ABSTRACT
RANK signaling in mouse mammary tumor cells exerts an immunosuppressive environment by promoting the infiltration of pro-tumorigenic neutrophils and preventing CD8 T cell recruitment. Single-agent denosumab led to an increased tumor immune infiltration by lymphocytes and CD8 + T cells in breast cancer patients, supporting the immunomodulatory role of RANK signaling.
The receptor activator of nuclear factor κB (RANK) signaling pathway is widely known for its role in bone remodeling. In osteoclasts, when RANK is activated by its ligand (RANKL), bone tissue resorption is promoted. Therefore, denosumab, a human monoclonal antibody against RANKL, is currently given in the clinic to treat osteoporosis and skeletal-related events caused by bone metastasis.Citation1
However, RANK has also been described to play other roles in several tissues and contexts, such as the mammary gland, breast cancer, and the immune system.Citation1 RANK acts as a paracrine mediator of progesterone, expanding mammary progenitor cells, and mediates the early steps of progesterone-driven mammary tumorigenesis.Citation2–4 Studies with preclinical models manifested that RANK plays a key role in tumor initiation in both genetic and carcinogen plus hormonal-induced murine tumor models.Citation2,Citation4,Citation5 Tumor latency is longer upon RL pharmacological inhibition and after genetic loss of either RANK or RL in several models of tumorigenesis.Citation2,Citation4–6 In the immune system, RANK pathway is involved in lymph node development,Citation7 the activation of dendritic cells, monocytes and T cells, and the establishment of central and peripheral tolerance.Citation1,Citation8,Citation9
Given these diverse roles of RANK pathway, the aim of this study was to evaluate the role of RANK pathway in the crosstalk between breast tumor cells and the immune microenvironment. Using tumor transplants from MMTV-PyMT murine breast cancer model from either control (RANK+/+) or RANK-depleted (RANK-/-) background, the effects of tumor-intrinsic RANK signaling on the immune microenvironment were evaluated.
Members of our group already reported that RANK-/- tumor transplants had a longer tumor latency than RANK+/+ tumors.Citation5 However, in this new publication, we observed that when implanted in immunodeficient hosts, RANK-/- tumors appear as fast as RANK+/+ tumors, implying that the differences in tumor latency are mediated by a stronger anti-tumor immune response against RANK-/- tumors.
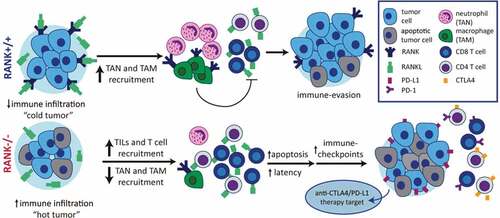
Indeed, RANK-/- tumor transplants presented a higher immune infiltration, enriched in anti-tumoral populations (T cells and CD8+ T cells) and with lower macrophage and neutrophil infiltration. By using depletion antibodies, neutrophils were identified as a key immune population promoting tumor growth and CD8 T cell recruitment, while CD8+ T cells were described to mediate the differences in tumor latency seen in RANK-/- tumor transplants. RANK-/- tumor transplants also showed upregulation of immune checkpoints. In fact, when combining RANKL inhibitors with immune checkpoint inhibitors (anti-CTLA4 or anti-PD-L1), RANK+/+ tumor transplants present a stronger attenuation of tumor growth, while RANK-/- tumors do not further benefit from the addition of anti-RANKL.
