ABSTRACT
Ipilimumab (IPI) can enhance immunity to the cancer-testis antigen NY-ESO-1. A clinical trial was designed to assess safety, immunogenicity, and clinical responses with IPI + NY-ESO-1 vaccines and effects on the tumor microenvironment (TME). Patients with measurable NY-ESO-1+ tumors were enrolled among three arms: A) IPI + NY-ESO-1 protein + poly-ICLC (pICLC) + incomplete Freund’s adjuvant (IFA); B) IPI + NY-ESO-1 overlapping long peptides (OLP) + pICLC + IFA; and C) IPI + NY-ESO-1 OLP + pICLC. Clinical responses were assessed by irRC. T cell and Ab responses were assessed by ex vivo IFN-gamma ELIspot and ELISA. Tumor biopsies pre- and post-treatment were evaluated for immune infiltrates. Eight patients were enrolled: 5, 2, and 1 in Arms A-C, respectively. There were no DLTs. Best clinical responses were SD (4) and PD (4). T-cell and antibody (Ab) responses to NY-ESO-1 were detected in 6 (75%) and 7 (88%) patients, respectively, and were associated with SD. The breadth of Ab responses was greater for patients with SD than PD (p = .036). For five patients evaluable in the TME, treatment was associated with increases in proliferating (Ki67+) CD8+ T cells and decreases in RORγt+ CD4+ T cells. T cell densities increased for those with SD. Detection of T cell responses to NY-ESO-1 ex vivo in most patients suggests that IPI may have enhanced those responses. Proliferating intratumoral CD8+ T cells increased after vaccination plus IPI suggesting favorable impact of IPI plus NY-ESO-1 vaccines on the TME.
List of Abbreviations: Ab = antibody; CTCAE = NCI Common Terminology Criteria for Adverse Events; DHFR/DHRP = dihydrofolate reductase; DLT = Dose-limiting toxicity; ELISA = enzyme-linked immunosorbent assay; IFA = incomplete Freund’s adjuvant (Montanide ISA-51); IFNγ = Interferon gamma; IPI = Ipilimumab; irRC = immune-related response criteria; mIFH = multispectral immunofluorescence histology; OLP = NY-ESO-1 overlapping long peptides; PBMC = peripheral blood mononuclear cells; PD = Progressive disease; pICLC = poly-ICLC (Hiltonol), a TLR3/MDA-5 agonist; RLT = Regimen-limiting Toxicity; ROI = regions of interest; RT = room temperature; SAE = serious adverse event; SD = stable disease; TEAE = treatment-emergent adverse events; TLR = toll-like receptor; TME = tumor microenvironment; TRAE = treatment-related adverse events.
Introduction
Ipilimumab, a monoclonal antibody (Ab) against CTLA-4, improves survival in patients with advanced and high-risk melanoma.Citation1–3 It also induces durable clinical responses in patients who progress on PD-1 antibody therapy,Citation4 and it improves clinical outcome when added to PD-1 blockade.Citation5 Its antitumor effect is believed to be mediated in part by amplifying T cell responses against tumor antigens. Thus, there is rationale for combining it with cancer vaccines. In murine models, CTLA-4 blockade has enhanced T cell responses to cancer vaccines and has enhanced tumor infiltration with immune cells and tumor control induced by vaccines.Citation6–8 However, adding ipilimumab to a short gp100 peptide vaccine in humans failed to enhance clinical benefit over ipilimumab alone.Citation3 That negative result may be attributable to the nature of the vaccine used in that trial, which only elicited CD8+ T cell responses to one epitope and lacked CD4+ T cell help. Ipilimumab’s antitumor effects appear to be mediated in large part through effects on CD4+ T cells.Citation9 Thus, vaccines that also induce CD4+ T cells as part of their response may benefit more from CTLA-4 blockade.
Cancer vaccines targeting the cancer-testis antigen NY-ESO-1 have induced integrated immune responses that include CD4+ and CD8+ T cells as well as antibody (Ab) responses.Citation10–12 Ipilimumab alone also enhances immune responses to NY-ESO-1Citation13,Citation14 and can induce cytotoxic NY-ESO-1-reactive CD4+ T cells.Citation15 An integrated Ab and T-cell response to NY-ESO-1 has also been associated with enhanced clinical benefit with ipilimumab therapy.Citation16 NY-ESO-1 is expressed in a range of cancers and may thus have broad relevance for cancer therapy, as evidenced by high clinical response rates to adoptive T cell therapy targeting NY-ESO-1 in patients with synovial cell sarcoma.Citation17 Similarly, CD8+ T lymphocytes specific for NY-ESO-1 from NY-ESO-1 seropositive patients can recognize NY-ESO-1+ melanoma cells in vitro. Thus, NY-ESO-1 may act as a tumor rejection antigen.
Vaccination with NY-ESO-1 protein or four overlapping long peptides (OLP) has been safe and immunogenic, when administered with adjuvants including an incomplete Freund’s adjuvant (IFA: Montanide ISA-51, Seppic) and a toll-like receptor (TLR) agonist.Citation10–12 Especially with IFA and the TLR3/MDA-5 agonist poly-ICLC (Hiltonol, Oncovir), vaccines with either NY-ESO-1 protein or OLP have induced integrated Ab, CD4+ and CD8+ T-cell responses.Citation10,Citation12,Citation18,Citation19 Both protein and OLP offer the potential to span multiple epitopes for T and B cells, across a wide range of HLA expression, permitting broad applicability.Citation10,Citation12,Citation18,Citation19 As monotherapy, ipilimumab induces objective clinical responses in a minority; thus, there is a rationale for its use with NY-ESO-1 vaccines. The present clinical trial was designed to evaluate the safety and immunogenicity of NY-ESO-1 vaccines (protein or OLP), with appropriate adjuvants, in combination with ipilimumab. The benefit of such combinations would be most significant if there were increases in both the circulating immune responses and in tumor-infiltrating lymphocytes. Thus, this trial incorporated pre-treatment and on-treatment tumor biopsies to evaluate changes in immune cell infiltrates in the tumor microenvironment.
Materials and methods
Clinical trial design and objectives: This was a phase I, open-label, non-randomized study. Patients were sequentially enrolled in Arms A, B, and C, alternating among treatment arms. The primary objectives were to evaluate (1) safety and tolerability of each regimen and (2) humoral and cellular immune responses to NY-ESO-1 with each regimen. Secondary objectives were to evaluate: (1) tumor response according to irRC, and (2) immunological changes in the tumor microenvironment. Target enrollment was a maximum of nine patients to each treatment arm (total 27). Each study arm is considered as a distinct pilot trial aimed at identifying a promising treatment. This sample size was chosen based on the methodology of Yao et al.Citation20 to minimize the total number of patients needed to identify promising treatment regimen(s). The target probability of achieving an immunological response for a promising vaccine was at least 50%. For each study arm, the treatment regimen would be considered promising if more than 4 of 9 patients have an immunological response defined as a NY-ESO-1 specific humoral response or NY-ESO-1 specific T cell response.
