ABSTRACT
The potential for durvalumab, a programmed cell death ligand-1 (PD-L1)-blocking monoclonal antibody, to treat head and neck squamous cell carcinoma (HNSCC) is being evaluated in multiple clinical trials. We assessed circulating proteins at baseline to identify potential biomarkers and to understand pathways related to clinical outcomes for durvalumab. Prior to treatment, 66 serum proteins were measured using multiplex immunoassays for 158 durvalumab-treated HNSCC patients in the phase II HAWK and CONDOR trials as a discovery dataset and 209 durvalumab-treated HNSCC patients in the phase III EAGLE trial as a validation dataset. Multivariate Cox modeling of HAWK and CONDOR datasets established that higher baseline concentrations of interleukin-6 (IL-6), C-reactive protein, S100 calcium-binding protein A12, and angiopoietin-2 (ANGPT2) were associated with shorter overall survival (OS), while higher concentrations of osteocalcin correlated with longer OS after durvalumab treatment (p < .05). All five proteins remained significantly correlated with OS after adjusting for baseline clinical factors, with consistent results across clinical efficacy endpoints based on univariate correlation analyses. The validation dataset from the EAGLE trial confirmed the independent association of IL-6 and osteocalcin with OS, and preserved directional trends for the other biomarkers identified in the discovery dataset. Our results demonstrate the important role of immunosuppressive proteins in the resistance of HNSCC to durvalumab treatment. Osteocalcin showed a positive correlation with clinical outcomes, which remains to be further investigated.
Introduction
Heterogeneity of tumor cell types and the aptitude of the immune surveillance system to recognize and eradicate cancer cells within a constantly evolving tumor microenvironment are established hallmarks of cancer immunotherapy.Citation1,Citation2 Patients who receive immunotherapy under current paradigms respond with high variability due to various underlying mechanisms of immune resistance. To achieve the full benefit of cancer immunotherapy, the intricate interplays between molecular and cellular moieties at the site of action, the disrupted homeostasis within local regions of the tumor, and the result of these disturbances (as reflected in the systemic circulation) need to be better understood.Citation3 Tumor growth and disease progression rely on both the intrinsic makeup of tumor cells and the tumor-induced systemic factors that affect immune cells in the primary tumor and the distant microenvironment.Citation4,Citation5
Programmed cell death ligand-1 (PD-L1) protein expression on tumor cells (TCs) and immune cells has emerged as the first predictive biomarker for sensitivity to anti–programmed cell death-1 (PD-1)/PD-L1 agents. While companion/complementary diagnostic testing for PD-L1 has been approved across 15 tumor types, PD-L1 positivity only predicted response in less than 30% of clinical studies.Citation6 The tumor and its microenvironment are difficult to access and neither lymph node nor tumor biopsies provide sufficiently robust quantitative data to determine the relative role of each factor on clinical outcomes. On the other hand, soluble biomarkers such as plasma circulating tumor DNA are easily accessible and can be measured accurately and reproducibly. They may provide new insights into the mechanism of action of cancer immunotherapies and can potentially be used as prognostic and/or predictive biomarkers for the optimization of oncology trial designs and precision medicine.Citation7 Peripheral protein biomarker profiling provides an opportunity to evaluate tumor-secreted factors and responsive systemic factors that are associated with clinical response following immunotherapy.Citation8 Numerous circulating biomarkers have shown prognostic value for many tumor types, and they have demonstrated value in deciphering efficacy outcomes based on several exploratory analyses from clinical data following immune checkpoint inhibitors (ICIs).Citation9–12 However, a comprehensive evaluation of the association between serum protein biomarkers and clinical response after PD-1/PD-L1 blockade therapy is lacking, especially for head and neck squamous cell carcinoma (HNSCC).
HNSCC arises in the mucosal linings of the upper aerodigestive tract and is remarkably heterogeneous in nature. Alcohol and tobacco use, along with human papillomavirus (HPV) infection, are well-known causative factors for HNSCC. It ranks sixth in incidence and eighth in mortality among all cancers. The prognosis for patients with HNSCC remains poor, with a 5-year survival rate of less than 50%.Citation13 ICIs offer a potentially new approach for the treatment of HNSCC and have demonstrated durable responses in these patients.Citation14 However, over 80% of patients with metastatic HNSCC do not respond to PD-1 blockade. For nonresponders, there are two possible ways to improve outcomes: rational combination approaches and improved biomarkers to inform patient selection.Citation15 Serum protein characterization may help identify clinical responders or rapid progressors. This will aid in understanding resistance mechanisms and in finding combination approaches to improve patient outcomes.Citation16
Here, we applied a robust statistical analysis to quantify the association of a panel of serum protein biomarkers with clinical outcomes following treatment with durvalumab, an anti–PD-L1 monoclonal antibody, in HNSCC. Baseline serum protein measurements from patients enrolled in three clinical trials of durvalumab in HNSCCCitation17-19 were analyzed together with clinical and recognized prognostic factors to characterize potential predictive biomarkers and inform rational combination therapies for HNSCC.
