphocytes and/or plasma cells that touched the BM or located within one lymphocyte cell thickness distance from BM. (f) The inset closer view of E for touching-TILs (x40, the inner red line: the location of BM; the outer red line: one lymphocyte cell thickness distance from BM; yellow arrows: touching-TILs; green arrows: lymphocytes located far than one lymphocyte cell thickness from BM)
Adjuvant radiotherapy and follow-up
For patients treated with WBI, a dose of 50 Gy in 25 fractions was prescribed to ipsilateral whole breast delivered by forward-planning field-in-field photons intensity-modulated radiation therapy using standard medial and lateral tangents. The decision of tumor bed boost was at the discretion of radiation oncologists. After surgery or WBI, patients were followed up every 3 months during the first 2 y, then every 6 months until 5 y and annually thereafter.
Ipsilateral breast tumor recurrence (IBTR) was defined as any pathologically confirmed recurrence of DCIS or invasive carcinoma in the ipsilateral breast. Follow-up time was calculated from the date of surgery to the date of the first event or last-confirmed date of breast cancer disease-free status.
Statistical analysis
Associations of clinicopathological features with the density of touching-TILs were examined using chi-square tests (Fisher’s exact test when necessary). The time-to-event curves were calculated by the Kaplan–Meier methods and compared by the log-rank test. Only the variables that showed evidence of association (p < .05) in the univariate analysis were tested in the multivariate analyses. All statistical tests were two-sided and p < .05 was considered significant. The software package SPSS 24.0 (IBM corporation, USA) was used for analysis.
Results
Patient and treatment characteristics
In total, 129 patients were enrolled in this analysis including 102 with pure DCIS and 27 with microinvasion tumors. The median age was 49 y old (range, 25–88). The median maximum tumor size was 1.5 cm (range, 0.2–4.8). In the whole cohort, there were 30 (23.3%) patients with the presence of comedo necrosis and 39 (30.2%) patients with high-grade tumors. Among 81 patients with ER-positive tumors, 69 (85.2%) received endocrine therapy. All of 98 patients treated with WBI completed radiotherapy as planned with 73.5% who received tumor bed boost. The patient and treatment characteristics are detailed in .
Table 1. Patient and treatment characteristics
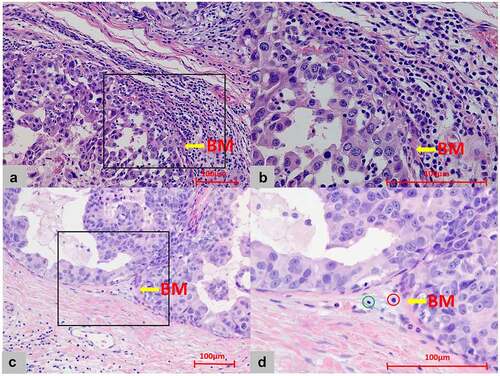
The density of touching-TILs was divided into two categories: sparse (≤5 touching-TILs per duct) and dense (>5 touching-TILs per duct). Dense touching-TILs were observed in 46 patients, while sparse touching-TILs were observed in 83 cases. Examples of dense and sparse touching-TILs are shown in .
Figure 2. Touching-TILs density around DCIS. (a) Dense infiltration: the mean number of touching-TILs was more than 5 cells/DCIS duct (x20). (b) The closer view of A for dense touching-TILs (x40). (c) Sparse infiltration: the mean number of touching-TILs was 5 cells or less/DCIS duct (x20). (d) The closer view of C for sparse touching-TILs (x40, green circle: lymphocyte; red circle: plasma cell. However, both the lymphocyte and plasma cell were not identified as touching-TILs for locating far than one lymphocyte cell thickness distance from basement membrane.)
Outcome analysis
With a median follow-up of 53.0 months (range, 0.6–124.2), there were 16 IBTR events and the 5-y rate of IBTR was 13.0% in the whole cohort. The 5-y rate of invasive-IBTR and DCIS-IBTR was 4.4% and 8.5%, respectively.
By univariate analysis, tumor size (≤2.5 cm vs. >2.5 cm), nuclear grade (low-intermediate vs. high), Ki67 index (≤14% vs. >14%), HER2 status (positive vs. negative), and density of touching-TILs (dense vs. sparse) were found to be significant prognostic factors for IBTR (p = .03, p = .03, p = .01, p = .01, and p < .01, respectively). In multivariate analysis, dense touching-ILs were the only independent risk factors for IBTR (HR = 6.17, 95%CI = 1.95–19.56, p < .01). The univariate and multivariate analyses are detailed in .
