ABSTRACT
Radiation therapy (RT) and cyclin-dependent kinase 4/6 (CDK4/6) inhibitors mediate poorly overlapping cytostatic and immunostimulatory effects, suggesting that combinatorial regimens may enable supra-additive tumor control. Our preclinical findings demonstrate that administration schedule stands out as a major determinant of efficacy when RT and CDK4/6 inhibitors are combined for breast cancer therapy.
Estrogen receptor (ER)+ breast cancer (BC) is the most common BC subtype, accounting for approximately 80% of all BC cases and the majority (>60%) of BC-related deaths in the US.Citation1 For decades, patients with resectable ER+ BC have been treated with surgery followed by endocrine therapy (ET), optionally combined with radiotherapy (in the case of conservative surgery) and/or chemotherapy (depending on risk for relapse).Citation2 Conversely, metastatic ER+ BC has classically been managed with ET or neoadjuvant chemotherapy, often based on rather immunostimulatory agents like taxanes or anthracyclines.Citation3,Citation4 Recently, the development of agents specific for cyclin-dependent kinase 4/6 (CDK4/6), which are key drivers of cell cycle progression in ER+ BC cells,Citation5 has considerably altered this approach.Citation6 Indeed, the addition of CDK4/6 inhibitors (i.e. palbociclib, abemaciclib, and ribociclib) to ET has been associated with a robust survival advantage over ET alone in women with metastatic ER+ BC,Citation7,Citation8 rapidly resulting in the implementation of CDK4/6 inhibitors plus ET as first-line therapeutic strategy for these patients. However, >50% of women with ER+ BC receiving CDK4/6 inhibitors plus ET experience disease progression within 24 months from treatment initiation,Citation7,Citation8 calling for the identification of novel strategies to maximize the therapeutic efficacy CDK4/6 inhibitors and further delay disease progression in this patient population.
Accumulating preclinical and clinical evidence suggests that the therapeutic synergy between CDK4/6 inhibitors and ET involves (at least in part) their ability to promote anticancer immunity. Indeed, letrozole-based ET has been associated with limited BC infiltration by immunosuppressive CD4+CD25+FOXP3+ regulatory T (TREG) cells.Citation9 Moreover, palbociclib and other CDK4/6 inhibitors not only resemble letrozole in its ability to deplete TREG cells, but also promote MHC Class I expression on malignant cells (de facto rendering them more visible to the immune system)Citation10 and favor the secretion of type III interferon (IFN), which is a potent immunostimulant.Citation11,Citation12 This suggests that immunological mechanisms including the establishment of an immunosuppressive tumor microenvironment may support clinical resistance to CDK4/6 inhibitors plus ET, and that additional immunostimulatory agents may be harnessed to circumvent it. In this context, radiation therapy (RT) stands out as an ideal partner for CDK4/6 inhibition as: (1) both RT and CDK4/6 inhibition have cytostatic effects, but operate at different phases of the cell cycle (i.e. G2/M and G1, respectively);Citation13,Citation14 (2) RT mediates pronounced immunostimulatory effects via pathways that are largely independent of those elicited by CDK4/6 inhibitors, including type I IFN secretion;Citation15,Citation16 and (3) the safety profile of RT is well established,Citation17 and preliminary retrospective data from a cohort of metastatic ER+ BC patients suggest that the administration of CDK4/6 inhibitors plus RT is reasonably well tolerated.Citation18