Methods
Study design and clinical outcomes
Three clinical trials investigating durvalumab in recurrent/metastatic (R/M) HNSCC were included in this analysis. HAWK was a phase II, single-arm study of durvalumab in patients with R/M HNSCC and tumor PD-L1 expression ≥25%.Citation17 CONDOR was a randomized, phase II study comparing durvalumab, tremelimumab, and the combination of durvalumab and tremelimumab in patients with R/M HNSCC and tumor PD-L1 expression <25%.Citation18 EAGLE was a randomized, open-label, phase III study comparing durvalumab, with or without tremelimumab, to standard-of-care (SoC) chemotherapy in patients with R/M HNSCC.Citation19 In all three trials, patients in the durvalumab monotherapy arm received the drug at 10 mg/kg intravenously every 2 weeks. Best overall response (BOR) was defined per Response Evaluation Criteria in Solid Tumors as progressive disease (PD), stable disease (SD), partial response (PR), or complete response (CR). The study protocols were approved by ethics committees at each participating site and each study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided informed consent to participate in the studies.
Only durvalumab monotherapy data were considered in this work. The EAGLE trial did not retain sufficient baseline samples in the SoC cohort of patients to generate a meaningful dataset of circulating biomarker results (<5 patients). Tremelimumab data were not considered in this work since this treatment was not evaluated in HAWK and a separate analysis would be needed due to the non-overlapping mechanism of action of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and PD-L1 inhibition.
Serum protein profiling
Prior to the start of durvalumab treatment, baseline serum samples were collected from patients with HNSCC enrolled in the three clinical trials. Samples were stored under controlled conditions when shipped to Myriad RBM (Austin, Texas, USA) for multiplex immunoassay testing, based on Luminex xMAP technology. Sixty-six cancer and immune-associated serum proteins were selected for measurement, which included chemokines, cytokines, adhesion molecules, and growth factors. Samples were processed, analyzed, and stored in accordance with standard operating procedures defined by the laboratory vendor. The distribution of all measured serum proteins is summarized with mean ± standard deviation in Supplementary Table S1.
Statistical analysis
Pooled data from the HAWK and CONDOR studies were used as a discovery dataset to identify potential associations among overall survival (OS), protein biomarkers, and various clinicopathological factors. Stepwise feature selection followed by multivariate Cox semiparametric regression was conducted to derive the survival hazard ratio (HR) of circulating biomarkers along with clinicopathological factors at baseline. Dichotomization of continuous variables was performed using the median as a cutoff for the “high” or “low” category prior to association with the OS time-to-event data. For both the continuous and categorical variables, a proportional HR was assumed between categories rather than estimating the hazard function, and log-rank tests were performed. A univariate survival analysis was conducted simultaneously by utilizing a Kaplan–Meier nonparametric test to corroborate Cox modeling results and to identify additional protein biomarkers involved in the same pathophysiological pathways. Furthermore, BOR association was assessed by comparing protein concentrations among patients with PD, SD, and clinical response (PR or CR) utilizing the Kruskal–Wallis H test followed by the Mann–Whitney U test.
In the validation step, the EAGLE trial dataset was used independently to confirm the results of the statistical analysis conducted on the HAWK and CONDOR discovery dataset. Cox modeling, Kaplan–Meier survival analysis, and the H and U tests of BOR correlation were used to assess the association of EAGLE trial outcomes with biomarkers identified in the discovery step.
Results
Characterization of durvalumab-treated HNSCC patients
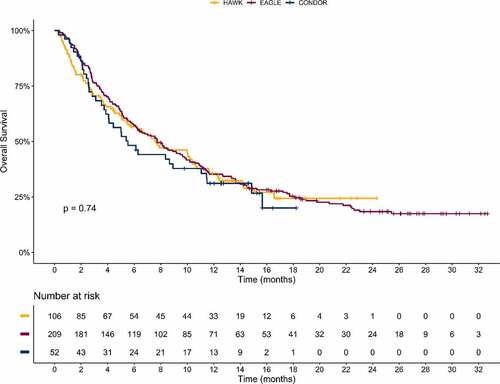
Among patients who received durvalumab monotherapy, median OS was 7.1 months (95% confidence interval [CI], 4.9–9.9) in the HAWK study (111 patients) and was 6.0 months (95% CI, 4.0–11.3 months) in the CONDOR study (65 patients). In the EAGLE trial, median OS was 7.6 months (95% CI, 6.1–9.8 months) among 237 durvalumab-treated patients. Among the 111 patients from HAWK, 106 had evaluable biomarker data, with a slightly higher proportion of missing biomarker data in CONDOR (52 patients out of 65) and EAGLE (209 patients out of 237). A log-rank test was performed and showed that OS for durvalumab-treated patients with evaluable biomarker data was similar between the three trials with no statistical difference (p = .74) ().
Figure 1. Kaplan–Meier survival curves of patients with HNSCC who received durvalumab and had evaluable biomarker data at baseline in HAWK (n = 106), CONDOR (n = 52), and EAGLE (n = 209) clinical trials. The p value obtained from log-rank test is p = .74
Baseline standard laboratory data with clinical and traditional prognostic factors for survival and clinical response analysis are summarized in . Overall, mean baseline characteristics were similar between the combined HAWK and CONDOR discovery datasets and the EAGLE validation dataset. Most patients were male, with a mean age of approximately 60 years. Approximately two-thirds of patients had a history of smoking and a similar proportion of patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Tumor size was consistent across trials with 73.1 ± 43.6 mm for HAWK and CONDOR and 67.5 ± 46.1 mm for EAGLE. Albumin, lactate dehydrogenase, and neutrophil counts were typical of the HNSCC patient population with signs of inflammation and high catabolic activity.
