ABSTRACT
Although PD-1/PD-L1 blockade therapy confers salutary effects across cancer types, their efficacy in Extranodal Natural killer/T-cell lymphoma (ENKTCL) patients is limited and unpredictable. Here, we comprehensively evaluated the expression profile of a panel of immune-regulatory makers to identify novel prognostic biomarkers and/or therapeutic targets for this malignancy. Using immunohistochemistry and multiplex immunofluorescence, we found that the expression of VISTA (88.1%) was predominantly in CD68+ macrophages and much higher than PD-L1 expression (68.7%) in ENKTCL. B7-H4 and HHLA2 proteins were not detected in ENKTCL. B7-H3 was expressed in minority of ENKTCL patients (13.7%) and mainly colocalized with CD31. A close correlation was detected between VISTA and PD-L1, but they were not co-expressed in the same cells. High expressions of VISTA or PD-L1 were significantly associated with detrimental clinicopathological characteristics, dismal prognosis, and high density of CD8+ TILs, and high VISTA expression was also significantly associated with high density of Foxp3+ TILs. VISTA combined with PD-L1 was an independent prognostic factor for PFS and OS. Moreover, the patients with high VISTA showed a poor response to PD-1 blockades in ENKTCL. In conclusion, these findings provide a rationale for VISTA as an ideal immunotherapeutic target next to PD-L1 for ENKTCL.
Introduction
Extranodal natural killer (NK)/T‑cell lymphoma (ENKTCL) is a rare but aggressive subtype of non-Hodgkin lymphoma, which is derived from NK cells or γδT cells.Citation1–4 ENKTCL is more prevalent in Asia and South America than that in European countries.Citation5 A typical immunophenotype of ENKTCL is positivity for CD3ε, CD56, and cytotoxic molecular such as granzyme B and TIA1.Citation6 Of note, ENKTCL is strongly associated with Epstein-Barr virus (EBV) infection and EBV encoded RNA (EBER) positive is an important auxiliary diagnostic maker for ENKTCL.Citation7 Unfortunately, the consensus on standard treatment strategy remains unavailable for ENKTCL patients, partially due to limited cases. Chemoradiotherapy or chemotherapy yield curative effects on ENKTCL patients in the early-stage.Citation8,Citation9 However, the outcomes of those treatments for patients with advanced or relapsed ENKTCL are disappointing, and the estimated 3-year overall survival is 25% in advanced patients.Citation10 Therefore, there is an urgent medical need to explore new therapeutic strategies for advanced ENKTCL.
Over the past decade, immunotherapy is, once again, a promising approach to cancer treatment, due to the remarkable salutary effectiveness of immune-checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1), in numerous solid and hematological malignancies.Citation11–14 Aberrant expression of PD-1/PD-L1 is also observed and associated with poor prognosis in these malignancies.Citation15–18 In terms of ENKTCL, the complete response rate of PD-1/PD-L1 blockades ranged from 24% to 71%.Citation14,Citation19,Citation20 However, the association between PD-L1 expression and effectiveness of the blockade of PD-1/PD-L1 is discrepant and there are still partial PD-L1 high expression ENKTCL cases suffering from disease progression following the blockades of PD-1/PD-L1 axis treatment.Citation14,Citation20 Therefore, it is speculated that the PD-1/PD-L1 axis independent immunosuppressive mechanisms that restrain T cell activation in ENKTCL and it is reasonable to develop alternative immune-modulatory therapies that may be functional in the non-responder cases.
Beyond PD-L1, several novel B7 family members, including B7-H3, B7-H4, V-domain immunoglobulin suppressor of T cell activation (VISTA) and HERV-H LTR-associating 2 (HHLA2), are aberrantly expressed and hamper anti-tumor immunity in cancers. Of these, VISTA, encoded by c10orf54 gene, is one of the most promising immunotherapeutic targets with sequence homology to PD-1 and PD-L1.Citation21,Citation22 As a negative immune checkpoint protein, VISTA displays an inhibitory role in the function of T cell via suppressing T cell proliferation and blunting cytokine production and makers activation.Citation23,Citation24 Of note, the mechanism of VISTA-induced T cell exhaustion is distinct from the PD-1/LD-L1 pathway.Citation25 VISTA is predominantly expressed within hematopoietic cells, with the highest expression level in myeloid cells.Citation21,Citation26 The high expression of VISTA is also observed in numerous cancers, including colorectal carcinoma, oral squamous cell carcinoma, melanoma and ovarian cancer.Citation27–30 Moreover, VISTA expression is elevated after treatment with an anti-PD-1 antibody and/or anti-CTLA4 antibody.Citation31,Citation32 Additionally, VISTA is preferentially expressed in pancreatic cancer, which is considered as “cold” tumor due to low response to ICIs.Citation33 Furthermore, previous in vivo experiment demonstrates that blockading of PD-L1 and VISTA shows a synergistic therapeutic effect in colon cancer models.Citation25 These findings indicate that VISTA may be a potential target for tumor immunotherapy. However, the expression and clinicopathological relevance of VISTA in ENKTCL remain unknown.
In the present study, we first examined the expression of PD-L1, B7-H3, B7-H4, VISTA and HHLA2 in ENKTCL cases using immunochemistry (IHC) and immunofluorescence (IF). Then, we evaluated the correlation between VISTA and PD-L1, clinicopathological characteristics, and the density of tumor-infiltrating lymphocytes (TILs). Finally, we evaluated the synergistic effect of PD-L1 and VISTA on predicting the prognosis and immunotherapeutic efficacy in ENKTCL patients.