Patients: Patients with advanced metastatic or unresectable AJCC (v7) stage IIIB-IV melanoma were eligible if they had one or more metastases available for biopsy, plus other measurable disease, and were eligible for treatment with ipilimumab, as indicated by the package insert. Also required was expression of NY-ESO-1 or LAGE-1 by immunohistochemistry (IHC) or RT-PCR, or IgG seropositivity to NY-ESO-1 or LAGE-1. Immunohistochemistry for NY-ESO-1 or LAGE-1 was performed using antibody clone E978 or ES121, and tumors with greater than 5% NY-ESO-1 or LAGE-1 positive cells were considered NY-ESO-1 or LAGE-1 positive and eligible for trial.Citation21 NY-ESO-1 antibody was detected by ELISA assay, usually performed on full length recombinant NY-ESO-1 protein from E.coli. Sera with reciprocal titers >100 were considered reactive.Citation22,Citation23 Details are provided in Supplemental Text.
Inclusion criteria also included age 18 years and above; ECOG performance status 0–2; adequate hematologic, liver and renal function; life expectancy of at least 4 months; and ability to give informed consent. Exclusion criteria included: contraindications to ipilimumab therapy, prior NY-ESO-1 vaccine, active autoimmune disease (other than vitiligo, type I diabetes, treated thyroiditis, asymptomatic laboratory evidence of autoimmune disease, or mild arthritis requiring no more than NSAIDs); unresolved immune-related adverse events from prior therapy; pregnancy or breast feeding; concurrent steroid therapy greater than 10 mg prednisone/day; cytotoxic chemotherapy, interferon, radiation, or experimental therapy within 4 weeks; history of severe allergy to vaccines or unknown allergens; untreated CNS metastases; significant heart disease; other serious illnesses; immune deficiency, HIV or active Hepatitis B or C. Patients were studied following informed consent, and with central Institutional Review Board approval at each site (MSKCC # 12–253, UPMC #13030240, UVA#16347, Northwell Health #14-133B, MSSM #13-00471) and FDA approval (BB-IND #10639). The trial was sponsored and coordinated by the Ludwig Institute for Cancer Research (New York, NY, USA). This was registered at ClinicalTrials.gov (NCT01810016) on March 13, 2013.
All vaccine components were prepared as cGMP agents.
NY-ESO-1 protein: NY-ESO-1 full-length protein was prepared from E. coli and filled into vials by Florida Biologic (Alachua, FL), and provided as single-use vials containing 0.65 ml of NY-ESO-1 protein at 500 mcg/ml in PBS with 4 M urea and 50 mM glycine at pH 6.5.
NY-ESO-1 overlapping long peptides (OLP): Four peptides, 30–32 amino acids long, were prepared with overlapping sequences of the NY-ESO-1 protein:
OLP1: NYESO-1 79–108 (GARGPESRLLEFYLAMPFATPMEAELARRS);
OLP2: NY-ESO-1100-129 (MEAELARRSLAQDAPPLPVPGVLLKEFTVS);
OLP3: NY-ESO-1 121–150 (VLLKEFTVSGNILTIRLTAADHRQLQLSIS); and
OLP4: NY-ESO-1 142–173 (HRQLQLSISSCLQQLSLLMWITQCFLPVFLAQ). The four peptides were manufactured by PolyPeptide Laboratories (San Diego, California) and were provided as a mixture in single use vials, containing 0.25 mg of each peptide (total 1 mg) lyophilized in 25 mg POPC manufactured by Baccinex SA (Courroux, Switzerland).
Poly-ICLC (Hiltonol). Poly-ICLC is formulated at 2 mg/ml poly-IC, 1.5 mg/ml poly-L-lysine, and 5 mg/ml sodium carboxymethylcellulose in 0.9% sodium chloride solution adjusted to pH 7.6–7.8 with sodium hydroxide, manufactured by Dalton Pharma Services (Toronto, Ontario, Canada), and purchased from Oncovir, Inc. (Washington, DC, USA).
Montanide ISA-51 VG. Montanide ISA-51 VG was provided as mannide oleate in mineral oil solution at 1 ml in single use glass vials from Seppic, Inc. (France).
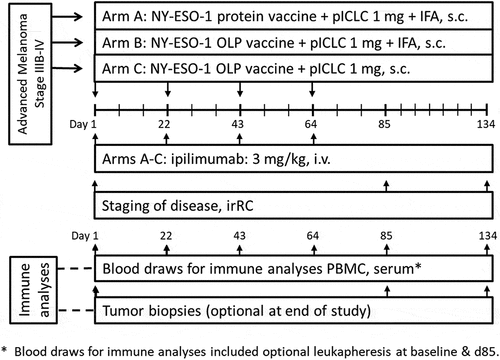
Vaccine composition and administration: Patients on arms A and B received vaccines containing either NY-ESO-1 protein (250 mcg, Arm A) or OLP (250 mcg each; total 1 mg, Arm B) mixed with 1 mg polyICLC and then emulsified with 1 ml Montanide ISA-51 (total volume 2 ml), which was confirmed to be a stable emulsion by a drop-test on water, then injected subcutaneously (s.c.), preferably in the upper arm. Patients in arm C received a vaccine containing OLP (250 mcg each) mixed with 1 mg polyICLC, then injected s.c. The vaccines were administered on days 1, 22, 43, and 64.
Ipilimumab administration. Ipilimumab (Bristol-Myers Squibb, Princeton, NJ) was administered i.v. over 90 minutes, at 3 mg/kg, in accordance with standard dosing, and representing standard of care therapy, every 3 weeks x 4 doses, on the same day as each vaccine.
Collection of peripheral blood mononuclear cells (PBMC), serum, and tumor biopsies: On days 1, 22, 43, 64, 85, and 134 (end of study), peripheral blood was drawn into heparinized tubes for isolation of PBMC, and 10 ml was drawn for serum studies at the time points shown in . When feasible within institutional guidelines, leukapheresis was also performed at baseline and day 85. Whole blood and leukapheresis samples were shipped overnight at ambient temperature in insulated containers to the central laboratory, where lymphocytes were isolated using Ficoll gradient centrifugation, and viably cryopreserved. Biopsies of tumor in skin, subcutaneous tissue, or lymph nodes were obtained by incisional, excision, or core needle biopsy on days 0, 85, and optionally at end of study, with separate portions a) placed in formalin, and later embedded in paraffin; b) quick frozen in OCT, c) quick frozen without media, and d) mechanically and then enzymatically dissociated to single cell suspensions. These were all sent to the core laboratory on dry ice for inventory management and analysis.