Based on these premises, we set to investigate the potential synergy between RT and the prototypic CDK4/6 inhibitor palbociclib, harnessing human ER+ and ER− BC cell lines and three different immunocompetent mouse models of BC. In particular, we aimed at identifying an optimal dosing and administration schedule with the ultimate objective to inform the design of clinical trials testing the combination of RT and palbociclib in patients with oligometastatic HR+ BC in the context of letrozole-based ET. Upon confirming that palbociclib and RT employed as standalone therapeutic agents arrest the proliferation of human ER+ BC MCF7 cells in G1 and G2/M, respectively, we combined these agents according to the following treatment schedules: (1) P→RT, RT delivered after palbociclib, or (2) RT→P, palbociclib delivered after RT. We found that RT→P mediates superior short-term (3 days) and long-term (14 days) cytostatic effects in MCF7 cells as compared to P→RT, reflecting the near-to-complete inhibition of cell proliferation. Similar data were obtained in human triple-negative breast cancer (TNBC) MDA-MB-231 cells (which at odds with MCF7 bear TP53 mutations and hence are expected to enable limited cooperativity between RT and palbociclib).Citation19,Citation20
We next evaluated the interactions between palbociclib and focal RT in (1) immunocompetent BALB/c mice bearing subcutaneous, syngeneic p53-incompetent TS/A mammary adenocarcinomas (a widely employed model of ER+ BC) or 4T1 mammary carcinomas (a widely employed model of metastatic TNBC), or (2) an endogenous model of mammary carcinogenesis driven by a synthetic progestin, i.e. medroxyprogesterone acetate (MPA) and a polycyclic aromatic hydrocarbon, i.e. 7,12-dimethylbenz[a]anthracene (DMBA), in immunocompetent C57BL/6J mice. The latter is a privileged platform for translational studies as it recapitulates key immunobiological features of luminal B ER+ BC in women, including reduced infiltration by immune cells, limited sensitivity to immunotherapy and (most often) wild-type TP53 status.Citation21,Citation22 In line with our in vitro data, RT→P also demonstrated superior therapeutic efficacy as compared to P→RT in vivo, irrespective of p53 competence. Indeed, RT→P was associated with (1) superior local disease control in all models, (2) extended overall survival in TS/A-bearing mice, and (3) limited metastatic dissemination to the lungs in 4T1-bearing mice.Citation19
Altogether, our findings support the contention that RT may be harnessed to improve the therapeutic benefits of CDK4/6 inhibitors in patients with metastatic ER+ BC, potentially linked to the initiation of superior tumor-targeting immune responses. Irrespective of this latter possibility (which we are actively investigating), our data strongly suggest that RT should be delivered before, not after, palbociclib to achieve superior disease control. These findings are in line not only with previous results from human glioblastoma and atypical-teratoid rhabdoid tumor (ATRT) cell lines, cultured in vitro and xenografted in immunodeficient mice,Citation23 but also with the notion that RT is preferentially active in the G2/M phase of the cell cycle, and hence that palbociclib may limit the fraction of cells responding to RT if administered beforehand. Consistent with this, it has recently been shown that CDK4/6 inhibitors should be delivered after, not before, DNA-damaging chemotherapeutics (which preferentially target cells in S and G2/M) to achieve an optimal control of cultured pancreatic ductal adenocarcinoma (PDAC) cells and an immunocompetent model of PDAC driven by Kras and Cdkn2a mutations.Citation24 Although also in this setting the contribution of the host immune system to treatment efficacy was not mechanistically dissected, various DNA-damaging chemotherapeutics resemble RT in promoting a variant of cell death that elicits robust immunostimulatory effects that can be accompanied by compensatory tumor infiltration by TREG cells,Citation3,Citation16,Citation25 and hence may set the stage for optimal immunostimulation by CDK4/6 inhibitors. Further supporting this possibility, multiple CDK4/6 inhibitors have been shown to cooperate with immunostimulatory targeted anticancer agents including MEK and PI3K inhibitors in preclinical models of lung and pancreatic cancer.Citation26
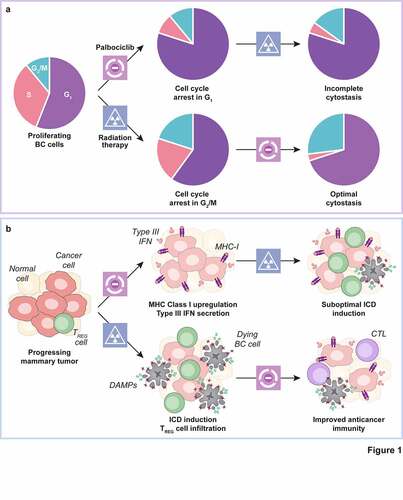