Methods
Patients and samples
Upon approval by the Institutional Ethical Boards of Sun Yat-sen University Cancer Center (SYSUCC). One hundred and nine consecutive ENKTCL cases diagnosed at SYSUCC between 2003 and 2019 were collected in this study. Written informed consent was obtained from all cases before enrollment. All enrolled cases met the following criteria: (1), pathologically confirmed ENKTCL; (2), received no anti-cancer treatments before biopsy; (3), Formalin-fixed, paraffin-embedded (FFPE) blocks were available; (4), no history and concurrence of other malignant tumors; (5), complete clinicopathological and follow-up data. Progression-free survival (PFS) was defined as the period span from the date of first treatment for ENKTCL to the date of cancer progression or death, and the overall survival (OS) was defined as the period span from the date of first treatment for ENKTCL to the date of death. The follow-up was censored on 31 December 2019, the date of the last follow-up for patients without progression or death event.
Immunochemistry and in situ hybridization
The immunochemistry (IHC) staining was performed on 4 μm FFPE ENKTCL sections by a professional pathologist according to our previous study.Citation34 Briefly, after deparaffinization, rehydration and endogenous peroxidase inactivation, antigen retrieval was performed using EDTA buffer (PH 9.0) in microwave, followed by blocking nonspecific binding with protein block (Novocastra, Newcastle, UK). Then, slides were incubated with primary antibody (anti-PD-L1: cell signaling technology (CST), #13684; anti-VISTA: CST, #54979; anti-B7-H3: CST, #14058; anti-B7-H4: CST, #14572; anti-HHLA2: Sigma-Aldrich, HPA055478; anti-CD8: CST, #85336; anti-Foxp3: Abcam, ab215206) at 4 °C overnight. After incubation with the corresponding second antibody, the slides were visualized with a DAKO EnVision Detection System (Dako). In situ hybridization (ISH) was performed to detect the EBV encoded small RNA (EBER) in FFPE tissue slides as previously described.Citation3
Quantification of PD-L1, VISTA and CD8+ or Foxp3 + T cells
The PD-L1 and VISTA expressions were evaluated by two senior pathologists separately, based on the percentage of positive cells (e.g., number of positive cells/numbers of total cells). CD8+ or Foxp3+ TILs were calculated in the same manner as described in the previous study.Citation16 In brief, the average of five independent high-power fields (400×), which represented the densest lymphocytic infiltrates, was used to reflect the extent of T cell infiltration.
Immunofluorescence
In line with our previous study,Citation35 immunofluorescence (IF) staining was performed on slides, which have been prepared in the same manner as for IHC before incubation of antibodies, by using primary and secondary antibodies as follows: PD-L1 (the same as IHC and ab210931 abcam), VISTA (the same as IHC), B7-H3 (the same as IHC), CD56 (Abcam, ab9272), CD68 (Abcam, ab955), CD31 (Abcam, ab9498), goat anti-rabbit (Alexa Fluor 488, ab150077; Alexa Fluor 594, ab150088, Abcam), goat anti-mouse (Alexa Fluor 488, ab150117; Alexa Fluor 594, ab150116, Abcam). DAPI (4′, 6-diamino-2-phenyindole) fluorochrome was used for nuclei staining of all cells.
Statistical analysis
Optimal cutoff values of PD-L1 or VISTA levels for predicting survival were determined via the receiver operating characteristics (ROC) curve analysis. Associations between PD-L1 and VISTA expression, and clinicopathological characteristics were calculated using the chi-square test or Fisher’s exact test, displayed by cross-table. The student’s t-test was used for two-group analysis. Kaplan–Meier method was performed to depict the survival curves of PFS and OS, and the intergroup differences of survival were determined with the log-rank test. Univariate and multivariate analyses were carried out based on the Cox proportional hazard model. All data were analyzed with SPSS V.24.0 software (SPSS, Chicago, IL, USA) and Graphpad Prism 7 software (La Jolla, California, USA). Statistical significance was defined as a p < .05.
Results
Patient clinicopathological characteristics
In the present study, a total of 109 patients with ENKTCL were included, and the baseline clinicopathological characteristics were summarized in supplementary table S1. Of those, 74 (67.9%) patients were male. The median age at diagnosis was 41 (range: 10–70) and 96 (88.1%) patients were younger than 60 years old. The primary lesion of 85 (78.0%) patients was present at nasal cavity and adjacent tissues (Table S1). Nineteen (17.4%) patients had distal lymph node (LN) involvement and 35 (32.1%) patients had stage III or IV disease (Table S1). Elevated lactate dehydrogenase (LDH) was detected in 34 (31.2%) patients (Table S1). Sixty-four (58.7%) patients were assigned to intermediate high-risk and high-risk groups based on the nomogram-revised index (NRI), and 26 (23.8%) patients were assigned to high prognostic index of natural killer/T cell (PINK) groupCitation10,Citation36 (Table S1). EBER positive was detected in 104 (95.4%) cases. Eighty-nine (81.6%) cases were positive for CD56. All patients had received chemotherapy and the chemotherapy can be categorized as anthracycline (ANT)-based (n = 19) and non-ANT-based regimens (n = 90) (Table S1). 17 out of 19 ANT-based regimens included CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone). Whereas the mainly used non-ANT-based regimens were asparaginase (ASP)-containing regimens, including ASP/ANT based (8.3%), ASP/gemcitabine (GEM) based (48.6%), ASP/methotrexate based (9.2%) and ASP/not otherwise specified based (2.8%) regimens, followed by platinum or other-containing regimens (13.8%)Citation37 (Table S1).