ELIspot assays. IFNγ-ELIspot assays were performed directly ex vivo, after cryopreservation (direct ELIspot) at the University of Virginia Human Immune Therapy Center laboratory, using published methodsCitation24,Citation25 and using NY-ESO-1 peptides listed in Supplemental , including 18-mers overlapping by 8 amino acids plus selected defined antigens for CD4 or CD8 T cells, and obtained from Genscript (Piscataway, NJ, USA). Immune responses were evaluated in response to each of five peptide pools, each with 4–6 of the NY-ESO-1 peptides.
Table 1. Patient demographics and outcomes
Negative controls included irrelevant peptide from HIV gag (residues 293–312; FRDYVDRFYKTLRAEQASQE; GenScript), and no peptide. Positive controls included each of the following: a mixture of viral peptides (CEF peptide poolCitation26), phorbol myristate acetate (PMA)-ionomycin and phytohemagglutinin (PHA). Evaluation of T-cell responses was based on the following definitions at each assay time point: Nvax = number of T-cells responding to NY-ESO-1 peptides; Nneg = number T-cells responding to maximum negative control; Rvax = Nvax/Nneg. A patient was considered to have a T-cell response to vaccination (binary yes/no) at each time point after baseline, by direct ELIspot assay only if all the following criteria were met: (1) Nvax exceeded Nneg by at least 10/100,000 cells (0.01%), (2) (Nvax- 1 SD) ≥ (Nneg + 1 SD), and (3) Rvax after vaccination ≥2 × Rvax pre-vaccine. Fold-increases less than one were set to one to indicate no response and to prevent overinflating adjusted fold-increases due to pre-vaccine ratios less than one, or division by zero, while not affecting the determination of response. Assay consistency is represented by interassay coefficients of variation (CVs) calculated for the response of normal donor PBMC to the CEF peptide pool. For that high responder normal donor, the mean number of spots was 328/100,000 cells plated, and the CV was 7%.
Ab responses to NY-ESO-1 peptides. Ab titers to NY-ESO-1 were determined using an enzyme-linked immunosorbent assay (ELISA) for reactivity to each pool of NY-ESO-1 peptides (Supplemental ), as described.Citation23,Citation27,Citation28 Briefly, 96-well half-area cluster plates (Costar) were coated with each NY-ESO-1 peptide pool (50 ng/well per peptide, in coating buffer: 15 mM Na2CO3, 30 mM NaHCO3, pH 9.4, Sigma, with 0.02% NaN3) or with either of 2 negative control peptides (HIV gag293–312; FRDYVDRFYKTLRAEQASQECitation29 and a dihydrofolate reductase (DHFR/DHRP) peptide (R&D Systems, Catalog# 84–56D-R100)Citation30). After overnight incubation at 4⁰C in a humidified chamber, wells were washed x5 with PBS containing 0.1% Tween 20 (wash buffer), then blocked 2 h at room temperature (RT) with 5% nonfat milk in PBS + 0.1% Tween 20 (blocking buffer). Participant serum was added in a four-fold dilution series in assay buffer (blocking buffer + 2% normal goat serum) from 1:100 to 1:6400. After overnight incubation at 4°C, plates were washed x5, and secondary Ab (Southern Biotech Goat anti-human IgG AP conjugate) was added and incubated at RT 60 min. After washing, Attophos substrate (Promega, Fisher Scientific) was added and incubated at RT 30 min in the dark. To stop the reaction, 3 N NaOH was added, and the plate read on a Fluorescent plate reader (Molecular Devices SPECTRAmax Gemini EM, excitation 450 nm, emission 580 nm). The FORECAST function in Microsoft Excel was used to calculate the Ab titer, defined as the reciprocal of the serum dilution that yielded a fluorescent intensity 10x the cutoff value.Citation23,Citation27,Citation28 The cutoff value was the average fluorescence of the first 4 serum dilutions of 2 normal donors.Citation23 Titers >100 were considered positive for induction of peptide-specific antibody if also at least 4x preexisting and 4x all negative controls. If the titer fell outside the dilution range (1:100–1:6400), a value was extrapolated, with the limitation that the FORECAST function is most accurate within the linear portion of the dilution curve.Citation23
Toxicity assessment and stopping rules: The trial was monitored for treatment-emergent and treatment-related adverse events (TEAEs and TRAEs, respectively), using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Dose-limiting toxicity (DLT) of NY-ESO-1 vaccine was defined as any grade 3 or greater hematologic or non-hematologic toxicity that was definitely, probably, or possibly related to administration of the NY-ESO-1 vaccine. DLT assessments were based on the combination of all vaccine components, not on individual components. DLT of ipilimumab was defined as any toxicity that was definitely, probably, or possibly related to ipilimumab and required permanent discontinuation of ipilimumab in accordance with the package insert (US) or product information (Australia). A RLT (Regimen-limiting Toxicity) was defined as a DLT of the vaccine/ipilimumab combination, i.e., where an observed DLT cannot be attributed solely and exclusively to either the vaccine or ipilimumab. Hence, a RLT was to be counted as DLT for both vaccine and ipilimumab. Protocol treatment was to be discontinued for any RLT or disease progression requiring other therapy. Study stopping rules included one death or two grade 4 AEs related to treatment. Changes in immune infiltrates and immune signatures in metastatic melanoma. The density (per mm2) of immune cells infiltrating melanoma metastases was measured by automated image analysis of formalin-fixed sections, using the Vectra system (Akoya Biosciences, Marlborough, Massachusetts, USA) for multispectral immunofluorescence histology (mIFH). Four-micron thick sections were cut from formalin-fixed paraffin-embedded tumor specimens, and human lymph node was used as a positive control. mIFH staining was performed according to the manufacturer’s protocol using the OPAL Multiplex Manual IHC kit, and antigen retrieval buffers AR6, AR9 (Akoya Biosciences), or DIVA Decloaker (Biocare Medical, Pacheco, CA). Staining sequence, antibodies, and antigen retrieval buffers are detailed in Supplemental Table 2.
