ABSTRACT
Immune checkpoint inhibitors (ICI) predispose patients to immune-related adverse events (irAEs). Although hepatitis is a potentially lethal toxicity, the timing and outcomes have not been well described. In this retrospective study, patients from six international institutions were included if they were treated with ICIs and developed immune-related hepatitis. Patient and tumor characteristics, and hepatitis management and outcomes were evaluated. Of the 164 patients included, most were male (53.7%) with a median age of 63.0 years. Most patients had melanoma (83.5%) and stage IV disease (86.0%). Median follow-up was 585 days; median OS and PFS were not reached. The initial grade of hepatitis was most often grade 2 (30.5%) or 3 (45.7%) with a median time to onset of 61 days. Patients were most commonly asymptomatic (46.2%), but flu-like symptoms, including fatigue/anorexia (17.1%), nausea/emesis (14.0%), abdominal/back pain (11.6%), and arthralgias/myalgias (8.5%) occurred. Most patients received glucocorticoids (92.1%); the median time to improvement by one grade was 13.0 days, and the median time to complete resolution was 52.0 days. Second-line immunosuppression was required in 37 patients (22.6%), and steroid-dose re-escalation in 45 patients (27.4%). Five patients (3%) died of ICI-hepatitis or complications of hepatitis treatment. Ninety-one patients (58.6%) did not resume ICI; of 66 patients (40 grade 1/2, 26 grade 3/4) that were rechallenged, only 25.8% (n = 17) had recurrence. In this multi-institutional cohort, immune-related hepatitis was associated with excellent outcomes but frequently required therapy discontinuation, high-dose steroids, and second-line immunosuppression. Rechallenge was associated with a modest rate of hepatitis recurrence.
Introduction
Immune checkpoint inhibitors (ICI) have revolutionized cancer management and are now approved in many different cancer types as single agents and in combination regimens. ICI treatment may be complicated by immune-related adverse events (irAEs), with increased frequency when used in combination. These irAEs can virtually affect any organ system, but usually present acutely and resolve with glucocorticoids.Citation1,Citation2 However, irAEs can be severe and lead to treatment cessation, hospitalization, long-term symptoms, and even death.Citation3,Citation4 Given the wide range of manifestations and organs impacted by ICIs, prior studies have characterized severe irAEs to help guide diagnosis and management decisions. As an example, pneumonitis, colitis, and myocarditis have been well described with large studies detailing incidence, risk factors, outcomes, and management.Citation5–10
Hepatitis is a relatively common, clinically significant irAE defined by elevations in alanine transaminase (ALT), aspartate transaminase (AST), and bilirubin.Citation11,Citation12 Severe presentations requiring prolonged immunosuppressants, hospitalization, and treatment discontinuation may occur. To date, however, many aspects of immune-related hepatitis have not been thoroughly characterized. Specifically, the clinical presentation, course, outcomes, and management of patients developing hepatitis on ICIs are not well described. We herein sought to analyze a large international, multi-institutional cohort of patients that developed hepatitis while treated with ICIs.
Methods
Study design
Institutional review board approval was obtained. Data were collected from electronic medical records in patients treated with ICIs at Vanderbilt University Medical Center, Massachusetts General Hospital, Northwestern Memorial Hospital, Duke University Medical Center, Rutgers University Hospital, and Melanoma Institute Australia. Patients were included if they were treated with ICIs (as monotherapy or combination therapy) and developed immune-related hepatitis, regardless of tumor type, treatment setting (standard of care vs. clinical trial), or dose. Included ICIs were monoclonal antibodies to programmed death-1/ligand-1 (PD-1/PD-L1) and cytotoxic T lymphocyte antigen-4 (CTLA-4). Patients who had received prior therapies or had preexisting liver-related comorbidities were also included. Patients with elevated liver function testing attributed to other causes by the investigator (e.g., disease-progression, other medications, liver hypoperfusion) were not included.