At the end of follow-up, 73 (67%) cases experienced disease progression and 58 (53.2%) cases had died. The median PFS time was 20 months (range: 1–111 months) and the median OS time was 60 months (range: 1–173 months). The 1-, 3-, and 5-year PFS rates were 60.6%, 43.8%, and 37.7%, respectively. The 1-, 3-, and 5- year OS rates were 89.0%, 56.8%, and 53.5%, respectively.
Expression of B7 family proteins in NK/T cell lymphoma
To explore the expression characteristics of B7 family proteins, including PD-L1, VISTA, B7-H3, B7-H4 and HHLA2, IHC, IF, and ISH were performed. Staining using serial sections showed that PD-L1 was expressed in tumor cells (marked with EBER) as well as in tumor-infiltrating immune cells (TIICs) (Figure S1A-B). The double IF for CD56 and PD-L1 further demonstrated that CD56 positive lymphoma cells partially co-expressed PD-L1 ()). Notably, the double IF staining for CD68 and PD-L1 also confirmed that CD68+ tumor associated macrophages (TAMs) were partially co-expressed PD-L1 ()). Through integral analysis of the PD-L1 expression in lymphoma cells and TAMs, 64 out of 109 cases (68.7%) were PD-L1 positive staining in ENKTCL.
Figure 1. Expression of PD-L1 and VISTA in tumor tissues from ENKTCL patients. A-E. Representative double immunofluorescence images of CD56 (green) and PD-L1 (red) (a), CD68 (green) and PD-L1 (red) (b), CD56 (green) and VISTA (red) (c), CD68 (green) and VISTA (red) (d), and PD-L1 (green) and VISTA (red) (e). The nucleus is labeled with DAPI (blue). Arrows indicate the colocation of indicated proteins. F. The representative IHC images of PD-L1 and VISTA from serial sections. G. The correlation between PD-L1 expression and VISTA expression depicted with scatter plot. Scale bar is 50 μm
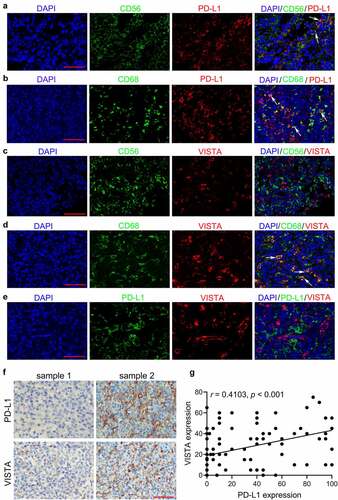
Unlike PD-L1 expression, both IHC and IF demonstrated that VISTA expression was not detected in lymphoma cells (marked with EBER or CD56) () and S1C-D). However, double IF staining showed that VISTA expression was predominantly present on CD68+ TAMs ()). VISTA positive staining was detectable to varying degrees in 96 cases (88.1%), which was more prevalent than PD-L1 expression in ENKTCL cases. Of note, double IF staining for PD-L1 and VISTA demonstrated that PD-L1 and VISTA were expressed on distinct cell subsets ()). However, they mainly co-existed in the same area within tumors and displayed a significant correlation (r = 0.4103, p < .001) ()).
Conversely, the IHC illustrated that the expression of B7-H3, B7-H4 and HHLA2 were observed neither in tumor cells nor TIICs (Figure S2A-C). While, B7-H3 expression was detected on partial membranes of vascular endothelial cells in 15 (13.7%) cases (Figure S2D-E). Taken together, these data demonstrated that, among B7 family members, only PD-L1 and VISTA were frequently expressed in ENKTCL.
Correlations between VISTA, PD-L1 expression, and clinicopathological characteristics
To explore the clinical relevance of PD-L1 and VISTA, we first divided cases into two subgroups based on the optimal cutoff values of percentage of PD-L1 (32.5%) and VISTA (27.5%) expression, which determined by ROC analysis, respectively. As shown in , high PD-L1 expression (≥32.5%) was significantly associated with distal LN metastasis (p = .033), advanced Ann Arbor stage (p = .006) and high NRI (p = .009) and PINK (p = .004) ().
Table 1. The correlation between PD-L1 and VISTA expression and clinicopathological characteristics
Similarly, high VISTA expression (≥27.5%) was also significantly correlated with the distal LN metastasis (p = .004), advanced Ann Arbor stage (p = .002), and high NRI (p = .001) and PINK (P = .003) (). Additionally, primary tumor in nasal had much higher VISTA expression, compared with primary tumor in others (p = .039) (). Moreover, a significant correlation was identified between PD-L1 and VISTA expression (p < .001) (). These results indicated that both high expressions of PD-L1 or VISTA were associated with detrimental clinicopathological characteristics.