Three antibody panels were used: T cell activation: CD4, CD8, CD45RO, Ki67, Granzyme B, ICOS-1, DAPI; Checkpoint 1: CD8, CD56, SOX10, PD-L1, IFNγ, DAPI; Helper T cell panel: CD4, CD8, FoxP3, Tbet, RORγt, GATA3, DAPI. Stained slides were mounted using prolong diamond antifade (Life Technologies, Carlsbad, California, USA) and scanned at 10x magnification using the PerkinElmer Vectra 3.0 system and Vectra software. Regions of interest in the tumor tissue were selected in Phenochart software, and 20x magnification images were acquired with the Vectra 3.0 system. These images were spectrally unmixed using single stain positive control images in the InForm software (Akoya Biosciences). Images were analyzed using HALO software (Indica Labs, Albuquerque, NM) and software developed at the University of Virginia for analyzing cells stained with multiple markers. Changes between intratumoral and peritumoral immune cells localized adjacent to tumor in pretreatment and d85 biopsies were assessed for effects of combination treatment. Enumerated dual expressing cell subsets for each panel are as follows: T cell activation panel: CD4+CD45RO+, CD8+CD45RO+, CD4+Granzyme-b+, CD8+Granzyme-b+, CD4+ICOS+, CD8+ICOS+, CD4+Ki67+, CD8+Ki67+; Checkpt1 Panel: Sox10+PDL1+, CD8+IFNy+, CD56+IFNy+; Helper T cell Panel: CD4+T-bet+, CD4+FoxP3+, CD4+GATA3+, CD8+RORyt+, CD8+T-bet+, CD8+FoxP3+, CD8+GATA3+, CD8+RORyt+. Changes between pretreatment and d85 were assessed for effects of combination treatment.
Clinical outcome. Clinical responses to therapy were assessed by immune-related response criteria (irRC).Citation31
Results
Patient population
Enrollment began in January 2014. As summarized in the CONSORT diagram (Supplemental material), 24 patients were screened for expression of NY-ESO-1 or LAGE-1: 13 were evaluated for NY-ESO-1 Ab, with 5 (38%) positive, and 12 were evaluated by IHC, with 3 (25%) positive. These included one patient who was screened by both methods and was negative by both methods. Thus, 8 (33%) expressed NY-ESO-1 by either serum Ab (n = 5) or IHC (n = 3). At final analysis, these eight eligible patients were enrolled among five participating institutions, with four enrolled at one site and one at each of the other sites. Five were enrolled in Arm A, 2 in Arm B, and 1 in Arm C. During the first 7 months of enrollment, five patients were enrolled sequentially to arms A, B, C, A, B, in that order. After August 2014, the company responsible for validating OLP could no longer perform it; so, enrollment on arms B and C was held until a new testing site was qualified. Through 2015, three additional patients were enrolled, all on arm A. By the time the OLP were revalidated, the study was stopped due to slow accrual. The protocol had been designed after the approval of ipilimumab as first-line therapy (March 2011), but PD-1 antibodies were approved as second-line therapy during the first year the protocol was open (pembrolizumab September 2014, and nivolumab December 2014). In practice, PD-1 antibody therapy replaced ipilimumab as first-line therapy, based on data presented in June 2015, which was confirmed by formal FDA approval as first-line therapy December 2015. In this changing landscape, it had been reasonable to offer ipilimumab monotherapy for patients who failed front-line PD-1 blockade, so the protocol was kept open, but on 10/1/2015, the combination of ipilimumab and nivolumab was approved and became the favored option for patients who failed PD-1 antibody monotherapy. Only three patients were enrolled during 2015, and with this changing landscape of therapy, it did not appear likely that the enrollment target could realistically be reached; so, the study was closed to enrollment at the end of 2015.
Seven patients received all four doses of ipilimumab and vaccine, and five completed the study per protocol including end of study visit. Reasons for early discontinuation are shown in , plus clinical and demographic details. Three patients had had prior immune therapy. Details of prior therapies, NY-ESO-1 expression, HLA expression, and sample weeks are shown in Supplemental Table 3.
Clinical toxicities
All patients experienced at least one TEAE: the most common were fatigue, injection site reaction, pruritus, diarrhea, rash, skin induration, and fever. Three (38%) patients experienced TEAEs with a maximum grade 3 (2 in Arm A; 1 in Arm C), and the patient in Arm C experienced a grade 4 TEAE (lactic acidosis, which was a serious adverse event [SAE] unrelated to study therapy) and died due to progressive disease. Treatment-related adverse events (TRAEs, i.e., TEAEs related to either or both treatments) are detailed in Supplemental Table 4. Seven (88%) patients experienced at least one ipilimumab-related TRAE, and 6 (75%) patients experienced at least one vaccine-related TRAE. Two (25%) patients experienced ipilimumab-related TRAEs with a maximum severity grade 3; no patient experienced ipilimumab-related TRAEs with a maximum grade ≥4. Grade 3 TRAEs considered related to ipilimumab included diarrhea, colitis and colon hemorrhage for one patient (diarrhea and colitis were also reported as SAEs); and hypophysitis (also an SAE), nausea, vomiting, fatigue, hyponatremia, dehydration, and hypotension for a second patient (this patient also experienced grade 2 adrenal insufficiency, which was considered an ipilimumab-related SAE). Two (25%) and three (38%) experienced ipilimumab-related TRAEs with a maximum severity grade 1 and 2, respectively. The most frequent grade 1 or 2 ipilimumab-related TRAEs included rash, pruritus, diarrhea, abdominal pain, and fatigue.
Four (50%) and two (25%) patients experienced vaccine-related TRAEs with a maximum severity grade 1 and 2, respectively; no patient experienced vaccine-related TRAEs with a maximum grade ≥3. The most frequently occurring grade 1 or 2 vaccine-related TRAEs included injection site reaction, pruritus, rash, skin induration, fatigue, fever, and myalgia.
Three (38%) patients (2 in Arm A and 1 in Arm C) experienced at least one SAE. No SAEs were assessed as vaccine-related. There were no DLTs or RLTs in the study.
Clinical responses. Clinical responses were assessed by irRC on day 85 (week 13) and end of study (weeks 19–21). No patients experienced objective clinical responses. Four (#4, 5, 6, 8) experienced SD as best response. Four patients had PD as best response ().
T cell responses to NY-ESO-1 peptides: direct ELIspot assay. All eight patients were evaluated for T cell responses to overlapping NY-ESO-1 peptides (Supplemental Table 1) in PBMC by direct ex vivo ELIspot assay. Immune responses were evaluated by ELIspot assay for production of IFN-gamma in response to each of five pools of the overlapping NY-ESO-1 peptides. Six patients (# 1, 4–8) had T cell responses to one or more peptide pools (). As shown in , the immune responses were primarily against pools 3 and 4 (4 patients each; 50%), representing NY-ESO-1 residues 71–107, and 101–178, respectively. Pools 3 and 4 also included 1–2 well-defined epitopes for helper T cells and a defined CD8 epitope, each. Responses were noted for two patients (25%) to peptides in pool 5, which spanned a similar range of the protein, and included the defined CD4 epitope NY-ESO-1 121–130.Citation32 One patient responded to peptides in pool 2 (spanning NY-ESO-151–88), but none responded to overlapping peptides spanning the first 58 residues of NY-ESO-1. The patients with T cell responses included 4/5 in arm A (#4-6, 8), 2/2 in arm B (#1, 7), and 0/1 in arm C (). Data for positive controls CEF, PHA, and PMA/ionomycin are shown in Supplemental Figure 1. The CEF responses vary substantially among the patients, as is expected. There are also variations over time and among patients in responses to PHA and to PMA-ionomycin, which may reflect differences in PBMC viability and function after shipping and cryopreservation. However, responses to NY-ESO-1 were detected even in patients with low responses to controls: for example, patient 4 had low responses to PHA and to PMA-ionomycin, but high responses to CEF, and responses to NY-ESO-1; and patient 7 had low responses to PHA and PMA-ionomycin were very low at weeks 3 and 6, and weak responses to CEF at all time points, but had T cell responses to NY-ESO-1 at weeks 9 and 12.