Data collection
Survival outcomes were assessed with overall survival (OS), progression-free survival (PFS), and follow-up time. Patient and tumor characteristics, liver-specific comorbidities, and extrahepatic toxicities to ICIs were collected. To characterize the clinical picture of hepatitis, initial grade (CTCAE version 5.0 as judged by elevations in alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST]), symptoms, biopsy results, and imaging results were collected. For patients with liver biopsies available, the hepatic injury was characterized by the following pathologic patterns: necroinflammatory (acute, chronic), zonal necrosis (with or without other injuries), and cholestatic as judged by a gastrointestinal pathologist. To evaluate the change over time and response to treatment, the following were tracked: time to hepatitis onset, time to any improvement (defined as a decrease by ALT or AST), time to improvement by one grade, and time to complete resolution (reflected by the decrease of liver function tests to normal or baseline). Treatment regimens were also characterized based on steroid dose (approximately ≥1 mg/kg vs. <1 mg/kg) and the need for second-line immunosuppressants (e.g., mycophenolate mofetil). After hepatitis resolution, side-effects to treatment, and outcomes to ICI rechallenge were also analyzed.
Statistical analysis
Categorical and continuous variables were analyzed with descriptive statistics. Chi-square and Mann–Whitney analyses were used to determine predictors of severe hepatitis, need for second-line immunosuppressants, hepatitis recurrence with ICI rechallenge, and time to hepatitis resolution. A p-value of <0.05 was considered statistically significant. The Kaplan–Meier method was used to visualize OS and PFS. All statistics were analyzed using Graph Pad Prism version 8.2.1.
Discussion
Herein, we present the largest multicenter characterization of immune-related hepatitis from ICI therapy. Most cases were severe (grade 3–4), and approximately 23% and 27% required escalation to second-line immunosuppression or increased glucocorticoid dosing. The median time to resolution was 52 days and most patients permanently discontinued ICI therapy. Four patients died directly from immune-related hepatitis and one patient died of complications of prolonged immunosuppression. Thus, hepatitis significantly impacted patients’ treatment courses.
Despite this, the overall cohort had excellent clinical outcomes with a 62% response rate and 80% of the cohort alive at last follow-up (median 585 days) despite most patients permanently discontinuing therapy. These findings are in agreement with prior studies that have suggested the development of irAEs correlate with improved survival outcomes.Citation13–15 The presence of liver metastases, which are generally associated with a poor prognosis, did not preclude a response. Conversely, liver metastases did not correlate with improved response, thus not suggesting an obvious mechanism for this association (e.g., cross-reactive T cells to both normal and neoplastic tissue in the liver).Citation16,Citation17 We observed that most patients presented asymptomatically, highlighting the importance of long-term liver enzyme monitoring (one case was 1000+ days after ICI initiation). Interestingly, most symptomatic patients had fairly nonspecific, flu-like symptoms (not generally associated with other forms of hepatitis), highlighting the importance of assessing liver enzymes in patients with these symptoms. Additionally, our results indicate that mild hepatitis (grade 1–2) is associated with a significantly faster time to resolution, further highlighting the importance of early detection.
Once hepatitis is suspected through laboratory evaluations, liver biopsy can confirm the diagnosis and further characterize the pattern of injury.Citation18,Citation19 Among 32 patients who underwent biopsies, the only treatment-related deaths were among the 10 patients with zonal necrosis or cholestatic liver injury (2 of 10) compared with none (of 22) patients with the necroinflammatory disorder. However, CT or ultrasound imaging did not reveal any specific findings characteristic of ICI-related hepatitis. Therefore, these adjunctive tests are primarily useful for confirming the diagnosis (biopsy) or ruling out other causes (imaging).