VISTA and PD-L1 synergistically predict a poor prognosis in ENKTCL
To further evaluate the prognostic significance of PD-L1 and VISTA, a univariate analysis was performed. In addition to lesion location (HR = 1.93, 95%CI: 1.14 to 3.19, p = .013), distal LN metastasis (HR = 2.17, 95%CI: 1.27 to 3.76, p = .006), Ann Arbor stage (HR = 2.34, 95%CI: 1.46 to 3.77, p < .001), NRI score (HR = 1.72, 95%CI: 1.06 to 2.78, p = .027), PINK (HR = 1.82, 95%CI: 1.09 to 3.02, p = .023), and chemotherapy regimens (HR = 0.38, 95%CI: 0.22 to 0.65, p < .001), patients with high PD-L1 or VISTA expression were associated with increased risks of disease progression, compared with patients with low PD-L1 or VISTA expression, respectively (PD-L1: HR = 1.93, 95% CI: 1.22 to 3.07, p = .005; VISTA: HR = 2.05, 95% CI: 1.29 to 3.25, p = .001) (). Kaplan-Meier analysis showed that the high PD-L1 or VISTA expression was significantly associated with shorter PFS (PD-L1: p = .004, VISTA: p = .002) ()). In terms of OS, univariate analysis demonstrated that patients with high PD-L1 or VISTA expression experienced significantly increased overall mortality than those with low PD-L1 or VISTA expression, respectively (PD-L1: HR = 2.44, 95% CI: 1.45 to 4.11, p = .001; VISTA: HR = 2.27, 95% CI: 1.34 to 3.83, p = .002), in line with the elevated LDH (HR = 1.74, 95%CI: 1.01 to 3.00, p = .048), distal LN metastasis (HR = 1.87, 95%CI: 1.01 to 3.48, p = .048), Ann Arbor stage (HR = 2.59, 95%CI: 1.53 to 4.38, p < .001), NRI score (HR = 2.24, 95%CI: 1.28 to 3.93, p = .005) and chemotherapy regimens (HR = 0.39, 95%CI: 0.22 to 0.69, p = .003) (). Kaplan-Meier analysis also confirmed that high PD-L1 or VISTA expression was significantly associated with worse OS (PD-L1: p < .002, VISTA: p = .001) (). Furthermore, multivariate analysis demonstrated that Ann Arbor stage (HR = 3.70, 95%CI: 1.57 to 8.73, p = .003), PINK (HR = 0.19, 95%CI: 0.05 to 0.73, p = .016) and chemotherapy regimens (HR = 0.33, 95%CI: 0.18 to 0.59, p = .001) remained significant independent factors for PFS and chemotherapy regimens (HR = 0.27, 95%CI: 0.14 to 0.52, p = .001) was the only significant factor for OS. However, neither PD-L1 nor VISTA was an independent prognostic factor for both PFS and OS ().
Table 2. Univariate analysis of prognostic factors correlated with PFS and OS in patients with ENKTCL
Table 3. Multivariate analysis of prognostic factors correlated with PFS and OS in patients with ENKTCL
Figure 2. Kaplan-Meier survival curves for progression-free survival (PFS) and overall survival (OS) of patients with ENKTCL according to PD-L1 and VISTA expression. A-B. PFS for ENKTCL patients based on PD-L1 (a) and VISTA (b) expression. C-D. OS for ENKTCL patients based on PD-L1 (c) and VISTA (d) expression. E-F. PFS (e) and OS (f) for ENKTCL patients based on combination of PD-L1 and VISTA expression. Type I, PD-L1low/VISTAlow; Type II, PD-L1low/VISTAhigh or PD-L1high/VISTAlow; Type III, PD-L1high/VISTAhigh
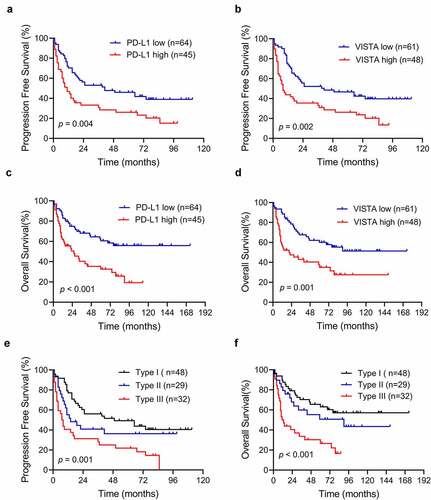
It is well known that both PD-L1 and VISTA are capable of suppressing antitumor T-cell responses. However, the combinatorial analysis of PD-L1 and VISTA expressions in prognosis of ENKTCL remained unknown. The patients were initially categorized as PD-L1low/VISTAlow, PD-L1low/VISTAhigh, PD-L1high/VISTA,low and PD-L1high/VISTAhigh. Interestingly, Kaplan–Meier analysis demonstrated that patients with PD-L1high/VISTAhigh had the shortest PFS and OS among the ENKTCL patients. Patients with PD-L1low/VISTAhigh or PD-L1high/VISTA low were associated significantly with worse PFS and OS than patients with PD-L1low/VISTAlow (Figure S3A-B). However, no differences in PFS and OS were noted between patients with PD-L1low/VISTAhigh or PD-L1high/VISTAlow (Figure S3A-B). Therefore, we further classified patients into three immune types: Type I, PD-L1low/VISTAlow; Type II, PD-L1low/VISTAhigh or PD-L1high/VISTA low; Type III, PD-L1high/VISTAhigh. As shown in Table S2, type III was significantly associated with distant lymph node metastasis (p = .009), advanced Ann Arbor stage (p = .002) and high NRI (p = .001) and PINK (p = .003). In terms of survival endpoints, both PFS and OS in type III were significantly shorter than the other two types (p < .001, )). Interestingly, multivariate analysis demonstrated that type III had a significant influence on PFS and OS, with high hazard ratios (PFS: HR = 1.47, 95% CI: 1.10–1.98, p = .010; OS: HR = 1.53, 95% CI: 1.10–2.12, p = .012) after adjusting for possible confounding variables (). In conclusion, PD-L1 and VISTA showed synergistic functions in predicting the poor prognosis in ENKTCL.