Figure 2. Cellular immune responses to NY-ESO-1. Direct ELISpot assay on PBMC pulsed with each of 5 pools of overlapping NY-ESO-1 peptides. The numbers of IFNγ-producing cells per 105 are shown for each condition and week on study for the 6 patients in whom T cell response criteria were met. Those without responses (patients 2 and 3) are not shown. Data are shown for patients 1, 4, 5, 6, 7, and 8 in panels A-F, respectively. For patients 5 and 8, data from weeks 21 and 13, respectively, are not shown because of high negative controls in those samples. some of the key values are written beside the bars
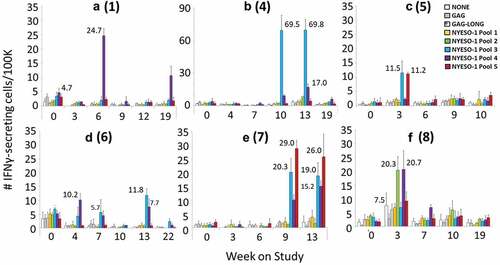
Table 2. T-cell, Ab, and clinical responses by patient
Table 3. Summary of T cell and Ab responses to NY-ESO-1 after vaccination plus IFA
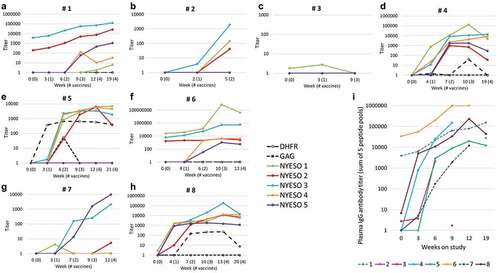
ELISA Results: Of the 8 patients, 7 (88%) demonstrated new or enhanced humoral immunity to NY-ESO-1 peptides induced by either the whole protein or by the OLP (, ). Patient 3 (Arm A) had no detectable Ab response to the protein-based vaccine and came off the study after two vaccines. Six of the seven serologic responders (4 of 5 in arm A and 2 of 2 in Arm B) also developed T cell responses to the vaccine as measured by the interferon-gamma ELIspot assay (, ). The one patient on arm C, without IFA (Patient 2) developed an Ab response to peptides in pools 3 and 4. Patient 6 (arm A) and 1 (arm B) had pre-vaccine responses to peptides in the first 104 amino acids, which increased in titer dramatically as a result of vaccination and thus are considered responders (). Overall, peptide pools 1–5 induced antibody in 4, 4, 7, 6, and 5 patients, respectively. Among the four patients with PD as best response, there were Ab responses by week 7 to a mean of 1 peptide pool (0, 2, 2, 4) whereas the four patients with SD had Ab responses by week 7 to a mean of 4.5 peptide pools (5, 4, 4, 5, ). These values differ based on a Mann–Whitney signed long rank test (p = 0.036). Thus, the breadth of early antibody response was greater for the four patients with SD than for the four patients with PD, as assessed by the number of peptide pools with Ab responses by week 7.
Figure 3. Serum Ab response to NY-ESO-1. Serum IgG Ab titers measured by ELISA are shown for all eight patients (1–8) in panels A-H, respectively. Each colored line represents the change in titers over time for each of the five NY-ESO-1 peptide pools (see legend), and negative control data are shown with solid or dotted black lines. Data are plotted on a log scale, with the range of Y-axis values varying from 1,000 to 1,000,000 among the patients, to illustrate fine details for each. Criteria for response are met by a titer >100 and increases of at least 4x if there is a preexisting response (as in A and F). Cumulative IgG response was calculated as the sum of the titers for all five peptide pools, and these are shown in panel I, with each line representing the cumulative titer over time for each patient. Note that the assays used plasma rather than serum for patient 6
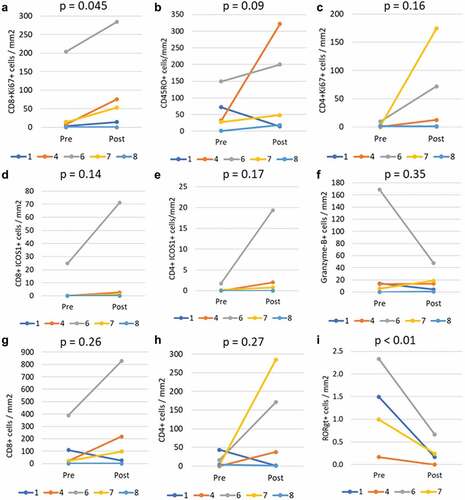
Assessment of melanoma metastases for immune cell infiltrates. Tumor biopsies were collected from all patients. However, two who discontinued early only had baseline samples collected (#2, #3), leaving six evaluable at baseline and day 85 (or day 128 for one patient). From one of those patients, there was insufficient tumor tissue for analysis, leaving five evaluable at both time points. FFPE slides were evaluated by mIFH for T cell activation by staining for CD8, CD4, CD45RO, Ki67, Granzyme B, and ICOS-1. Representative images for patients 4 and 7 are shown in Supplemental Figures 2 and 3, respectively. Stained cells were enumerated by automated image analysis in multiple regions of interest (ROI) totaling 2 to 42 mm2 total tumor area (mean 15 mm2, median 11 mm2) for the 10 samples. Changes from baseline to on-study biopsies were evaluated by paired T-tests after square root transformation. There was a significant increase in proliferating (Ki67+) CD8+ T cells (p = .045, ) and trends to increases in total antigen-experienced (CD45RO+) cells (p = .09), proliferating CD4+ T cells (p = .158), CD8+ICOS1+ cells (p = .142), and CD4+ICOS1+ cells (p = .166, -e). Significant differences were not observed for other markers in this panel: as an example, granzyme B data are shown in . For most of the evaluable patients, there were increases in the densities of total CD4+ and CD8+ T cells in the tumors, but decreases in both cell densities were observed for patient 1; so these were not statistically significant in this small study sample (, h). Evaluation of T cells for transcription factors FoxP3, Tbet, GATA3 and RORγt identified no consistent changes from pre- to post- other than a decrease in the number of RORγt+ cells/mm2 (p < .01, ). No consistent or significant changes were observed in a mIFH panel staining for PDL1, Sox10, CD56, CD8, IFNγ. These latter two panels were limited further by additional samples that were not evaluable (only 4 and 3 pairs evaluable, respectively).