Our findings inspired the design of a Phase II clinical trial (CIMER, NCT04563507) that randomizes patients with newly diagnosed oligometastatic ER+ BC (up to 5 metastases) to either palbociclib plus letrozole or palbociclib plus letrozole preceded by stereotactic body RT to each metastatic lesion. As the CIMER protocol involves the collection of tumor biopsies at baseline and at progression, it will be interesting not only to see whether the addition of RT improves disease control by palbociclib plus letrozole, but also to investigate the immunological correlates of progression in both treatment arms. Irrespective of these impending studies, data from us and others provide solid preclinical grounds to harnessing immunostimulatory regimens such as RT as tools for improving the clinical efficacy of CDK4/6 inhibitors in patients with metastatic ER+ BC, as they highlight treatment schedule as a potential key determinant of therapeutic success ().
Figure 1. Impact of treatment schedule on the efficacy of radiation therapy combined with CDK4/6 inhibitors. a. Palbociclib administration arrests breast cancer (BC) cells in the G1 phase of the cell cycle, resulting in the generation of at least some radioresistance upon the depletion of cells in G2/M (a). While palbociclib may per se mediate some immunostimulatory effects, including MHC Class I upregulation and the secretion of type III interferon (IFN), the virtually pure cytostasis imposed by palbociclib may prevent the initiation of optimal anticancer immunity upon irradiation, which is at least partially dependent on immunogenic cell death (ICD) (b). Radiation therapy (RT) blocks BC cells in G2/M (a), a process that (1) at least in some cells is leaky, meaning that such cells can complete mitosis and resume cycling at G1, and (2) is associated with at least some degree of ICD-driven immunostimulation coupled to compensatory tumor infiltration by regulatory T (TREG) cells. In this context, the administration of palbociclibafter RT not only mediates superior cytostasis as it arrests BC cells potentially escaping the RT-induced arrest in G2/M, but may also boost anticancer immunity elicited by ICD as a consequence of TREG cell depletion (b). CTL, cytotoxic T lymphocyte; DAMP, damage-associated molecular pattern
Competing interests
GP has no conflicts of interest to declare. LG reports research funding from Lytix, and Phosplatin (completed), consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, The Longevity Labs, Inzen, and the Luke Heller TECPR2 Foundation.
Acknowledgments
The LG lab is supported by a Breakthrough Level 2 grant from the US Department of Defense (DoD), Breast Cancer Research Program (BRCP) (#BC180476P1), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukemia and Lymphoma Society (LLS), by a startup grant from the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US), by a Rapid Response Grant from the Functional Genomics Initiative (New York, US), by industrial collaborations with Lytix (Oslo, Norway) and Phosplatin (New York, US), and by donations from Phosplatin (New York, US), the Luke Heller TECPR2 Foundation (Boston, US) and Sotio a.s. (Prague, Czech Republic).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019, 69(1):7–4. doi:10.3322/caac.21551.
- Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT, Hurria A, Openshaw TH, Krop IE. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: American society of clinical oncology endorsement of cancer care ontario guideline recommendations. J Clin Oncol. 2016;34(19):2303–2311. doi:10.1200/JCO.2015.65.8609.
- Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. 2020. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 17:725–741.
- Hart CD, Migliaccio I, Malorni L, Guarducci C, Biganzoli L, Di Leo A. 2015. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin Oncol. 12:541–552.
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al. 2009. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11:R77.
- O’Leary B, Finn RS, Turner NC. 2016. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 13:417–430.
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, et al. 2020. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 382:514–524.