Table 4. Multivariate analysis of prognostic factors correlated with PFS and OS in patients with ENKTCL
High expression of PD-L1 or VISTA were associated with infiltration of TILs
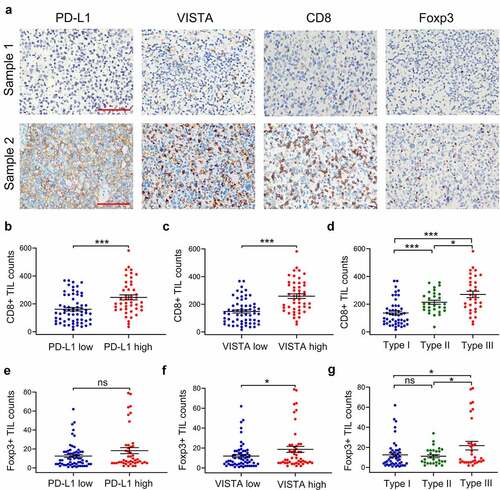
Given that both PD-L1 and VISTA display suppressive effects on TILs, we evaluated the correlation between PD-L1 and VISTA, and TILs, including CD8+ TILs and Foxp3+ TILs by IHC in serial slides ()). As shown in ), the density of CD8+ TILs was significantly higher in tumors with PD-L1 or VISTA high expression than that in corresponding PD-L1 or VISTA low expression tumor (PD-L1, p < .001; VISTA, p < .001). Moreover, the counts of CD8+ TILs were much higher in both PD-L1 and VISTA high expression tumors than that in single maker high expression tumors ()). No significant difference of Foxp3+ TIL infiltration was detected between PD-L1 high expression tumors and PD-L1 low expression tumors ()). However, the count of Foxp3+ TILs was significantly increased in VISTA high expression tumors, compared with that in VISTA low expression tumors (p = .03) ()). Additionally, the count of Foxp3+ TILs was significantly higher in both maker high expression tumors compared with the other two types (p < .05 )). These data showed high expression of PD-L1 or VISTA was associated with infiltration of TILs.
Figure 3. The correlation between the expression of VISTA and PD-L1, and the density of tumor-infiltrating lymphocytes (TILs). A. The representative images of PD-L1, VISTA, CD8 and Foxp3 from serial sections from two samples, sample 1(up) and sample 2 (down). B-D. The correlations between CD8+ TILs and PD-L1 expression (b), VISTA expression (c) or novel immune classification (d) depicted with scatter plot. E-G. The correlations between Foxp3+ TILs and PD-L1 expression (e), VISTA expression (f) or novel immune classification (g) depicted with scatter plot. All data display mean ± SEM. Scale bar is 50 μm. *p < .05, ***p < .001. ns: no significance. Type I, PD-L1low/VISTAlow; Type II, PD-L1low/VISTAhigh or PD-L1high/VISTAlow; Type III, PD-L1high/VISTAhigh
The PD-L1 and VISTA expression predicted the response to blockade of PD-1
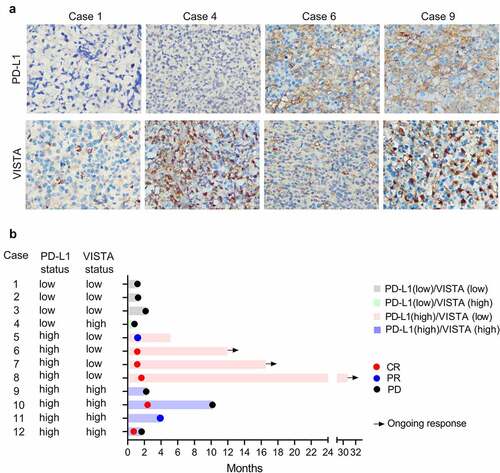
To further evaluate the clinical relevance of PD-L1 and VISTA for predicting the immunotherapeutic effect, we retrospectively investigated the PD-L1 and VISTA expression in tumors from 12 refractory and recurrent ENKTCL patients who were treated with PD-1 blockade in a validation cohort (Table S3). 2-deoxy-2-fluorine-18-fluoro-D-glucose positron emission tomography integrated with computed tomography (PET-CT) was used to assess the response to PD-1 blockade. As shown in ), all three patients with PD-L1low/VISTAlow showed no response to PD-1 blockade. Similarly, one patient with PD-L1low/VISTAhigh did not respond to PD-1 blockade. Conversely, three of the four patients with PD-L1high/VISTAlow displayed complete remission (CR) to PD-1 blockade and maintained this status for more than 12 months. However, another patient with PD-L1high/VISTA low showed partial response (PR) after the treatment of PD-1 blockade. Intriguingly, among the four patients with PD-L1high/VISTAhigh, two patients achieved CR, but the maintain time of CR was less than that in two patients with PD-L1high/VISTA low; however, the other two patients unfortunately showed transient PR or no response to PD-1 blockade ()). The data indicated that treatment with PD-1 blockade alone might not achieve a satisfying effect in patients with VISTA high expression. Thus, combination treatment with PD-L1 and VISTA blockade might be a potential option for ENKTCL patients with PD-L1high/VISTAhigh.