Table 2. The univariate and multivariable analyses for IBTR
Association of touching-TILs density with clinicopathological and treatment parameters
Dense touching-TILs were associated with unfavorable biologic characteristics including high Ki67 index, HER2 positivity, and presence of microinvasion. Half of the cases (52.2%) with dense touching-TILs had a high Ki67 index, while this was observed only in 31.3% of the patients with sparse touching-TILs (p = .02). Approximately 34.8% of DCIS cases with dense touching-TILs were HER2-positive tumors compared with 20.5% of the patients with sparse touching-TILs (p = .08). Microinvasion was observed in 30.4% of DCIS cases with dense touching-TILs compared with 15.7% of DCIS cases with sparse touching-TILs (p = .05).
The choice of WBI was marginally higher in patients with dense touching-TILs than sparse touching-TILs (84.8% vs. 71.1%, p = .08). The association between TILs density and various clinicopathological parameters is also summarized in .
Risk of IBTR according to density of touching-TILs
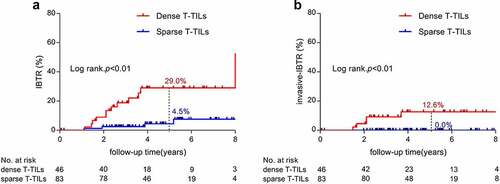
There were 4 and 12 IBTR events in patients with sparse and dense touching-TILs, respectively. The 5-y rate of IBTR was significantly higher in the dense touching-TILs group compared with the sparse touching-TILs group (29.0% vs. 4.5%, p < .01, as shown in )). A similar result was observed in the 5-y rate of invasive-IBTR between the two groups, with all the invasive-IBTR events occurring in the dense touching-TILs group (12.6% vs. 0.0%, p < .01, as shown in )).
Figure 3. Cumulative incidence of IBTR according to density of touching-TILs (A: IBTR; B: invasive-IBTR)
Among 33 patients with HER2-positive tumors, there were 17 patients with sparse touching-TILs and 16 with dense touching-TILs. Consistent with the results in the whole cohort, the 5-y rate of IBTR was also significantly higher in the dense touching-TILs group comparing with those with sparse touching-TILs (54.5% vs. 6.2%, p = .01).
Benefit from WBI according to the density of touching-TILs
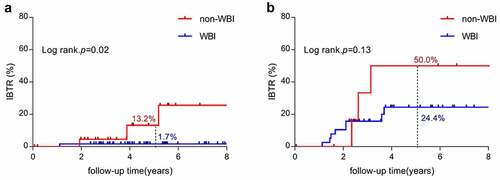
In the whole cohort, the 5-y rate of IBTR between the non-WBI and WBI groups was 22.6% versus 10.3% (p = .04). However, WBI was associated with a significantly different IBTR risk modification among individuals between the two groups. For sparse touching-TILs group, WBI significantly reduced the rate of 5-y-IBTR risk from 13.2% to 1.7% (p = .02, as shown in )), but among 46 patients with dense touching-TILs tumors, WBI showed no significant benefit in reducing IBTR (p = .13, as shown in )).
Discussion
Clinicopathological factors including age, nuclear grade, necrosis, and tumor size have been successfully used to predict outcomes in patients with DCIS. Recent research suggests that assessment of the immune microenvironment may add information beyond the traditional clinicopathological parameters and help to stratify the risk of local recurrence.Citation14,Citation22 To our best knowledge, this is the first study to assess the prognostic and predictive significance of touching-TILs density in DCIS patients who received BCS. The results of the present study demonstrated that touching-TILs density not only separated patients into different risk groups of IBTR but also helped to predict the benefit of WBI in decreasing IBTR. The DCIS patients with sparse touching-TILs were associated with better prognosis and significant benefit from WBI, while these results were not found in the dense touching-TILs group.