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. 2018. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 379:1926–1936.
- Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, et al. 2009. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res. 15:1046–1051.
- Kotsias F, Cebrian I, Alloatti A. 2019. Antigen processing and presentation. Int Rev Cell Mol Biol. 348:69–121.
- Petroni G, Formenti SC, Chen-Kiang S, Galluzzi L. Immunomodulation by anticancer cell cycle inhibitors. Nat Rev Immunol. 2020;20(11):669–679. doi:10.1038/s41577-020-0300-y.
- Ameratunga M, Kipps E, Okines AFC, Lopez JS. To cycle or fight-CDK4/6 inhibitors at the crossroads of anticancer immunity. Clin Cancer Res. 2019;25(1):21–28. doi:10.1158/1078-0432.CCR-18-1999.
- Chiron D, Martin P, Di Liberto M, Huang X, Ely S, Lannutti BJ, Leonard JP, Mason CE, Chen-Kiang S. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3Kδ inhibition through PIK3IP1. Cell Cycle. 2013;12(12):1892–1900. doi:10.4161/cc.24928.
- Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7(11):861–869. doi:10.1038/nrc2248.
- Yamazaki T, Kirchmair A, Sato A, Buque A, Rybstein M, Petroni G, Bloy N, Finotello F, Stafford L, Navarro Manzano E, et al. 2020. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 21(10):1160–1171. doi:10.1038/s41590-020-0751-0.
- Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21(2):120–134. doi:10.1038/s41590-019-0561-4.
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5(1):13. doi:10.1038/s41572-019-0064-5.
- Beddok A, Xu HP, Henry AA, Porte B, Fourquet A, Cottu P, Kirova Y. Concurrent use of palbociclib and radiation therapy: single-centre experience and review of the literature. Br J Cancer. 2020;123(6):905–908. doi:10.1038/s41416-020-0957-9.
- Petroni G, Buque A, Yamazaki T, Bloy N, Di Liberto M, Chen-Kiang S, Formenti SC, Galluzzi L. 2021. Radiotherapy delivered before CDK4/6 inhibitors mediates superior therapeutic effects in ER+ breast cancer. Clin Cancer Res. clincanres.3871.2020. doi:10.1158/1078-0432.CCR-20-3871.
- Naz S, Sowers A, Choudhuri R, Wissler M, Gamson J, Mathias A, Cook JA, Mitchell JB. Abemaciclib, a Selective CDK4/6 Inhibitor, enhances the radiosensitivity of non–small cell lung cancer in vitro and in vivo. Clin Cancer Res. 2018;24(16):3994–4005. doi:10.1158/1078-0432.CCR-17-3575.
- Buque A, Bloy N, Perez-Lanzon M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A, et al. 2020. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 11(1):3819. doi:10.1038/s41467-020-17644-0.
- Abba MC, Zhong Y, Lee J, Kil H, Lu Y, Takata Y, Simper MS, Gaddis S, Shen J, Aldaz CM. DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations. Oncotarget. 2016;7(39):64289–64299. doi:10.18632/oncotarget.11733.
- Hashizume R, Zhang A, Mueller S, Prados MD, Lulla RR, Goldman S, Saratsis AM, Mazar AP, Stegh AH, Cheng SY, et al. 2016. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 18(11):1519–1528. doi:10.1093/neuonc/now106.
- Salvador-Barbero B, Alvarez-Fernandez M, Zapatero-Solana E, El Bakkali A, Menendez MDC, Lopez-Casas PP, Di Domenico T, Xie T, VanArsdale T, Shields DJ, et al. 2020. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer Cell. 37(3):340–53 e6. doi:10.1016/j.ccell.2020.01.007.
- Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. 2013. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 210:2435–2466.
- Petroni G, Buque A, Zitvogel L, Kroemer G, Galluzzi L. 2020. Immunomodulation by targeted anticancer agents. Cancer Cell.