Table 1. Covariate distribution of patients with HNSCC at baseline split by study and dataset (discovery and validation, respectively)
PD-L1 expression status on TCs and tumor-infiltrating immune cells has been approved as a companion diagnostic for selecting patients with HNSCC for treatment with an anti–PD-1 agent.Citation20 The HAWK trial enrolled PD-L1–high patients,Citation17 while only PD-L1–low/negative patients were eligible for the CONDOR trial.Citation18 The PD-L1 TC score was markedly higher in the HAWK dataset (63.1 ± 26.2%) compared with the CONDOR dataset (3.3 ± 5.5%). The EAGLE studyCitation19 enrolled both patients with high – and low–PD-L1 expression with a TC score of 19.6 ± 29%. Due to the high imbalance in PD-L1 TC score, we did not include PD-L1 status in our integrated analysis of the three HNSCC clinical trials. An independent analysis of data from EAGLE with PD-L1 status and additional biomarker data is ongoing and will be reported separately.
Multivariate Cox modeling of protein biomarkers for the discovery HAWK and CONDOR dataset
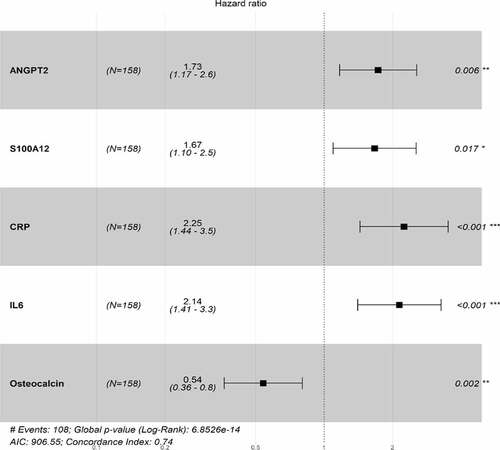
Most protein concentrations at baseline were comparable among the three trials (Supplementary Table S1), and were elevated compared with healthy controls based on published observational data.Citation21,Citation22 Stepwise feature selection and multivariable Cox modeling identified five proteins that were independently associated with OS based on the HAWK and CONDOR datasets (). These included three proinflammatory and immunosuppressive proteins (C-reactive protein [CRP], interleukin-6 [IL-6], S100 calcium-binding protein A12 [S100A12]) and an angiogenic protein [angiopoietin-2 (ANGPT2)]. High CRP, IL-6, and S100A12 protein levels were associated with a higher HR compared with their lower level categories based on median cutoff, with an HR of 2.25 (95% CI, 1.44–3.5), 2.14 (95% CI, 1.41–3.3), and 1.67 (95% CI, 1.1–2.5), respectively. Of note, the Spearman’s correlation coefficient for IL-6 and CRP was 0.64. High ANGPT2 protein levels were associated with shorter OS, with an HR of 1.73 (95% CI, 1.17–2.6) when compared with lower levels of ANGPT2. High osteocalcin hormone levels were associated with longer OS, with an HR of 0.54 (95% CI, 0.3–0.8) compared with low levels of osteocalcin.
Figure 2. Correlation of angiogenic and immunomodulatory proteins with OS of durvalumab-treated HNSCC patients in HAWK and CONDOR trials. Stepwise feature selection and Cox proportional hazards modeling identified independent association of baseline concentrations of an angiogenic biomarker (ANGPT2), three immunosuppressive proteins (IL-6, CRP, S100A12), and an immunostimulatory hormone (osteocalcin) with OS in 158 patients with HNSCC receiving durvalumab. Only statistically significant factors are presented with p values shown on the right side of the HR forest plot. Global p value from the log-rank test, Akaike Information Criteria (AIC), and concordance index of the model are shown at the bottom of the plot
Various laboratory measurements, demographics, and clinical factors have been reported to be prognostic factors in patients with HNSCC.Citation23 Using stepwise Cox modeling, we evaluated the association of OS with age, gender, smoking history, extent of HPV status, ECOG status, tumor size, albumin levels, hemoglobin levels, lactate dehydrogenase levels, neutrophil counts, lymphocyte counts, and platelet counts of HNSCC patients enrolled in the HAWK and CONDOR trials. Three baseline clinical factors (ECOG status, tumor size, and neutrophil counts) were found to be independently associated with OS among durvalumab-treated patients. Although HPV is a well-known prognostic factor for HNSCC, the virus status was not associated with OS in our multivariate model. Kaplan–Meier survival analysis also indicated the lack of association between HPV status and OS (p = .35).