We also assessed for predictors of high-grade hepatitis or the need for second-line immunosuppressants. Age, time to hepatitis onset, or the presence of liver-related comorbidities were not predictors of severe clinical outcomes. We observed a higher rate of severe (21%) extrahepatic toxicities than previously described.Citation20,Citation21 While combination therapy with CTLA-4 inhibitors has been known to increase the risk of severe irAEs, we found that combination therapy did not predict hepatitis severity, the need for second-line immunosuppressants, or time to resolution. However, the presence of any-grade extrahepatic irAEs was a predictor for mild (grade 1–2) hepatitis compared to severe (grade 3–5). The exact mechanism behind this finding is unclear but is perhaps related to immunosuppressant treatment for extrahepatic irAEs blunting the severity of subsequent hepatitis onset.
A subset of our cohort (66 patients) were subsequently rechallenged with an ICI. Previous studies have assessed outcomes to rechallenge following irAEs and found relatively high rates of recurrent or new irAEs (~20-50%).Citation22–24 Our results were similar with 26% developing subsequent hepatitis, which was particularly common in combination CTLA-4/PD-1 blockade rechallenge (60%), uncommon with the resumption of PD-1 blockade after combination-induced hepatitis (9%), and intermediate risk following monotherapy hepatitis with monotherapy rechallenge (32%). Most recurrent cases were mild grade 1–2 (58%) and only 2 patients required second-line immunosuppressants. Providers should thus weigh the risk of recurrence and expected benefit from additional ICI therapy (which may be questionable in a patient with a long-standing complete response or, conversely, early disease progression) when considering management options.
This study has several limitations, mainly related to the multi-institutional retrospective design. First, there is potential for recall bias, as patients with mild, non-clinical (grade 1) hepatitis may not have been routinely captured. Additionally, while the standardized CTCAE grading system was used, variations among institutions may exist in the characterization of hepatitis severity and management protocols. For time to resolution analyses, unless the patient was hospitalized, labs were often only collected on a weekly or bimonthly basis. Therefore, conclusions for time to improvement and resolution may be limited. Further, it is difficult to conclusively exclude other contributors or causes for hepatitis outside ICI, although each case was judged by investigators as due to ICI. Finally, most patients had stage IV melanoma, but there was a small subset of patients with stage III disease and other types of primary cancer. Therefore, while all patients were treated with an ICI, there is potential that disease-specific clinical factors may impact outcomes in this cohort.
To date, this is the largest characterization of hepatitis related to ICI therapy. While this cohort did display a favorable long-term response and survival, the rate of early treatment disruption, permanent discontinuation, and even death due to hepatitis was significant. Therefore, physicians should routinely monitor for hepatitis through both clinical and laboratory assessments, particularly in the first months of treatment. This study should inform future studies in determining the long-term impacts of immune-related hepatitis on survival outcomes, hepatic function, and quality of life.
Results
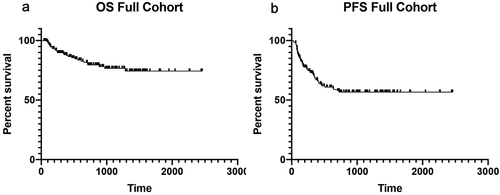
Of the 164 patients included, most were male (53.7%) with a median age of 63.0 years. Most patients had melanoma (83.5%) or lung cancer (7.3%) and were treated with ipilimumab and nivolumab (59.2%), pembrolizumab (20.7%), or nivolumab (11.6%) monotherapy. At ICI initiation, most patients had stage IV disease (86.0%) and the most common liver-specific comorbidity was liver metastases (25.6%). Extrahepatic irAEs developed in 65.9% of the cohort, with 20.7% being severe (grade 3–4), and 37.2% requiring glucocorticoids. The response rate was 61.6% (27.4% complete response and 34.1% partial response); only 14.6% had primary disease progression. During available follow-up, 35.4% of the patients experienced disease progression. Response rates were high both in patients with liver metastases at baseline (25/42; 59.5%) and those with metastatic disease but without liver metastases (74/119; 62.2%). At last follow-up, 130 patients (79.3%) were alive, with an ECOG of 0 (n = 65, 50%) or 1 (n = 42, 32%). The median duration of follow-up after ICI initiation was 585 days, and median OS and PFS were not reached (, ). There was no difference in OS or PFS based on the initial grade of hepatitis (grade 1–2 vs. grade 3–4).