Figure 4. Immunological changes in the tumor microenvironment. Tumor biopsies were evaluable for five patients pre and post treatment (d85+), with significant increases in CD8+Ki67+ cell density (p = .045) on study (a), increases for some patients in total CD45RO+ cells (b), CD4+Ki67+ cells (c), CD8+ ICOS1+ cells (d), and CD4+ ICOS1+ cells. No significant changes were observed in cells expressing granzyme B (f). Total CD8+ (g) and CD4+ (h) cells also increased for patients 4, 6, and 7, but not overall. There was a consistent and significant decrease in RORγt+ cell density (p < .01). All comparisons used paired T-tests on square-root transformed values
Discussion
The primary goal of this clinical trial was to test the safety and immunogenicity of each of three NY-ESO-1 vaccines combined with ipilimumab. There were no DLTs, and the toxicities were mostly attributable to ipilimumab. The study design to evaluate immunogenicity included a target of a 50% immune response rate, defined as either a T cell response or an Ab response, in more than 4 of 9 patients per arm. The study closed to enrollment prior to enrolling nine patients on any arm, which limits the ability to complete these assessments. However, five patients were enrolled in Arm A, of which four had both T cell and Ab responses and one had neither; thus, 80% had an immune response. Only two patients were enrolled in Arm B, but each had both T cell and Ab responses. Only one patient was enrolled in Arm C, and an Ab response was induced in that patient. Thus, the data provide preliminary data supporting immunogenicity for each vaccination strategy.
Tumor tissue pre- and post-treatment was evaluable for five patients, with a significant enhancement in intratumoral Ki67+ (proliferating) CD8+ cells (p = .045) and trends to increases in antigen-experienced (CD45R0+) cells overall, in proliferating CD4 T cells, and in ICOS+ CD4 and CD8 T cells, along with a significant decrease in the density of RORγt+ cells (p < .01). These findings suggest that the combination of ipilimumab plus any of the NY-ESO-1 vaccines may increase the density of proliferating intratumoral CD8+ T cells within metastatic lesions.
T-cell responses to NY-ESO-1 OLP vaccines have been reported with IFA and with IFA + polyICLC,Citation10 but those data were based on ELIspot assays after in vitro stimulation (IVS). In another prior trial, vaccination with whole protein for MAGE-A3 has required IVS to detect T cell responses.Citation33 Also, a study of vaccination with NY-ESO-1 protein in Iscomatrix has induced high rates of Ab, CD8 and CD4 T cell responses, where the T cell responses were detected after in vitro sensitization.Citation34 Ex vivo assays are less sensitive than assays after in vitro sensitization;Citation24 thus, the 80% T cell response rate for the present study in direct (ex vivo) ELIspot assays for Arm A is promising and suggests that the ipilimumab may well have enhanced responses to NY-ESO-1. Ipilimumab monotherapy can enhance immune responses to NY-ESO-1;13,Citation14 so, it is difficult to tease apart the relative impact of ipilimumab on spontaneous immune responses to NY-ESO-1 vs the impact of ipilimumab on enhancing the response to vaccines. Similarly, favorable induction of T cell responses was observed for both patients on arm B, using OLP and IFA plus polyICLC. However, the one patient on arm C, who was vaccinated with polyICLC without IFA developed an Ab response but not a T cell response. We did not assess responses specifically among CD4+ T cells or CD8+ T cells. However, a prior report using NY-ESO-1 OLP4 demonstrated both CD4 and CD8 T cell reactivity after vaccination with IFA or IFA + polyICLCCitation10 and a follow-up report further assessed the specificity of Th1 CD4 + T cell responses to NY-ESO-1.Citation35 Also, vaccination with NY-ESO-1 protein and Iscomatrix adjuvant induced CD4+ and CD8+ T cells as well, with more dominant CD4 + T cell responses than CD8+ responses.Citation34 Data from murine models suggest that CTLA4 blockade acts predominantly on CD4+ T cells;Citation9 thus, combination with vaccines may be most promising for its effect on those cells. There would be value, for future studies, in understanding more about the specificity of T cell responses to NY-ESO-1 induced by vaccination plus CTLA-4 blockade.
In recent trials, immune responses to peptide and protein vaccines have been less effective at inducing CD8+ T cell responses when using polyICLC alone than when using polyICLC + IFA.Citation12,Citation36 One reason for including arm C was because murine data have suggested that IFA-containing vaccines may sequester T cells to the vaccine site and away from tumor.Citation37 Enrollment on arm C was not sufficient to address that question directly, but a high proportion of patients on arms A and B had T cell responses persisting to week 13, through multiple vaccines, supporting the ability of IFA-containing vaccines to induce durable T cell responses in humans, especially with protein and long peptides.
T cell responses for two of the patients were delayed (week 9 or later, after at least 3 vaccines), as has been observed in patients on some prior trials of MAGE and NY-ESO-1 vaccines.Citation38 Interestingly, in both of these patients (#4, #7), antibody responses to NY-ESO-1 were observed earlier than the T cell responses, which suggests that low levels of CD4+ Th2 responses may have occurred prior to the Th1/Tc1 responses detected by IFN-gamma ELIspot assays in those patients.
The objective clinical response rate with standard of care ipilimumab is about 11%; thus, it would not have been surprising for 1 of the 8 study patients to have experienced an objective clinical response; however, the lack of objective response in 8 patients is within the range of expected outcomes. Four patients experienced SD, which may reflect some clinical benefit. The study was not designed to address whether any of the three vaccine regimens may improve clinical outcome with ipilimumab, and the sample size is too small to make meaningful conclusions. All 4 of the patients with SD were in Arm A; so, 4 of 5 patients in that arm had SD, and all had both T-cell and Ab responses. The one patient with PD had neither. These are provocative associations, but a larger dataset would be required to evaluate this definitively. Vaccination against NY-ESO-1 can induce CD8+ T-cells that upregulate PD-1 and Tim-3;Citation39 thus, future combinations with blocking antibodies to PD-1 and/or Tim-3 may offer promise to enhance tumor control.
NY-ESO-1 is well known for its induction of antibody responses in cancer patients, and these were observed in 5 of the 8 patients (2–4, 6, 8), during initial screening; however, in the ELISA assays done to assess for immunogenicity after treatment, IgG responses to NY-ESO-1 peptides were detected at baseline in only two patients (#1 and 6). The assay to screen for Ab as evidence of tumor expression of NY-ESO-1 used full-length NY-ESO-1 protein as the immunogen, whereas the assays done in the present study used pools of peptides from the protein. This likely explains differences in the pre-treatment serology results. On this study, all but one patient developed new or enhanced IgG Ab responses to NY-ESO-1 peptides, and there was a greater breadth of the response in patients with SD than in those with PD, which suggests either that the Ab response to NY-ESO-1 in responders is a marker for responsiveness to ipilimumab, as has been reported,Citation16 or that the induced Ab response itself may have value in tumor control.