Immunological parameters, especially high percentage of stromal TILs, have been validated by several trials to play a role in the tumor progression and associated with higher pCR for IBC.Citation23,Citation24 However, data on the prognostic and predictive significance of immune microenvironment for DCIS were much less documented. There is currently no consensus to evaluate TILs in the context of DCIS in clinical significance. Previous studies of TILs evaluation in DCIS neither used clear or uniform definition of the stromal area surrounding DCIS for TILs assessment nor identified the cutoff points that can prognostically stratify DCIS. Darvishian et al. evaluated the percentage of TILs from the densest focus (hotspot) in one high-power field of stroma touching the basement membrane and found the mean time to recurrence was longer in the sparse TILs group (TILs <45%) than dense TILs group (TILs≥45%)(97.9 m vs. 73.5 m, p < .01).Citation25 The largest sample study to date found no significant associations between TILs (as a continuous variable or stratified by percentage) and the 10-y cumulative incidence of IBTR events, which enrolled 1488 DCIS patients and used the International Working TILs Group guidelines with modification to DCIS to assess the percentage of stromal TILs.Citation15 After validation in 666 DCIS patients, Toss et al. recommended that compared with the percentage of stromal TILs and hotspot TILs touching-TILs were the optimal method for TILs scoring in DCIS due to the highest concordance rate between observers and the most significant association with DCIS local outcome.Citation17 Thus, in this study, we adopted the methodology described by Toss and also found that density of touching-TILs was significantly associated with IBTR risk.
One of the important findings in our study was that, in contrast to favorable prognosis and better therapeutic response to NAC observed in IBC with dense TILs, we found that dense TILs in DCIS were associated with increased risk of tumor recurrence. In the 1488 DCIS cohort, there were no significant associations between TILs and the incidence of IBTR events.Citation15 However, there existed several differences between theirs and our study. In terms of systemic treatment, only 40.4% of Pruneri’s cohort received endocrine therapy while in our cohort it was 85.2%. Adjuvant endocrine therapy has kept the risk of IBTR in ER-positive DCIS to a considerably low level.Citation26,Citation27 For local treatment, only 75.5% of the patients were treated with BCS in Pruneri’s cohort while in our series BCS was 100%. We used touching-TILs as the method of TILs scoring in our study, while Pruneri et al. used the percentage of TILs. Compared with other methods of TILs scoring, touching-TILs seemed like a better method with the highest concordance rate between observers and the strongest association with DCIS local outcome.Citation17 These differences in treatment characteristics and assessment method of TILs may account for the variant results between these two studies. To explain the correlation between dense TILs and high IBTR in our population, one postulation was that dense TILs were an independent biomarker for aggressiveness in DCIS. Dense touching-TILs were significantly associated with higher Ki67 index, HER2-positive status, and presence of microinvasion in our cohort. Consistent with our results, Beguinot et al. observed that compared with pure DCIS, DCIS with microinvasion contained significantly more cases with high TIL density (>50%).Citation28 Alcazar et al. found that TILs density was higher in HER2-positive tumors than other molecular subtypes in DCIS patients, which implied that increased HER2 levels stimulate to form a more immunogenic microenvironment.Citation29 Our result also revealed that among HER2-positive subgroup, dense touching-TILs were also significantly associated with worse local prognosis. Several studies found that trastuzumab could induce active immunity by promoting a significant T-cell response in HER2-positive breast tumors.Citation30 Recently, the NRG-NSABP B-43 trial reported that two doses of trastuzumab concurrently with WBI could reduce the risk of IBTR compared with WBI alone (5-yrate of IBTR: 4.9% vs. 3.9%, p = .26) in patients with HER2-positive DCIS.Citation31 We hypothesized that the benefits of trastuzumab observed in this trial attributed more likely to the immunological effects rather than the direct anti-tumor effect. Furthermore, Komforti et al. revealed that the presence of touching-TILs (>5 touching TILs per DCIS duct) was significantly associated with high or intermediate Oncotype DCIS Score, hence worse outcomes in DCIS.Citation32
In several previous studies, the intrinsic immunological associations between dense TILs and worse prognosis in the DCIS population have been preliminarily described. In a study enrolling 138 patients with pure DCIS, both the fraction of the genome altered (FGA) and the number of telomeric imbalances were found to be positively associated with touching-TILs (both p < .001), which implies that the touching-TILs could be influenced by the antigens produced by copy number variation. Such changes in the immune microenvironment might lead to immunoediting of the tumor and therefore promote the progression of DCIS to IBC.Citation33 Teresa et al.Citation34 examined data from 5255 tumor/normal samples representing 12 cancer types and found that the tumor-immune microenvironment of high FGA level tumors was more protumorigenic and immunosuppressive. In particular, the ratio of CD8/Treg was reduced in IBC with high FGA. As the progression of DCIS to IBC requires tumor immunoediting driven by FGA, the increased touching-TILs might present the transitional phase in evasion of the immune system during the progression of DCIS to IBC. A previous study of IBC has observed that dense TILs were more prevalent in molecular subtypes with aggressive biological behaviors such as TNBC and HER2-positive breast cancer. In ER-positive tumors, higher TILs were associated with unfavorable prognosis and worse therapeutic response which were different to higher therapeutic response with chemotherapy and anti-HER2 targeted therapy in TNBC and HER2-positive tumors with dense TILs.Citation8 Our results suggested that DCIS tumors might display a comparable tumor-immune microenvironment to that of less aggressive IBC subtypes such as ER-positive IBC.