Table 1. Patient, tumor, and treatment characteristics
The initial grade of hepatitis at presentation was 2 in 50 (30.5%), 3 in 75 (45.7%), and 4 in 23 (14.0%) (). The median time to onset was 61 days after starting therapy. At presentation, patients were most often asymptomatic (46.3%), but flu-like symptoms, including fatigue/anorexia (17.1%), nausea/emesis (14.0%), abdominal/back pain (11.6%), arthralgias/myalgias (8.5%), and rarely jaundice (3.7%) occurred. Imaging (with ultrasound and/or computed tomography) was performed in 73 (44.5%) patients. Imaging was unremarkable in essentially all patients, frequently revealing preexisting and/or incidental imaging findings including concurrent liver metastases, cysts, or hemangiomas; there were no characteristic findings of immune-related hepatitis. Of 32 patients who underwent liver biopsy, 22 (68.8%) displayed a necroinflammatory pattern (acute or chronic), while cholestatic (n = 5, 15.6%), cholestatic and zonal necrosis (n = 3, 9.4%) and zonal necrosis (n = 2, 6.3%) alone were also observed. Patients that were biopsied had a higher chance of receiving second-line immunosuppressants (62.5% vs 12.9%, p < .001), had a longer time to complete resolution (90 days vs 50 days, p = .019), and had marginally higher grades of initial hepatitis presentation (p = .06). None of the 22 patients with necroinflammatory pattern died of hepatitis, whereas 1/5, 0/3, and 1/2 patients with cholestatic, mixed cholestatic and zonal necrosis, and pure zonal necrosis died.
Table 2. Hepatitis characteristics
Patients were initially managed with glucocorticoids in 90.9% of cases while 7.9% resolved with observation alone; two additional patients who were initially observed ultimately were treated with corticosteroids. Of patients initially treated with glucocorticoids, 56.4% were treated with high-dose oral (at least approximately 1 mg/kg), 12.8% were treated with low-dose oral (<1 mg/kg), and 30.9% were treated with intravenous steroids. Due to lack of response to glucocorticoids, 37 patients (22.6%) were given additional immunosuppressants (mycophenolate mofetil [MMF] alone n = 31; MMF + tacrolimus n = 3; MMF + tacrolimus + IVIG n = 1; MMF + abatacept n = 1; infliximab n = 1) and 45 (27.4%) required an escalation of their initial glucocorticoid dose.
Conflicts of interest:
DBJ serves on advisory boards for Array Biopharma, BMS, Catalyst, Iovance, Jansen, Merck, and Novartis, and receives research funding from BMS and Incyte.
AKS receives research funding (paid to institution) from Bristol-Myers Squibb, Immunocore, Merck, Pfizer and serves as a consultant/advisory board member for Array, Novartis, Iovance, and Regeneron.
AMM has served on advisory boards for BMS, MSD, Novartis, Roche, Pierre-Fabre, and QBiotics.
GVL is consultant advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma inc, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Highlight Therapeutics S.L., Merck Sharpe & Dohme, Novartis Pharma AG, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX B.V.
Supplemental Material
Download ()Acknowledgments
AMM is supported by a Cancer Institute NSW Fellowship and Melanoma Institute Australia.GVL is supported by an NHMRC Practitioner Fellowship and the University of Sydney Medical Foundation.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schandendorf D, Wagstaff J, Dummer R, et al. 2019. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine. 381(16):1535–7. doi:10.1056/NEJMoa1910836.
- Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. 2017. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 35(7):785–792. doi:10.1200/JCO.2015.66.1389.