Analysis of tumor tissue pre- and post-therapy revealed some encouraging findings with the combination of systemic CTLA-4 blockade plus an NY-ESO-1 vaccine in patients with NY-ESO-1+ tumors: There were significant increases in the density of proliferating CD8 T cells. The cells may well include vaccine-induced NY-ESO-1-reactive T cells that infiltrate tumors. However, they could also represent preexisting tumor-infiltrating CD8+ T cells that expanded in response to ipilimumab. A prior murine study suggests that tumor regression with checkpoint blockade depends primarily on T cells already infiltrating the tumors.Citation40 Questions remain about the ability of vaccine-induced T cells to infiltrate cancers de novo, and to induce tumor regressions alone or in combination with checkpoint blockade. Technologies for T-cell receptor sequencing may enable a better understanding of whether vaccine induced T cells can be found in tumors after combination therapy.
Evaluation of tumor biopsies was limited by sample size so that increases in total CD8+ T cells and CD4+ T cells were observed in several patients but were not consistently observed. Similarly, several tumors had increased ICOS1+ T cells, which is consistent with the impact of CTLA4 blockade on increasing ICOS1 expression.Citation41 We had expected to observe increases in proportions of Th1/Tc1 T cells, marked by nuclear expression of T-bet (TBX21); however, the only significant change observed in T cell transcription factors (T-bet, GATA-3, FoxP3, RORγt) was a consistent decrease in expression of RORγt, which is a driver of Th17 function. The role of Th17 cells in cancer remains unclear, as they can have effector function or regulatory function. This finding should be assessed further in other studies of cancer vaccines and checkpoint blockade.
In summary, this study does not raise any safety concerns of combining ipilimumab (3 mg/kg) x 4 doses with each of 3 NY-ESO-1 vaccines: no study patients discontinued early for toxicity. However, the enrollment on each arm fell short of target, especially for arms B and C. Thus, a formal conclusion about safety cannot be made for arms B and C. On the other hand, safety adverse event data from the five patients enrolled to arm A are more supportive of safety of ipilimumab (3 mg/kg) plus vaccination with NY-ESO-1 protein emulsified in IFA plus 1 mg polyICLC. The study supports immunogenicity of vaccination with either NY-ESO-1 protein or OLP, with immune responses detected ex vivo in most patients. The fact that all 4 patients with SD were on arm A provides further support for vaccination with NY-ESO-1 protein plus IFA and polyICLC, though the lack of objective responses still leaves open the question of whether the combination of CTLA-4 blockade plus vaccine will improve clinical outcomes. The safety and immunogenicity of the regimens tested here do support continued investigation, especially in patients who have progressed on PD-1 antibody therapy, with ipilimumab plus NY-ESO-1 vaccines. While there will be value in studying tumor samples pre- and post-therapy to understand whether vaccine-induced T cells infiltrate tumors, there also will be value in larger studies that are not limited to patients with biopsy-accessible tumor, to evaluate the clinical benefit of adding an NY-ESO-1 vaccine (protein or OLP) to ipilimumab.
Declaration of interests statement:
Dr Slingluff discloses research funding to his University from Merck, Celldex, and GlaxoSmithKline; research support in kind to his University from Theraclion and 3M; Scientific Advisory Board role with Immatics (prior), and Curevac (planned); PI role for Polynoma with compensation to his University; and patent royalties as co-inventor of peptides for use in cancer vaccines (patents held by the UVA Licensing and Ventures Group).
Dr Zarour: research support: Bristol-Myers Squibb, Checkmate Pharmaceuticals, GlaxoSmithKline; consulting: Bristol-Myers Squibb, Checkmate Pharmaceuticals, GlaxoSmithKline, Vedanta.
Dr Tawbi: Consulting Honoraria: BMS, Novartis, Merck, Genetech, Eisai, Iovance. Research Funding to Institution: BMS, Novartis, Merck, Genentech, GSK, Celgene.
Dr Kirkwood: Consulting/SAB: Amgen, Bristol Myers Squibb, Checkmate Pharmaceuticals, Harbour BioMed, Iovance Biotherapeutics, Istari Oncology, OncoSec, Novartis Pharmaceuticals, Scopus BioPharma, Pfizer; Research Trial Support to Institution: Amgen, Inc., Bristol Myers Squibb, Castle Biosciences, Checkmate, Immunocore, Iovance, Novartis.
Dr Postow: Consulting fees: BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, Eisai; Honoraria: BMS and Merck; Institutional Support: RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca.
Dr Devoe: consultant with Pfizer.
Dr Friedlander: Advisory board: Sanofi, Castle Biosciences. Equity: Gilead Sciences, Thermo Fisher Scientific, adverum biotechnology, iovance, Clovis Oncology.
Dr Wolchok (over the past 12 months): Consultant for: Amgen; Apricity; Arsenal IO; Ascentage Pharma; AstraZeneca; Astellas; Boehringer Ingelheim; Bristol Myers Squibb; Chugai; Dragonfly; F Star; Eli Lilly; Georgiamune; Imvaq; Merck; Polynoma; Psioxus, Recepta; Trieza; Truvax; Sellas, Werewolf Therapeutics. Grant/Research Support from: Bristol Myers Squibb; Sephora. Equity in: Tizona Pharmaceuticals; Imvaq; Beigene; Linneaus, Apricity, Arsenal IO; Georgiamune. All other authors have no competing interests or conflicts to disclose.
Ethics approval and consent to participate:
Patients involved in this study signed informed consent for the clinical trial in which they participated. The clinical trial was performed with IRB and FDA approval (IND 10369) and was registered with Clinicaltrials.gov (NCT01810016, first posted March 13, 2013). The institutional review boards (IRB) approvals were obtained from Memorial Sloan Kettering Cancer Center’s IRB (# 12-253), the University of Pittsburgh and Magee-Womens Health Corporation IRB (MOD13030240-02/PRO13030240), the University of Virginia IRB-Health Sciences Research (#16347), Northwell Health IRB (# 14-133B), and Mount Sinai School of Medicine IRB (# 13-00471).
Consent for publication:
All authors consent to publication of the manuscript.
Prior abstract publication. Preliminary data were published in association with the 2018 meeting of the American Society of Clinical Oncology (J Clin Oncol 36:15S, abstract e15175, 20 May 2018) and the 2019 meeting of the Society for Immunotherapy of Cancer (J Immunother Cancer 7:S1, abstract P366, Nov 6, 2019)
DeclarationDisclosure of potential conflicts of interest
Data from this study will be made available through ClinicalTrials.gov and upon written request, after the main findings have been accepted for publication. Such research data will be redacted to prevent disclosure of personal identifiers.