Figure 4. The PD-L1 and VISTA expression were associated with the response to blockade of PD-1. (a). The representative images of the PD-L1 and VISTA expression in indicated groups. (b). Swimmer’s plot showing the response to PD-1 blockade in extranodal NK/T-cell lymphoma patients, who were classified into four groups as indicated. CR, complete response; PR, partial response; PD, progressive disease
Discussion
With the development of research for immune checkpoints, it is increasingly clear that immune checkpoints can facilitate tumor escape from immune surveillance via inhibiting the T cell response. Thus, the ICIs, particularly PD-L1 and CTLA-4, have been the focus of investigation in basic and clinical research. Nevertheless, the response rate following ICIs, targeting CTLA-4, PD-1 or PD-L1, is generally less than 30%. Similarly, the efficacy of PD-1/PD-L1 blockades is limited in relapsed or refractory ENKTCL cases, indicating that alterative immune checkpoint pathways hamper antitumor immunity.Citation20 To our knowledge, this is the first study to survey the expression of a panel of immune checkpoint markers in ENKTCL. We found that PD-L1 and VISTA were variably expressed, while B7-H3 was sporadically expressed in endothelial cells in partial ENKTCL. However, no expression of B7-H4 and HHLA2 was detected in ENKTCL. These data indicate that, among the B7 family proteins, perhaps only PD-L1 and VISTA could perform the immune-modulatory function in ENKTCL.
Previous studies consistently demonstrated that PD-L1 was expressed variably by tumor cells and CD68+ TAMs in ENKTCL, which has been further confirmed in the present study.Citation38,Citation39 However, the clinical relevance and prognostic significance of PD-L1 in ENKTCL remain controversial. Two studies from South Korea showed that high PD-L1 expression was significantly associated with better clinical outcomes and a prolonged OS in ENKTCL.Citation40,Citation41 In this study, however, based on a more lager cohort, we confirmed that high PD-L1 expression in all cells (including tumor cells and immune cells) was correlated with worse clinical outcomes and shorter PFS and OS. Similarly, two studies from our center also determined that high PD-L1 expression in tumor cells or TIICs were associated with poor prognosis.Citation17,Citation18 Moreover, we previously found that patients with the high level of soluble PD-L1 in serum showed significantly worse prognosis, which was confirmed by another study.Citation17,Citation39 Given this contradiction, it is possible that the expression of other immune checkpoints may affect the prognostic significance of PD-L1 in ENKTCL.
As a novel immune checkpoint of B7 family, VISTA has received an increasing amount of attention. The VISTA protein was detected in tumor cells and immune cells in melanoma, colorectal cancer, ovarian cancer, and craniopharyngioma.Citation27,Citation28,Citation42 However, this study found that the VISTA was predominantly expressed in CD68+ TAMs but not in tumor cells, and VISTA expression was more prevalent, compared with PD-L1 expression, in ENKTCL. Taking this further, VISTA overexpression was common in PD-L1 low expression ENKTCL, and positively associated with detrimental clinical features. As for survival, previous studies demonstrated that VISTA expressed in TCs and ICs displayed discrepant prognostic significance in cancers. VISTA expression in TCs was identified as a favorable prognostic factor in patients with hepatoma, non-small cell lung cancer (NSCLC), or advanced ovarian cancer.Citation27,Citation43,Citation44 In contrast, VISTA expression in ICs was associated with poor prognosis in patients with melanoma.Citation28 Consistent with the latter, this study also confirmed VISTA mainly on ICs as an extremely poor prognostic factor in ENKTCL. Thus, VISTA expressed in TCs or ICs may exert distinct immunomodulatory functions in malignancies and a great deal of effort is necessary to define the exact mechanism of VISTA for distinct cell types.
A positive correlation between PD-L1 expression and VISTA expression was detected in this study, which corresponds to the findings in NSCLC and craniopharyngioma.Citation42,Citation44 Furthermore, Wang et al. reported that VISTA and PD-L1 co-expression was detected in the same TIICs but not in tumor cells.Citation42 In contrast, another study showed a differential expression of PD-L1 and VISTA on distinct CD68+ subsets.Citation33 In this study, we also demonstrated that PD-L1 and VISTA were expressed in distinct immune cells. Despite this, the regional co-localization of PD-L1 and VISTA proteins was frequently observed within ENKTCL. Additionally, PD-L1 and VISTA proteins displayed a synergistic effect on predicting PFS and OS in ENKTCL. These data indicate that VISTA upregulation may be mediated by locally secreted cytokines, which could also induce PD-L1 overexpression, but VISTA and PD-L1 are capable of facilitating immune escape via separate inhibitory pathways. Of note, Bharaj et al. showed that VISTA in monocytes was prominently upregulated by IL-10 and IFN-γCitation26 CD8 + T cells are the main IFN-γ-producing immune cells in tumors. In this study, we found that CD8 + T cells were mainly aggregated in the region of high PD-L1 and/or VISTA expression and the mean counts of CD8 + T cells was highest in patients with both PD-L1 and VISTA high expression. Besides, a higher mean count of Foxp3+ Tregs was detected in patients with high VISTA expression. Considering both PD-L1 and VISTA function as brakes for T-cell activation, we speculate that there is a negative feedback immunomodulatory mechanism in ENKTCL as follows: activated CD8 + T cells induce the upregulation of PD-L1 and VISTA expression via secreting IFN-γ; then, overexpressed PD-L1 and VISTA, in turn, suppress the proliferation and activation of CD8 + T cells through nonredundant inhibitory pathways, and finally undermine antitumor immune response in ENKTCL.