- Patrinely JR, Young AC, Quach H, William GR, Ye F, Fan R, Horn L, Beckermann KE, Gillaspie EA, Sosman, JA, et al. 2020. Survivorship in immune therapy: assessing toxicities, body composition and health-related quality of life among long-term survivors treated with antibodies to programmed death-1 receptor and its ligand. Eur J Cancer. 135:211–220. doi:10.1016/j.ejca.2020.05.005.
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. 2018. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4(12):1721–1728. doi:10.1001/jamaoncol.2018.3923.
- Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunnigham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al. 2017. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 35(7):709–717. doi:10.1200/JCO.2016.68.2005.
- Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Griglio A, et al. 2019. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol. 37(30):2738–2745. doi:10.1200/JCO.19.00320.
- Abu-Sbeih H, Herrera LN, Tang T, Chaftari AP, Okhuysen PC, Jeng RR, Wang Y. 2019. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J Immunother Cancer. 7(1):242. doi:10.1186/s40425-019-0714-x.
- Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. 2018. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 391(10124):933. doi:10.1016/S0140-6736(18)30533-6.
- Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, Mehta R, Silk AW, Zhou A, Compton ML, et al. 2019. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. 8(1):e1524695. doi:10.1080/2162402X.2018.1524695.
- Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling KL, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, et al. 2018. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 71(16):1755–1764. doi:10.1016/j.jacc.2018.02.037.
- Thompson JA, Schneider BJ, Brahmer J, Andrew S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, et al. 2020. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 18(3):230–241. doi:10.6004/jnccn.2020.0012.
- Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. 2017. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 5(1):95. doi:10.1186/s40425-017-0300-z.
- Das S, Johnson DB. 2019. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 7(1):306. doi:10.1186/s40425-019-0805-8.
- Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, Johnson DB. 2019. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 5(6):906–908. doi:10.1001/jamaoncol.2019.0046.
- Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N, et al. 2017. Liver metastasis and treatment outcome with anti-pd-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 5(5):417–424. doi:10.1158/2326-6066.CIR-16-0325.
- Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. 2016. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 375(18):1749–1755. doi:10.1056/NEJMoa1609214.
- Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, et al. 2019. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5(7):1043–1047. doi:10.1001/jamaoncol.2019.0402.
- Cohen JV, Misdraji J, Dougan M, Fadden R, Rubin KM, Lawless A, Reynolds KL, Lawrence DP, Mooradian M, Flaherty K, et al.. 2018. Characterization of immune-related hepatitis (irH) from immune checkpoint inhibitors (ICIs). Journal of Clinical Oncology. 36(15_suppl):3087–13087. doi:10.1200/JCO.2018.36.15_suppl.3087.
- Imoto K, Kohjima M, Hioki T, KurashigeT, KurokawaM, Tashiro S, Suzuki, H, Kuwano A, Tanaka M, Okada S, et al. 2019. Clinical features of liver injury induced by immune checkpoint inhibitors in japanese patients. Can J Gastroenterol Hepatol. 2019:6391712. doi:10.1155/2019/6391712.
- Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al. 2017. Adjuvant nivolumab versus ipilimumab in resected stage iii or iv melanoma. N Engl J Med. 377(19):1824–1835. doi:10.1056/NEJMoa1709030.
- lEggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, CarlinoMS, et al. 2018. Adjuvant pembrolizumab versus placebo in resected stage iii melanoma. New England Journal of Medicine. 378(19):1789–1801. doi:10.1056/NEJMoa1802357.
- Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV,Menzies AM, Eroglu Z, et al. 2018. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 29(1):250–255. doi:10.1093/annonc/mdx642.
- Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A, Varga A, et al. 2019. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 5(9):1310–1317. doi:10.1001/jamaoncol.2019.1022.
- Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, et al. 2018. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 6(9):1093–1099. doi:10.1158/2326-6066.CIR-17-0755.