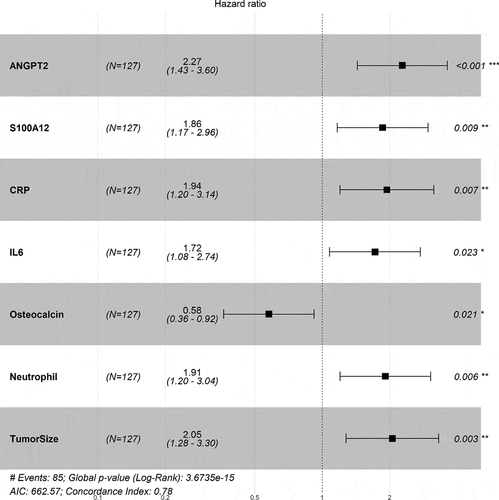
To evaluate the relative contributions of known prognostic factors and novel protein biomarkers to patient survival, we repeated the Cox modeling with the three clinical factors and five protein biomarkers indicated previously. Importantly, after stepwise selection, all five protein biomarkers remained significantly correlated with OS (p < .05) and with the same directional trend after adjusting for the clinical factors (). Of note, ECOG status was removed from the final Cox model, suggesting redundant informative value.
Figure 3. Significant association of protein biomarkers with OS maintained after adjusting for clinical factors in HAWK and CONDOR dataset. Forest plot of HRs is shown with individual p values on the right side. Global p values, AIC, and concordance index are shown at the bottom
Osteocalcin is a versatile, bone-derived, immunostimulatory hormone,Citation24 and bone is one of the most common sites of distant metastases from HNSCC.Citation25 Therefore, using osteocalcin as a marker, we sought to determine whether bone metastasis underlies the relationship between osteocalcin and OS. Thirty-five patients demonstrated evidence of bone metastases in our dataset, and Cox modeling indicated that the survival association of osteocalcin was independent of bone metastasis. High osteocalcin levels at baseline were significantly associated with favorable OS (p = .005), while bone metastasis was not associated with favorable OS in the Cox model (Supplementary Figure S1).
Kaplan–Meier survival and response analysis of protein biomarkers for the discovery HAWK and CONDOR dataset
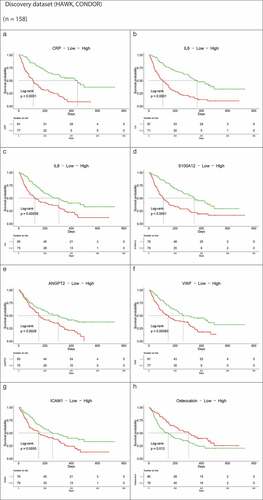
In addition to Cox modeling, we applied a univariate Kaplan–Meier survival analysis to evaluate all the protein biomarkers for their association with OS. Consistent with multivariate analysis results, higher levels of CRP, IL-6, S100A12, and ANGPT2 correlated with poorer survival. Interestingly, more immunosuppressive and angiogenic proteins, including IL-8, von Willebrand factor (vWF), and vascular endothelial growth factor (VEGF) demonstrated similar patterns with a significant association between higher baseline protein concentrations and shorter OS. A higher level of intercellular adhesion molecule-1 (ICAM-1), an endothelial protein involved in leukocyte migration from the vascular wall to tissues upon cytokine stimulation, was also associated with shorter OS. In contrast, higher osteocalcin levels correlated with longer OS ()).
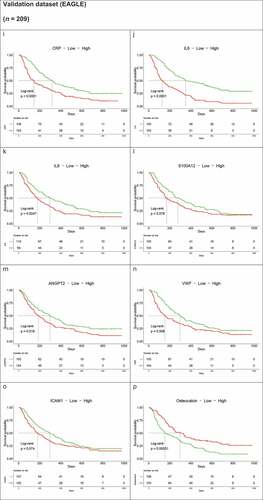
Figure 4. Kaplan–Meier OS curves of immunomodulatory and angiogenic protein concentrations above or below median at baseline for the HAWK and CONDOR discovery dataset (a–h). (b) Kaplan–Meier OS curves of immunomodulatory and angiogenic protein concentrations above or below median at baseline for the EAGLE validation dataset (i–p)
Comparison of biomarker concentrations as continuous variables among patients with different response rates provides another way to evaluate the impact of biomarkers on disease progression or clinical responses after treatment. A Mann–Whitney U test indicated that IL-6, CRP, S100A12, IL-8, ICAM-1, ANGPT2, vWF, and VEGF concentrations were higher in patients with PD than in those with SD or an objective response (PR/CR), while osteocalcin levels were higher in patients who achieved an objective response than in patients with a BOR of PD or SD (Supplementary Figure S2). A similar analysis confirmed the correlation of circulating proteins at baseline with progression-free survival (PFS; data not shown).