Supplemental Material
Download ()Acknowledgments
We acknowledge the contributions of Geoffrey Weiss in patient enrollment, Anna Dickinson in participation in assays of antibody responses to NY-ESO-1, to Donna Deacon in participation in ELIspot assays, and to Emily Allred for coordination of clinical research care at the University of Virginia.
Supplementary material
Supplementary material can be accessed here
Additional information
Funding
References
- Robert C, Thomas L, Bondarenko I, O’Day S, Jw Md GC, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–12. doi:10.1056/NEJMoa1104621.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto, PA, Richards, JM, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi:10.1016/S1470-2045(15)70122-1.
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466.
- Ochoa CE, Joseph RW. Utility of ipilimumab in melanoma patients who progress on anti-PD-1 therapy. Melanoma Management. 2017;4(3):143–145. doi:10.2217/mmt-2017-0010.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaaff J, Dummer R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi:10.1056/NEJMoa1910836.
- Zaidi N, Quezada SA, Kuroiwa JMY, Zhang L, Jaffee EM, Steinman RM, Wang B. Anti–CTLA-4 synergizes with dendritic cell–targeted vaccine to promote IL-3–dependent CD4+ effector T cell infiltration into murine pancreatic tumors. Ann N Y Acad Sci. 2019;1445(1):62–73. doi:10.1111/nyas.14049.
- Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, Ellenhorn JDI. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003;170(6):3401–3407. doi:10.4049/jimmunol.170.6.3401.
- Mougel A, Terme M, Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019;10:467. doi:10.3389/fimmu.2019.00467.
- Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7(6):885–895. doi:10.1016/S1074-7613(00)80406-9.
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18:6497–6508. doi:10.1158/1078-0432.CCR-12-2189.
- Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A. 2007;104(21):8947–8952. doi:10.1073/pnas.0703395104.
- Pavlick A, Blazquez AB, Meseck M, Lattanzi M, Ott PA, Marron TU, et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res. 2020;8:70–80. doi:10.1158/2326-6066.CIR-19-0545.
- Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105(51):20410–20415. doi:10.1073/pnas.0810114105.
- Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35(1):89–97. doi:10.1097/CJI.0b013e31823aa41c.
- Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, et al. Enhancement of tumor-reactive cytotoxic CD4+T-cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res. 2013;1(4):235–244. doi:10.1158/2326-6066.CIR-13-0068.
- Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–16728. doi:10.1073/pnas.1110814108.
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019–1027. doi:10.1158/1078-0432.CCR-14-2708.
- Wada H, Isobe M, Kakimi K, Mizote Y, Eikawa S, Sato E, et al. Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. J Immunother. 2014;37(2):84–92. doi:10.1097/CJI.0000000000000017.
- Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F, et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res. 2015;3(3):278–287. doi:10.1158/2326-6066.CIR-14-0202.
- Yao TJ, Begg CB, Livingston PO. Optimal sample size for a series of pilot trials of new agents. Biometrics. 1996;52(3):992–1001. doi:10.2307/2533060.
- Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. International Journal of Cancer. 2001;92(6):856–860. doi:10.1002/ijc.1282.
- Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens [see comments]. J Exp Med. 1998;187(8):1349–1354. doi:10.1084/jem.187.8.1349.
- Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. 11-9. doi:10.1007/978-1-60327-811-92.
- Slingluff CL Jr., Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+and CD4+T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15(22):7036–7044. doi:10.1158/1078-0432.CCR-09-1544.
- Slingluff CL Jr., Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29(21):2924–2932. doi:10.1200/JCO.2010.33.8053.
- Currier JR, Kuta EG, Turk E, Earhart LB, Loomis-Price L, Janetzki S, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260(1–2):157–172. doi:10.1016/S0022-1759(01)00535-X.
- Reed CM, Cresce ND, Mauldin IS, Slingluff CL Jr., Olson WC. Vaccination with melanoma helper peptides induces antibody responses associated with improved overall survival. Clin Cancer Res. 2015;21(17):3879–3887. doi:10.1158/1078-0432.CCR-15-0233.
- Slingluff CL Jr., Petroni GR, Chianese-Bullock KA, Wages NA, Olson WC, Smith KT, et al. A trial to evaluate the immunogenicity and safety of a melanoma helper peptide vaccine plus incomplete Freund’s adjuvant, cyclophosphamide, and polyICLC (Mel63). J Immunother Cancer. 2021;9(1):e000934. in press. doi:10.1136/jitc-2020-000934.
- Vingert B, Perez-Patrigeon S, Jeannin P, Lambotte O, Boufassa F, Lemaitre F, et al. HIV controller CD4+ T cells respond to minimal amounts of Gag antigen due to high TCR avidity. PLoS Pathog. 2010;6(2):e1000780. doi:10.1371/journal.ppat.1000780.
- Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res. 2014;2(1):37–49. doi:10.1158/2326-6066.CIR-13-0126.
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi:10.1158/1078-0432.CCR-09-1624.
- Zeng G, Touloukian CE, Wang X, Restifo NP, Rosenberg SA, Wang RF. Identification of CD4+T cell epitopes from NY-ESO-1 presented by HLA-DR molecules. Journal of Immunology. 2000;165(2):1153–1159. doi:10.4049/jimmunol.165.2.1153.
- Slingluff CL Jr., Petroni GR, Olson WC, Smolkin ME, Chianese-Bullock KA, Mauldin IS, et al. A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites. Cancer Immunol Immunother. 2016;65(1):25–36. doi:10.1007/s00262-015-1770-9.
- Cebon JS, Gore M, Thompson JF, Davis ID, McArthur GA, Walpole E, et al. Results of a randomized, double-blind phase II clinical trial of NY-ESO-1 vaccine with ISCOMATRIX adjuvant versus ISCOMATRIX alone in participants with high-risk resected melanoma. J Immunother Cancer. 2020;8(1):8. doi:10.1136/jitc-2019-000410.
- Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, et al. Effect of montanide and poly-iclc adjuvant on human self/tumor antigen-specific CD4+T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res. 2013;1(5):340–350. doi:10.1158/2326-6066.CIR-13-0089.
- Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N, et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J Immunother Cancer. 2019;7(1):163. doi:10.1186/s40425-019-0625-x.
- Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi:10.1038/nm.3105.
- Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64(10):1251–1260. doi:10.1007/s00262-015-1731-3.
- Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, et al. PD-1 and tim-3 regulate the expansion of tumor antigen–specific CD8+T cells induced by melanoma vaccines. Cancer Res. 2014;74(4):1045–1055. doi:10.1158/0008-5472.CAN-13-2908.
- Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2(1):3. doi:10.1186/2051-1426-2-3. eCollection;%2014.3–2
- Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, et al. Increased frequency of ICOS+CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1(4):229–234. doi:10.1158/2326-6066.CIR-13-0020.