Actually, Liu et al. reported that a combination of PD-L1 and VISTA blockade showed a synergistic therapeutic effect in a colon cancer mouse model.Citation25 In this study, we retrospectively analyzed PD-L1 and VISTA expression in ENKTCL patients who received monoclonal therapy against PD-1. We found that ENKTCL patients with low PD-L1 expression, regardless of VISTA expression, could not benefit from the immunotherapy of PD-1 blockade. Three out of four patients with PD-L1 high expression alone achieved CR, but one patient with PD-L1 high expression alone did not respond to PD-1 blockade. These data indicated that other non-B7 family proteins, such as IDO1, TIM3, might also display an immunosuppressive role within ENKTCL. More importantly, despite the certain effect of PD-1 blockade in patients with both high expression of PD-L1 and VISTA, it was not as effective as that in patients with PD-L1 high expression alone, indicating that VISTA may be involved in resistance to PD-1 blockade. Therefore, simultaneous blockade of both PD-L1 and VISTA pathways may be a potential therapeutic regimen for ENKTCL patients bearing the high expression of PD-L1 and VISTA.
Despite a series of novel findings, our study also had some limitations as follows: First, given the tumor heterogeneity, the biopsy sample may not able to represent the immune microenvironment of the entire tumor. Second, although we conducted the largest exiting cohort study to analyze PD-L1 expression in ENKTCL, this is a retrospective, single-center study and the selection bias was inevitable. Third, this study only analyzed the expression of B7 family proteins but not other immunosuppressive factors, such as IDO1 and adenosine, in ENKTCL. Finally, because only 12 ENKTCL cases were enrolled in the validation cohort, it is hard to accurately evaluate the clinical reliability of predicting immunotherapeutic effectiveness based on the expression of PD-L1 and VISTA.
In conclusion, we comprehensively explore the expression profile of numerous B7 family proteins and elucidated that VISTA expression was more prevalent than PD-L1 in ENKTCL. Both high expressions of PD-L1 and VISTA were significant associated with worse clinical outcomes and more infiltrating CTLs. VISTA synergized with PD-L1 was an independent predictor of poor prognosis, and patients with both high expressions of PD-L1 and VISTA suffered from the worst prognosis. More importantly, VISTA was involved in the resistance to PD-1/PD-L1 blockades in ENKTCL. These findings indicate VISTA might be an ideal target for therapeutic modulation alone or in combination with PD-L1 blockade for ENKTCL immunotherapy.
Authors’ contributions
Conception and Design: Gao Yan, Xu Chen and Hui-qiang Huang; Performing experiments: Hai-xia He, Qiang-hua Zhou and Jian-chang Fu; Statistical analyses: Hai-xia He, Qi-xiang Rong, Cheng Huang and Yan-xia He; Drafting of the article: Hai-xia He and Qiang-hua Zhou; Critical revision of manuscript: all authors; All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
None potential conflicts of interest were disclosed
Ethics approval
This study was approved by the Clinical Research Ethics Committee of Sun Yat-sen University Cancer center (B202100301).
Supplemental Material
Download ()Acknowledgments
This work was supported by a grant from Guangdong Science and Technology Department. We would like to thank Dr. Xinke Zhang and Dr. Yuanzhong Yang from Department of Pathology, SYSUCC for their assistance in IHC score.
Availability of data and materials
All datasets used and/or analyzed during the current study are available in the Research Data Deposit public platform (www.researchdata.org.cn, number RDDB2021001093) from the corresponding author on request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Cazzola M. Introduction to a review series: the 2016 revision of the WHO classification of tumors of hematopoietic and lymphoid tissues. Blood. 2016;127:2361–11. doi:10.1182/blood-2016-03-657379.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569.
- Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, Kon S. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996;77(10):2137–2149. doi:10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V.
- Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, Sekine T, Yamamoto K, Shimizu N. Characterization of novel natural killer (NK)–cell and γδ T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. 2001;97(3):708–713. doi:10.1182/blood.V97.3.708.
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim W-S, Sng I, Vose J, Armitage JO, Liang R. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood. 2009;113(3931–7):3931–3937. doi:10.1182/blood-2008-10-185256.
- Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017;10(85). doi:10.1186/s13045-017-0452-9.
- Hu B, Oki Y. Novel immunotherapy options for extranodal NK/T-Cell Lymphoma. Front Oncol. 2018;8(139). doi:10.3389/fonc.2018.00139.
- Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, Leung AYH, Chim C-S. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia lymphoma study group. Blood. 2012;120(2973–80):2973–2980. doi:10.1182/blood-2012-05-431460.
- Li YX, Yao B, Jin J, Wang W-H, Liu Y-P, Song Y-W, Wang S-L, Liu X-F, Zhou L-Q, He X-H, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(181–9):181–189. doi:10.1200/JCO.2005.03.2573.
- Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, Park Y, Chang KM, Maeda Y, Ishida F, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17:389–400. doi:10.1016/S1470-2045(15)00533-1.
- Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi:10.1016/S1470-2045(15)70076-8.
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(2455–65):2455–2465. doi:10.1056/NEJMoa1200694.
- Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(2698–704):2698–2704. doi:10.1200/JCO.2015.65.9789.
- Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, Zhang X, Chang Y, Sun Z, Yu H, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11(1). doi:10.1186/s13045-018-0559-7.
- Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, Xu H, Ogurtsova A, Bleich KB, Cornish TC, et al. PD-L1 expression in melanoma: a quantitative immunohistochemical antibody comparison. Clin Cancer Res. 2017;23(4938–4944):4938–4944. doi:10.1158/1078-0432.CCR-16-1821.
- Zhou QH, Li K-W, Chen X, He H-X, Peng S-M, Peng S-R, Wang Q, Li Z-A, Tao Y-R, Cai W-L, et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer. 2020;8:e000157. doi:10.1136/jitc-2019-000157.