Validation with the EAGLE dataset
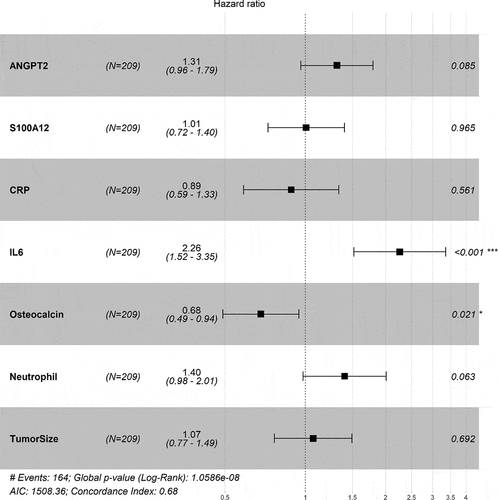
To validate our discovery results, we measured the same set of proteins at baseline for durvalumab-treated patients with HNSCC enrolled in the phase III EAGLE trial. Correlation matrix estimates of the discovery and validation datasets were broadly similar between baseline patient’s characteristics. The same set of immunosuppressive, proinflammatory, and angiogenic proteins showed a negative correlation with OS, while osteocalcin demonstrated a positive correlation with OS among durvalumab-treated patients based on a Kaplan–Meier survival analysis (). The same pattern was also observed for the correlation between baseline protein levels and objective response rate (Supplementary Figure S2) and PFS (data not shown). Cox proportional hazards modeling indicated an independent association of two proteins (IL-6 and osteocalcin) with OS of durvalumab-treated patients in the EAGLE trial () and the same directional trends for ANGPT2 and neutrophils as in the discovery dataset but without reaching statistical significance (p > .05). The proinflammatory and immunosuppressive protein IL-6 demonstrated a 2.26-fold hazard for patients with higher concentrations compared with lower concentrations (95% CI, 1.52–3.35), whereas higher osteocalcin levels continued to show significant correlation with OS benefit with a 32% lower risk (95% CI, 0.49–0.94; p = .021). ANGPT2 showed a directionally consistent trend (high levels at baseline were associated with a 1.31-fold hazard [95% CI, 0.96–1.79] compared with low levels) with a p value of 0.085. Neutrophil counts were negatively correlated with OS, with an HR of 1.40 (95% CI, 0.98–2.01) for above versus below the median (p = .063). CRP and S100A12 belong to the same proinflammatory and immunosuppressive pathway as IL-6. Their correlation with OS no longer held in the multivariable model.
Discussion
In this study, we applied a stepwise modeling strategy to analyze circulating protein biomarkers for their association with OS and BOR in patients with HNSCC receiving durvalumab treatment. Similar results were found for PFS correlation (data not shown). Our results demonstrated differential contributions of proinflammatory and immunosuppressive cytokines and an immunostimulatory hormone to the clinical outcomes after PD-L1 blockade in patients with HNSCC, while angiogenic proteins such as ANGPT2 appear to play an important roleCitation26 despite not reaching statistical significance in the validation set. The first pathway was shown to be statistically associated with OS in both our discovery and validation dataset: a proinflammatory and immunosuppressive pathway represented by IL-6,Citation27,Citation28 Other proinflammatory biomarkers, such as CRP and S100A12 were found statistically associated with OS in the training dataset but findings were not confirmed in the validation step. Univariate Kaplan–Meier survival analysis and BOR analysis support the association of durvalumab outcomes with additional protein biomarkers, such as IL-8.
Circulating IL-6 and CRP levels have been correlated with poorer outcomes in patients with urothelial bladder cancer, metastatic triple-negative breast cancer, and melanoma treated with anti–PD-1/PD-L1 agents.Citation29–31 Increased on-treatment IL-6 and CRP levels were also associated with shorter survival of nivolumab or ipilimumab-treated melanoma patients.Citation32 IL-6 is the main inducer of CRP production, and their serum levels are closely correlated in different cancer types but the results of this study points to a direct role of IL-6 circulating levels as a prognostic marker of OS, with high levels associated with poor outcomes. IL-6 promotes tumor growth and metastatic potential in multiple ways, from modification of T cell and dendritic cell functionCitation33,Citation34 to induction of expression of angiogenic molecules, such as VEGF, that promotes the growth, survival, migration, and invasion of cancer cells.Citation35 Although IL-6 and CRP are not predictive biomarkers specific to immunotherapy,Citation32 blockage of IL-6 and CRP synthesis and/or activity may enhance responses to ICIs in patients with different cancers, including HNSCC. Preclinical results have demonstrated that combined blockade of IL-6 and PD-1/PD-L1 signaling abrogated mutual regulation of their immunosuppressive effects in the tumor microenvironment and exerted a synergistic antitumor effect.Citation16,Citation36 Recently, clinical trials combining either anti-IL-6 or anti-IL-6 receptor with anti-PD-(L)1 have started or are being initiated for patients with cancer (NCT03999749, NCT04258150, and NCT03821246). Interestingly, the clinical relevance of circulating IL-6 levels with OS appears independent of the prognostic role of high neutrophils at baseline that did not reach statistical significance in the multivariate model derived from the validation dataset.