Importantly, in this manuscript, preclinical data are supported by the clinical observations of the D-BEYOND clinical trial (NCT01864798*), where the effects of denosumab on tumor immune infiltration are evaluated. Twenty-four premenopausal women diagnosed with early breast cancer were recruited in the D-BEYOND clinical trial. Patients undergo biopsy and then receive two doses of denosumab before having surgical removal of the primary tumor. The design of the trial allowed for evaluation of the same tumor before and after denosumab treatment. Both by immunohistochemistry and RNAseq deconvolution analysis, denosumab treatment increased tumor infiltrating lymphocytes (TILs), in particular T cells and CD8 T cells, resembling the observations with the preclinical models. Patients were classified into two groups based on their increase in TILs, with those presenting ≥10% increase classified as “responders” and the rest as “non-responders”. Several baseline parameters were analyzed to identify the putative predictors of denosumab’s immunomodulatory response. Higher T regulatory cell (Treg) tumor infiltration, higher serum RANKL levels, and higher tumor RANK activation (as measured with the RANK metagene) were observed to correlate with the responders’ group.
Overall, the data presented in this manuscript suggest that RANK signaling in tumor cells promotes the establishment of an immunosuppressive environment. Inhibition of the pathway would lead to an increased infiltration of anti-tumoral immune populations (see ). This is of special relevance for breast cancer tumors, which have not shown so much benefit from immunotherapy, due to their low immune infiltration.Citation10 Denosumab might improve the response to immunotherapy in those patients with high serum RANKL, Treg tumor infiltration, or tumor RANK pathway activation.
Figure 1. The RANK pathway as immune modulator in breast cancer. RANK expression in luminal breast cancer cells leads to the expression of pro-inflammatory cytokines/chemokines favoring recruitment of tumor associated-macrophages (TAMs) and neutrophils (TANs), immunosuppressive populations which interfere with lymphocyte T cell recruitment and/or activity. Denosumab (anti-RANKL) or RANK signaling inhibition results in increased TILs, lymphocytes and CD8+ T cell infiltration, transforming immune “cold” tumors into “hot” ones and attenuating tumor growth. Eventually the exacerbated immune response driven by RANK inhibition will induce the expression of immune checkpoints evading immune surveillance and allowing tumor growth. These results support the benefit of combining RANKL and immune checkpoint inhibitors in luminal breast cancer
Excitingly, our own group is currently running a clinical trial with denosumab (D-BIOMARK, NCT03691311), in a similar setting to D-BEYOND, but also including a control arm and postmenopausal patients. With D-BIOMARK, we aim to corroborate and expand our knowledge of the immunomodulatory effects of denosumab and potential biomarkers of response. Based on the results reported in this publication and the future observations on D-BIOMARK, new clinical trials for denosumab in combination with immune checkpoint inhibitors will be designed to explore this new and promising indication for RANKL inhibitors.
The D-BEYOND clinical trial was approved by the Ethics Committee of the trial sponsor, the Medical Ethics Committee of the Institute Jules Bordet (IJB No.: 2064) and the Melbourne Health Human Research Ethics Committee. All patients provided written informed consent prior to study entry.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- González-Suárez E, Sanz-Moreno A. RANK as a therapeutic target in cancer. FEBS J. 2016;283:2018–2. doi:10.1111/febs.13645.
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi:10.1038/nature09495.
- Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi:10.1038/nature09091.
- Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi:10.1038/nature09387.
- Yoldi G, Pellegrini P, Trinidad EM, Cordero A, Gomez-Miragaya J, Serra-Musach J, Dougall WC, Muñoz P, Pujana M-A, Planelles L, et al. RANK signaling blockade reduces breast cancer recurrence by inducing tumor cell differentiation. Cancer Res. 2016;76:5857–5869. doi:10.1158/0008-5472.CAN-15-2745.
- Nolan E, Vaillant F, Branstetter D, Pal B, Giner G, Whitehead L, Lok SW, Mann GB, Rohrbach K, Huang L-Y, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22:933–939. doi:10.1038/nm.4118.
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi:10.1101/gad.13.18.2412.
- Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi:10.3389/fimmu.2014.00511.
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi:10.1038/36593.
- Gatti-Mays ME, Balko JM, Gameiro SR, Bear HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky K, et al. If we build it they will come: targeting the immune response to breast cancer. npj Breast Cancer. 2019;5:37. doi:10.1038/s41523-019-0133-7.