- Bi XW, Wang H, Zhang -W-W, Wang J-H, Liu W-J, Xia Z-J, Huang H-Q, Jiang W-Q, Zhang Y-J, Wang L, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. doi:10.1186/s13045-016-0341-7.
- Zhang XW, Bi X-W, Liu P-P, Liu Z-L, Nie M, Yang H, Lei D-X, Xia Y, Jiang W-Q, Zeng W-A, et al. Expression of PD-L1 on monocytes is a novel predictor of prognosis in natural Killer/T-cell lymphoma. Front Oncol. 2020;10:1360
- Kwong YL, Chan TSY, Tan D, Kim SJ, Poon L-M, Mow B, Khong P-L, Loong F, Au-Yeung R, Iqbal J, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437–2442. doi:10.1182/blood-2016-12-756841.
- Kim SJ, Lim J-Q, Laurensia Y, Cho J, Yoon S-E, Lee J-Y, Ryu K-J, Ko Y-H, Koh Y, Cho D, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136(24):2754–2763. doi:10.1182/blood.2020007247
- Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L-F, Gondek D, Wang Y, Fava RA, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi:10.1084/jem.20100619.
- Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(1537–41):1537–1541. doi:10.4049/jimmunol.1100660.
- Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, Guan J, Singh R, Rollins S, Solorz A, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(74–85):74–85. doi:10.1111/imm.13001.
- Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74(7):1924–1932. doi:10.1158/0008-5472.CAN-13-1504.
- Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A. 2015;112(21):6682–6687. doi:10.1073/pnas.1420370112.
- Bharaj P, Chahar HS, Alozie OK, Rodarte L, Bansal A, Goepfert PA, Dwivedi A, Manjunath N, Shankar P. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS One. 2014;9(10):e109103. doi:10.1371/journal.pone.0109103
- Zong L, Zhou Y, Zhang M, Chen J, Xiang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother. 2020;69(33–42):33–42. doi:10.1007/s00262-019-02434-5.
- Kuklinski LF, Yan S, Li Z, Fisher JL, Cheng C, Noelle RJ, Angeles CV, Turk MJ, Ernstoff MS. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother. 2018;67(7):1113–1121. doi:10.1007/s00262-018-2169-1.
- Wu L, Deng -W-W, Huang C-F, Bu -L-L, Yu G-T, Mao L, Zhang W-F, Liu B, Sun Z-J. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(5):627–636. doi:10.1007/s00262-017-1968-0.
- Xie S, Huang J, Qiao Q, Zang W, Hong S, Tan H, Dong C, Yang Z, Ni L. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother. 2018;67(11):1685–1694. doi:10.1007/s00262-018-2227-8.
- Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–1676. doi:10.1038/modpathol.2017.89.
- Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–555. doi:10.1038/nm.4308.
- Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, Kim J, Sepulveda AM, Sharp M, Maitra A, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(5):1692–1697. doi:10.1073/pnas.1811067116.
- Zhou Q-H, Han H, Lu J-B, Liu T-Y, Huang K-B, Deng C-Z, Li Z-S, Chen J-P, Yao K, Qin Z-K, et al. Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients. Cancer Commun (Lond). 2020;40(1):3–15. doi:10.1002/cac2.12001.
- Xie R, Chen X, Cheng L, Huang M, Zhou Q, Zhang J, Chen Y, Peng S, Chen Z, Dong W, et al. NONO inhibits lymphatic metastasis of bladder cancer via alternative splicing of SETMAR. Mol Ther. 2020;29:291–307. doi:10.1016/j.ymthe.2020.08.018.
- Chen SY, Yang Y, Qi S-N, Wang Y, Hu C, He X, Zhang -L-L, Wu G, Qu B-L, Qian L-T, et al. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making. Leukemia. 2021;35(130–142):130–142. doi:10.1038/s41375-020-0791-3.
- Qi SN, Yang Y, Song Y-Q, Wang Y, He X, Hu C, Zhang -L-L, Wu G, Qu B-L, Qian L-T, et al. First-line non-anthracycline-based chemotherapy for extranodal nasal-type NK/T-cell lymphoma: a retrospective analysis from the CLCG. Blood Adv. 2020;4(3141–3153):3141–3153. doi:10.1182/bloodadvances.2020001852.
- Cho J, Kim SJ, Park W-Y, Kim J, Woo J, Kim G, Yoon SE, Ko YH, Kim WS. Immune subtyping of extranodal NK/T-cell lymphoma: a new biomarker and an immune shift during disease progression. Mod Pathol. 2020;33(4):603–615. doi:10.1038/s41379-019-0392-8.
- Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877–890. doi:10.1007/s00262-017-1987-x.
- Jo J-C, Kim M, Choi Y, Kim H-J, Kim JE, Chae SW, Kim H, Cha HJ. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2017;96(1):25–31. doi:10.1007/s00277-016-2818-4.
- Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim C-W, Jeon YK. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. 2016;469(5):581–590. doi:10.1007/s00428-016-2011-0.
- Wang Y, Deng J, Wang L, Zhou T, Yang J, Tian Z, Yang J, Chen H, Tang X, Zhao S, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J Immunother Cancer. 2020;8(2):e000406. doi:10.1136/jitc-2019-000406.
- Zhang M, Pang H-J, Zhao W, Li Y-F, Yan L-X, Dong Z-Y, He X-F. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer. 2018;18: 511
- Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, Syrigos K, Toki M, Zhao H, Chen L, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24(1562–1573):1562–1573. doi:10.1158/1078-0432.CCR-17-2542.