Angiogenesis has increasingly been considered as an immune modulator with the potential for combinatorial use with ICIs. In our analysis, an angiogenic pathway denoted by ANGPT2Citation26 was found to be significantly correlated with OS in the discovery dataset on top of IL-6, and showed a similar trend in the validation dataset with a p-value of 0.085. The important role of pro-angiogenesis markers in the resistance of durvalumab treatment is supported by the combination strategy of anti-angiogenic and ICI treatment that has demonstrated more potent antitumor effects and favorable clinical outcomes in multiple tumor types.Citation37–41 Recently, a VEGF-A targeting antibody, bevacizumab, has been approved in combination with an anti–PD-L1 antibody, atezolizumab, for the treatment of metastatic non-squamous non-small-cell lung carcinoma and unresectable hepatocellular carcinoma.Citation42
ANGPT2 is a multifaceted cytokine that functions in both angiogenesis and inflammation.Citation43 It is a target of active immunotherapy and involved in the resistance to anti-VEGF treatment.Citation44 High circulating ANGPT2 concentrations at baseline and early increases of ANGPT2 during treatment has been shown to be a poor prognostic and predictive biomarker for PD-1 ICI-treated melanoma patients. The contribution of ANGPT2 to ICI resistance may be due to its role in the recruitment of monocytes/macrophages into the tumor microenvironment and PD-L1 induction in tumor-associated macrophages. ICIs also elicited humoral immune responses to ANGPT2, which were long lasting and robust in long-term melanoma survivors.Citation28 ANGPT2 antibodies have demonstrated antitumor activities in animal studies and clinical trials,Citation45–48 but none were approved for cancer treatment. The combination with ICIs may improve the efficacy of either treatment alone. Our results demonstrated the association of ANGPT2 with clinical outcomes in durvalumab-treated patients with HNSCC, suggesting potential benefits of combining ICIs with ANGPT2 blockade therapy for patients with HNSCC.
The third pathway involves the non-collagenous protein hormone osteocalcin, which is found in bone and dentin. The discovery and validation datasets indicated a positive association between higher baseline osteocalcin levels and favorable OS in durvalumab-treated patients with HNSCC. This relationship was found to be independent of the presence of bone metastasis, despite the known role of osteocalcin in bone homeostasis. Recently, osteocalcin has been reported to exert multiple antitumor effects through cellular immunostimulatory effects in melanoma.Citation49 Osteocalcin increased T cell proliferation and IFN production but had no effect on IL-6 production by splenocytes. To our knowledge, no prognostic significance has been reported for osteocalcin in HNSCC.Citation50 However, due to the limited samples available for biomarker study in the control arm of the EAGLE trial, the specificity and predictive value of osteocalcin in durvalumab-treated patients remain to be further explored in comparison with control samples.
One limitation of our study is the lack of control arm samples to compare the predictive value of protein biomarkers for ICIs with other treatment regimens. Many proteins included in this study are well-known prognostic biomarkers for various tumors including HNSCC.Citation51 Our results demonstrated the association of several prognostic biomarkers with clinical outcomes in the context of PD-L1 blockade, but the specificity of the biomarkers is unknown and the lack of biomarker data in the SoC cohort of patients did not allow us to disentangle the predictive or prognostic value of our findings. In our analysis, Cox regression was performed using the median baseline value of the biomarkers to dichotomize. The choice of this approach was governed by the exploratory nature of the analysis. A receiver-operating-characteristic analysis would allow a better determination of the cutoff value for patient selection and clinical utility but was outside the scope of this work. Another limitation is the exclusion of PD-L1 TC scores from our current analysis. The potential correlation between soluble protein biomarkers and PD-L1 status, along with their contribution to clinical outcomes after ICI treatment, is of significant interest. Unfortunately, PD-L1 staining results were not included in our analysis because of the high imbalance of PD-L1 scores between the three trials. Finally, to fully understand the intricate interrelationship among different pathophysiological pathways, tumor growth, and OS, we may need to apply novel analytical approaches beyond traditional survival analysis. To that end, tumor kinetic and OS modeling were applied using the same pooled dataset, including PD-L1 status, clinical factors, and protein biomarker levels treated as continuous variables.Citation52 Interestingly, it confirmed the negative association of OS with IL-6 and the positive association with osteocalcin among other pro-angiogenic proteins.Citation52 To gain additional insights, we are conducting a comprehensive analysis of the EAGLE dataset in conjunction with data from the ongoing phase III trial, KESTREL (NCT02551159), which is investigating durvalumab with or without tremelimumab versus SoC in patients who have not received prior systemic chemotherapy for R/M HNSCC.
In conclusion, circulating IL-6 and osteocalcin concentrations at baseline have been shown to be independently associated with clinical outcomes with trends of additional effects for ANGPT2 in durvalumab-treated patients with HNSCC across three clinical trials. Measurements of these biomarkers before and after treatment should be prioritized in clinical trials of ICI monotherapy and combination therapy. These areas of research warrant further investigation for the potential enhancement of precision medicine and patient outcomes.
Declaration of interest statement
R. H. Arends, J. Xie, I. González-García, N. Morsli, and A. Yovine are employees of AstraZeneca. X. Guo, P.G. Baverel, and L.K. Roskos are former employees of AstraZeneca.
Data sharing
Data reported in this article may be obtained in accordance with AstraZeneca’s data sharing policy available at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplemental Material
Download ()Acknowledgments
The contents of this paper were partially presented by X. Guo et al. at the ASCO 2019 Annual Meeting (May 31–June 4, 2019). The CONDOR, HAWK, and EAGLE studies were sponsored by AstraZeneca. The authors would like to thank the patients, their families and caregivers, and all investigators involved in this study. Editorial assistance, in accordance with Good Publication Practice (GPP3) guidelines, was provided by Parexel (Hackensack, NJ) and was funded by AstraZeneca.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Fleuren GJ, Gorter A, Kuppen PJ, Litvinov S, Warnaar SO. Tumor heterogeneity and immunotherapy of cancer. Immunol Rev. 1995;145:91–11. doi:10.1111/j.1600-065x.1995.tb00078.x.