Different stromal lymphocyte composition between DCIS and IBC may be another answer for the potential different roles in their prognostic significance and relation with the underlying genomic instability.Citation35 Immunofluorescence analysis showed more activated CD8 + T cells in DCIS than in IBC whereas T cells of IBC samples were more similar to Tregs, suggesting a stronger immunosuppression in IBC compared with DCIS.Citation10 Modern immune researches have focused on the characteristics of different subtypes of TILs in DCIS and their relation with recurrence risk.Citation22 Low CD8 + T cells, high FOXP3+ regulatory T cells, high numbers of B cells, and high density of CD68+ and CD163+ macrophages have been validated to be associated with increased recurrence risk in the DCIS population.Citation12,Citation22,Citation36,Citation37 After a retrospective analysis of 117 DCIS patients, Campbell et al. found that not only high cytotoxic T lymphocytes (CTL) but also high numbers of CD115+ macrophage cells were predictive of high recurrence risk.Citation38 A study with subset analysis of TILs has shown an association between low CD8+/FOXP3+ ratio and high risk of ipsilateral recurrence. These results indicated that, as the lymphocytic composition of the DCIS immune microenvironment shifted from a pro-inflammatory (high CD8, low FOXP3) to an anti-inflammatory (low CD8, high FOXP3) signature, the immune surveillance weakened, thus leading to a higher risk of recurrence.Citation37 Furthermore, a study by Ishigami et al. reported for the first time that metastasis-free survival was significantly shorter for patients with coexistence of Tregs and Bregs aggregates in TILs than in those with Tregs alone without Bregs (p = .047).Citation39 Our further studies including TILs subset analysis and immune checkpoint expression are currently ongoing.
Interestingly, we found that the benefit of WBI was limited to patients in the sparse touching-TILs subgroup with a significant reduction of a 5-y rate of IBTR from 13.2% to 1.7% (p = .02), while the IBTR rate remains high regardless of WBI in patients with dense touching-TILs. This is one of the first studies, to our knowledge, to try to illustrate the association between density of TILs and the benefit from radiotherapy on local control in the DCIS population. Similar to our finding, in the subanalysis of the SweBCG91RT trial, in which 936 patients with stage I–II IBC were randomized into BCS plus WBI or BCS only, stromal TILs were assessed using a dichotomized cutoff of 10%. The study showed that WBI was significantly beneficial in the low-TILs group (HR 0.37, 95%CI 0.24–0.58, P < .01) but not in the high TILs group (p = .32).Citation40 The potentially larger benefit from WBI in the low-TILs group could be hypothesized as enhanced radiation-induced antitumoral immune response through the immunogenic transformation of tumor cells. Several preclinical studies have supported the theory that a large number of activated CD8 + T cells are recruited to the tumor microenvironment after radiation.Citation41,Citation42 Based on our present study, the density of touching-TILs could serve as a reference in tailoring local therapy decisions that DCIS with sparse touching-TILs might be better candidates for WBI while BCS should be cautious in DCIS with dense touching-TILs despite the addition of WBI. All of these results need to be validated by a prospective study.
As a single-center retrospective study, our study has some inherent limitations including limited sample size and biases; thus, a large prospective investigation will be needed to confirm the value of touching TILs in tailoring local treatment decisions. Also, we did not report the information of TILs subset and immune checkpoint expression, which may bring additional information on the significance of TILs for DCIS patients.
Conclusion
The density of TILs was heterogeneous in DCIS, and touching-TILs density showed a potential not only to stratify the risk of IBTR but also to predict the benefit of adjuvant radiotherapy for DCIS patients receiving BCS. Touching-TILs may serve as a biological surrogate to optimize local therapy decisions in patients with DCIS and await further validation.
Disclosure of potential conflicts of interest
All authors declare that they have no conflicts of interest concerning this study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Funding
References
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–9. doi:10.3322/caac.21583.
- Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232. doi:10.7150/jca.2.232.
- Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, Peto R, Bijker N, Solin L, Darby S. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177.
- Benson JR, Jatoi I, Toi M. Treatment of low-risk ductal carcinoma in situ: is nothing better than something? Lancet Oncol. 2016;17:e442–e451. doi:10.1016/S1470-2045(16)30367-9.
- Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol. 2013;3:107. doi:10.3389/fonc.2013.00107.
- Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0152500. doi:10.1371/journal.pone.0152500.
- Hendrickx W, Simeone I, Anjum S, Mokrab Y, Bertucci F, Finetti P, Curigliano G, Seliger B, Cerulo L, Tomei S, et al. Identification of genetic determinants of breast cancer immune phenotypes by integrative genome-scale analysis. Oncoimmunology. 2017;6(2):e1253654. doi:10.1080/2162402X.2016.1253654.
- Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/S1470-2045(17)30904-X.
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi:10.1200/JCO.2011.41.0902.
- Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, Zi X, Kwak M, Bergholtz H, Su Y, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1098–1115. doi:10.1158/2159-8290.CD-17-0222.
- Kim M, Chung YR, Kim HJ, Woo JW, Ahn S, Park SY. Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res. 2020;22(1):32. doi:10.1186/s13058-020-01267-w.
- Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, Argani P, Cimino-Mathews A, Emens LA. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29(3):249–258. doi:10.1038/modpathol.2015.158.
- Morita M, Yamaguchi R, Tanaka M, Tse GM, Yamaguchi M, Otsuka H, Kanomata N, Minami S, Eguchi S, Yano H, et al. Two progressive pathways of microinvasive carcinoma: low-grade luminal pathway and high-grade HER2 pathway based on high tumour-infiltrating lymphocytes. J Clin Pathol. 2016;69(10):890–898. doi:10.1136/jclinpath-2015-203506.
- Agahozo MC, Hammerl D, Debets R, Kok M, van Deurzen CHM. Tumor-infiltrating lymphocytes and ductal carcinoma in situ of the breast: friends or foes? Mod Pathol. 2018;31(7):1012–1025. doi:10.1038/s41379-018-0030-x.
- Pruneri G, Lazzeroni M, Bagnardi V, Tiburzio GB, Rotmensz N, DeCensi A, Guerrieri-Gonzaga A, Vingiani A, Curigliano G, Zurrida S, et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann Oncol. 2017;28(2):321–328. doi:10.1093/annonc/mdw623.
- Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, Pruneri G, D’Alfonso TM, Demaria S, Castaneda C, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol. 2018;52:16–25. doi:10.1016/j.semcancer.2017.10.003.
- Toss MS, Miligy I, Al-Kawaz A, Alsleem M, Khout H, Rida PC, Aneja R, Green AR, Ellis IO, Rakha EA, et al. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod Pathol. 2018;31(8):1226–1236. doi:10.1038/s41379-018-0040-8.
- Li X, Schwartz MR, Ro J, Hamilton CR, Ayala AG, Truong LD, Zhai QJ. Diagnostic utility of E-cadherin and P120 catenin cocktail immunostain in distinguishing DCIS from LCIS. Int J Clin Exp Pathol. 2014;7:2551–2557.
- Dewar R, Fadare O, Gilmore H, Gown AM. Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135:422–429. doi:10.1043/2010-0336-CP.1.
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harbor Perspect Biol. 2011;3. doi:10.1101/cshperspect.a004911.
- Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–251. doi:10.1097/PAP.0000000000000162.
- Chen XY, Yeong J, Thike AA, Bay BH, Tan PH. Prognostic role of immune infiltrates in breast ductal carcinoma in situ. Breast Cancer Res Treat. 2019;177:17–27. doi:10.1007/s10549-019-05272-2.
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959. doi:10.1200/JCO.2013.55.0491.
- Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64. doi:10.1001/jamaoncol.2015.3239.
- Darvishian F, Ozerdem U, Adams S, Chun J, Pirraglia E, Kaplowitz E, Guth A, Axelrod D, Shapiro R, Price A, et al. Tumor-infiltrating lymphocytes in a contemporary cohort of women with ductal carcinoma in situ (DCIS). Ann Surg Oncol. 2019;26:3337–3343. doi:10.1245/s10434-019-07562-x.
- Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, Forbes JF, Bishop H, Fentiman IS, George WD, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi:10.1016/S1470-2045(10)70266-7.
- Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, Albain KS, Whitworth PW, Cianfrocca ME, Brufsky AM, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–856. doi:10.1016/S0140-6736(15)01168-X.
- Beguinot M, Dauplat MM, Kwiatkowski F, Lebouedec G, Tixier L, Pomel C, Penault-Llorca F, Radosevic-Robin N. Analysis of tumour-infiltrating lymphocytes reveals two new biologically different subgroups of breast ductal carcinoma in situ. BMC Cancer. 2018;18:129. doi:10.1186/s12885-018-4013-6.
- Nelson AC, Machado HL, Schwertfeger KL. Breaking through to the other side: microenvironment contributions to DCIS initiation and progression. J Mammary Gland Biol Neoplasia. 2018;23:207–221. doi:10.1007/s10911-018-9409-z.
- Kuerer HM, Buzdar AU, Mittendorf EA, Esteva FJ, Lucci A, Vence LM, Radvanyi L, Meric-Bernstam F, Hunt KK, Symmans WF, et al. Biologic and immunologic effects of preoperative trastuzumab for ductal carcinoma in situ of the breast. Cancer. 2011;117:39–47. doi:10.1002/cncr.25399.
- Cobleigh MA, Anderson SJ, Siziopikou KP, Arthur DW, Julian TB, Rabinovitch R, Parda DS, Seaward SA, Carter DL, Lyons JA, et al. Primary results of NRG Oncology/NSABP B-43: phase III trial comparing concurrent trastuzumab (T) and radiation therapy (RT) with RT alone for women with HER2-positive ductal carcinoma in situ (DCIS) after lumpectomy. J Clin Oncol. 2020;38:508. doi:10.1200/JCO.2020.38.15_suppl.508.
- Komforti M, Badve SS, Harmon B, Lo Y, Fineberg S. Tumour-infiltrating lymphocytes in ductal carcinoma in situ (DCIS)—assessment with three different methodologies and correlation with Oncotype DX DCIS Score. Histopathology. 2020;77:749–759. doi:10.1111/his.14181.
- Hendry S, Pang JB, Byrne DJ, Lakhani SR, Cummings MC, Campbell IG, Mann GB, Gorringe KL, Fox SB. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin Cancer Res. 2017;23:5210–5217. doi:10.1158/1078-0432.CCR-17-0743.
- Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355:eaaf8399. doi:10.1126/science.aaf8399.
- Toss MS, Abidi A, Lesche D, Joseph C, Mahale S, Saunders H, Kader T, Miligy IM, Green AR, Gorringe KL, et al. The prognostic significance of immune microenvironment in breast ductal carcinoma in situ. Br J Cancer. 2020;122:1496–1506. doi:10.1038/s41416-020-0797-7.
- Thike AA, Chen X, Koh VCY, Binte Md Nasir ND, Yeong JPS, Bay BH, Tan PH. Higher densities of tumour-infiltrating lymphocytes and CD4 + T cells predict recurrence and progression of ductal carcinoma in situ of the breast. Histopathology. 2020;76(6):852–864. doi:10.1111/his.14055.
- Semeraro M, Adam J, Stoll G, Louvet E, Chaba K, Poirier-Colame V, Sauvat A, Senovilla L, Vacchelli E, Bloy N, et al. The ratio of CD8 +/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology. 2016;5:e1218106. doi:10.1080/2162402X.2016.1218106.
- Campbell MJ, Baehner F, O’Meara T, Ojukwu E, Han B, Mukhtar R, Tandon V, Endicott M, Zhu Z, Wong J, et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2017;161(1):17–28. doi:10.1007/s10549-016-4036-0.
- Ishigami E, Sakakibara M, Sakakibara J, Masuda T, Fujimoto H, Hayama S, Nagashima T, Sangai T, Nakagawa A, Nakatani Y, et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer. 2019;26(2):180–189. doi:10.1007/s12282-018-0910-4.
- Kovács A, Stenmark Tullberg A, Werner Rönnerman E, Holmberg E, Hartman L, Sjöström M, Lundstedt D, Malmström P, Fernö M, Karlsson P, et al. Effect of radiotherapy after breast-conserving surgery depending on the presence of tumor-infiltrating lymphocytes: a long-term follow-up of the SweBCG91RT randomized trial. J Clin Oncol. 2019;37:1179–1187. doi:10.1200/JCO.18.02157.
- Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15:411–421. doi:10.1007/s10911-010-9194-9.
- Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu Y-X, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi:10.1158/0008-5472.CAN-10-2820.