- Williams JB, Li S, Higgs EF, Cabanov A, Wang X, Huang H, Gajewski TF. Tumor heterogeneity and clonal cooperation influence the immune selection of IFN-gamma-signaling mutant cancer cells. Nat Commun. 2020;11(1):602. doi:10.1038/s41467-020-14290-4.
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. doi:10.1038/s41568-019-0116-x.
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi:10.1016/j.ccr.2012.02.022.
- Zhang H, Lyden D. Bone voyage-osteoblasts remotely control tumors. Science. 2017;358(6367):1127–1128. doi:10.1126/science.aar2640.
- Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi:10.1186/s40425-019-0768-9.
- Lee EY, Kulkarni RP. Circulating biomarkers predictive of tumor response to cancer immunotherapy. Expert Rev Mol Diagn. 2019;19(10):895–904. doi:10.1080/14737159.2019.1659728.
- Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, Fleener C. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer. 2019;7(1):325. doi:10.1186/s40425-019-0799-2.
- Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114(3):256–261. doi:10.1038/bjc.2015.467.
- Koguchi Y, Hoen HM, Bambina SA, Rynning MD, Fuerstenberg RK, Curti BD, Urba WJ, Milburn C, Bahjat FR, Korman AJ, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res. 2015;75(23):5084–5092. doi:10.1158/0008-5472.CAN-15-2303.
- Boutsikou E, Domvri K, Hardavella G, Tsiouda D, Zarogoulidis K, Kontakiotis T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238. doi:10.1177/1758835918768238.
- Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Onate C, Perez G, Alfaro C, Martin-Algarra S, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28(8):1988–1995. doi:10.1093/annonc/mdx190.
- Du E, Mazul AL, Farquhar D, Brennan P, Anantharaman D, Abedi-Ardekani B, Weissler MC, Hayes DN, Olshan AF, Zevallos JP. Long-term survival in head and neck cancer: impact of site, stage, smoking, and human papillomavirus status. Laryngoscope. 2019;129(11):2506–2513. doi:10.1002/lary.27807.
- Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184. doi:10.1186/s40425-019-0662-5.
- Bauml JM, Aggarwal C, Cohen RB. Immunotherapy for head and neck cancer: where are we now and where are we going? Ann Transl Med. 2019;7(Suppl3):S75. doi:10.21037/atm.2019.03.58.
- Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi:10.1158/0008-5472.CAN-18-0118.
- Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, Ready NE, Clement PM, Even C, Jang RW, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–152. doi:10.1016/j.ejca.2018.11.015.
- Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, Krauss J, Saba NF, Nabell L, Ready NE, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195–203. doi:10.1001/jamaoncol.2018.4628.
- Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–950. doi:10.1016/j.annonc.2020.04.001.
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, De Castro G Jr., Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi:10.1016/s0140-6736(19)32591-7.
- Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi:10.1155/2013/434010.
- Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med. 2011;9:113. doi:10.1186/1479-5876-9-113.
- Cadoni G, Giraldi L, Petrelli L, Pandolfini M, Giuliani M, Paludetti G, Pastorino R, Leoncini E, Arzani D, Almadori G, et al. Prognostic factors in head and neck cancer: a 10-year retrospective analysis in a single-institution in Italy. Acta Otorhinolaryngol Ital. 2017;37(6):458–466. doi:10.14639/0392-100X-1246.
- Coleman RE, Mashiter G, Fogelman I, Whitaker KD, Caleffi M, Moss DW, Rubens RD. Osteocalcin: a potential marker of metastatic bone disease and response to treatment. Eur J Cancer Clin Oncol. 1988;24(7):1211–1217. doi:10.1016/0277-5379(88)90130-7.
- Grisanti S, Bianchi S, Locati LD, Triggiani L, Vecchio S, Bonetta A, Bergamini C, Conte P, Airoldi M, Merlano M, et al. Bone metastases from head and neck malignancies: prognostic factors and skeletal-related events. PLoS One. 2019;14(3):e0213934. doi:10.1371/journal.pone.0213934.
- Wu X, Giobbie-Hurder A, Liao X, Connelly C, Connolly EM, Li J, Manos MP, Lawrence D, McDermott D, Severgnini M, et al. Angiopoietin-2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol Res. 2017;5(1):17–28. doi:10.1158/2326-6066.CIR-16-0206.
- Tsai MS, Chen WC, Lu CH, Chen MF. The prognosis of head and neck squamous cell carcinoma related to immunosuppressive tumor microenvironment regulated by IL-6 signaling. Oral Oncol. 2019;91:47–55. doi:10.1016/j.oraloncology.2019.02.027.
- Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: the imbalance between phosphorylated STAT1 and phosphorylated STAT3. Oncoimmunology. 2013;2(12):e27215. doi:10.4161/onci.27215.
- Powles T, Nickles D, Van Allen E, Chappey C, Zou W, Kowanetz M, Kadel E, Denker M, Boyd Z, Vogelang N, et al. Immune biomarkers associated with clinical benefit from atezolizumab (MPDL3280a; anti-PD-L1) in advanced urothelial bladder cancer (UBC). J Immunother Cancer. 2015;3(S2):83. doi:10.1186/2051-1426-3-S2-P83.
- Li Y, Fassò M, Emens LA, Molinero L. Biomarkers of systemic inflammation associated to reduced clinical activity of atezolizumab monotherapy in patients with metastatic triple negative breast cancer. Cancer Res. 2019;79(S13):CT001. doi:10.1158/1538-7445.AM2019-CT001.
- Weber JS, Tang H, Hippeli L, Qian M, Wind-Rotolo M, Larkin JMG, Wolchok JD, Sznol M, Robert C, Woods DM, et al. Serum IL-6 and CRP as prognostic factors in melanoma patients receiving single agent and combination checkpoint inhibition. J Clin Oncol. 2019;37:S15. doi:10.1200/JCO.2019.37.15_suppl.100.
- Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, Weber J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1). doi:10.1136/jitc-2020-000842.
- Bergmann J, Muller M, Baumann N, Reichert M, Heneweer C, Bolik J, Lucke K, Gruber S, Carambia A, Boretius S, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology. 2017;65(1):89–103. doi:10.1002/hep.28874.
- Ohno Y, Toyoshima Y, Yurino H, Monma N, Xiang H, Sumida K, Kaneumi S, Terada S, Hashimoto S, Ikeo K, et al. Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci. 2017;108(10):1959–1966. doi:10.1111/cas.13330.
- Tawara K, Scott H, Emathinger J, Ide A, Fox R, Greiner D, LaJoie D, Hedeen D, Nandakumar M, Oler AJ, et al. Co-expression of VEGF and IL-6 family cytokines is associated with decreased survival in HER2 negative breast cancer patients: subtype-specific IL-6 family cytokine-mediated VEGF secretion. Transl Oncol. 2019;12(2):245–255. doi:10.1016/j.tranon.2018.10.004.
- Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486(2):239–244. doi:10.1016/j.bbrc.2017.02.128.
- Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15(5):310–324. doi:10.1038/nrclinonc.2018.9.
- Reck M, Shankar G, Lee A, Coleman S, McCleland M, Papadimitrakopoulou VA, Socinski MA, Sandler A. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med. 2020;14(2):125–136. doi:10.1080/17476348.2020.1701439.
- McGregor BA, McKay RR, Braun DA, Werner L, Gray K, Flaifel A, Signoretti S, Hirsch MS, Steinharter JA, Bakouny Z, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol. 2020;38(1):63–70. doi:10.1200/JCO.19.01882.
- Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer. 2020;30(1):139–143. doi:10.1136/ijgc-2019-000880.
- Zhang L, Chen Y, Li F, Bao L, Liu W. Atezolizumab and bevacizumab attenuate cisplatin resistant ovarian cancer cells progression synergistically via suppressing epithelial-mesenchymal transition. Front Immunol. 2019;10:867. doi:10.3389/fimmu.2019.00867.
- Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi:10.1016/j.ctrv.2020.102017.
- Scholz A, Plate KH, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347:45–51. doi:10.1111/nyas.12726.
- Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, Neuberg D, Mihm M, Hodi FS, Dranoff G. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70(24):10150–10160. doi:10.1158/0008-5472.CAN-10-1852.
- Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, Rasmussen E, Sun YN, Zhong D, Hwang YC, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27(21):3557–3565. doi:10.1200/JCO.2008.19.6683.
- Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, Herting F, Yu S, The HH, Martarello L, et al. Ang-2-VEGF-A crossmab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res. 2013;19(24):6730–6740. doi:10.1158/1078-0432.CCR-13-0081.
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. doi:10.1016/j.ccr.2011.02.005.
- Thomas M, Kienast Y, Scheuer W, Bahner M, Kaluza K, Gassner C, Herting F, Brinkmann U, Seeber S, Kavlie A, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared to pan-angiopoietin-1/-2 inhibitors. PLoS One. 2013;8(2):e54923. doi:10.1371/journal.pone.0054923.
- Hayashi Y, Kawakubo-Yasukochi T, Mizokami A, Hazekawa M, Yakura T, Naito M, Takeuchi H, Nakamura S, Hirata M. Uncarboxylated osteocalcin induces antitumor immunity against mouse melanoma cell growth. J Cancer. 2017;8(13):2478–2486. doi:10.7150/jca.18648.
- Valero C, Olmos JM, Rivera F, Hernandez JL, Vega ME, Macias JG. Osteoprotegerin and bone mass in squamous cell head and neck cancer patients. Calcif Tissue Int. 2006;78(6):343–347. doi:10.1007/s00223-005-0237-y.
- Schaaij-Visser TB, Brakenhoff RH, Leemans CR, Heck AJ, Slijper M. Protein biomarker discovery for head and neck cancer. J Proteomics. 2010;73(10):1790–1803. doi:10.1016/j.jprot.2010.01.013.
- Pierre V, Guo X, Gonzalez-Garcia I, Morsli N, Yovine AJ, Li W, Narwal R, Roskos L, Baverel P. Overall survival modeling and association with serum biomarkers in durvalumab-treated patients with head and neck cancer. J Clin Oncol. 2020;38(15Suppl). Abstract6549. doi:10.1200/JCO.2020.38.15_suppl.6549