ABSTRACT
Preclinical studies suggest that some effects of conventional chemotherapy, and in particular, gemcitabine, are mediated through enhanced antitumor immune responses. The objective of this study was to use material from a randomized clinical trial to evaluate whether patients with preexisting immune infiltrates responded better to treatment with gemcitabine + docetaxel (GD) compared to docetaxel alone. Formalin fixed, paraffin-embedded breast cancer tissues from SBG0102 phase 3 trial patients randomly assigned to treatment with GD or docetaxel were used. Immunohistochemical staining for CD8, FOXP3, LAG3, PD-1, PD-L1 and CD163 was performed. Tumor infiltrating lymphocytes (TILs) and tumor associated macrophages were evaluated. Prespecified statistical analyses were performed in a formal prospective-retrospective design. Time to progression was primary endpoint and overall survival secondary endpoint. Correlations between biomarker status and endpoints were evaluated using the Kaplan–Meier method and Cox proportional hazards models. Biomarker data was obtained for 237 patients. There was no difference in treatment effect according to biomarker status for the whole cohort. In planned subgroup analysis by PAM50 subtype, in non-luminal (basal-like and HER2E) breast cancers FOXP3 was a significant predictor of treatment effect with GD compared to docetaxel, with a HR of 0.22 (0.09–0.52) for tumors with low FOXP3 compared to HR 0.92 (0.47–1.80) for high FOXP3 TILs (Pinteraction = 0.01). Immune biomarkers were not predictive of added benefit of gemcitabine in a cohort of mixed breast cancer subtypes. However, in non-luminal breast cancers, patients with low FOXP3+ TILs may have significant benefit from added gemcitabine.
Introduction
Globally, breast carcinoma is the most common malignancy amongst women and accounts for more than two million new cases diagnosed each year. Despite targeted treatment options and the relatively high efficacy of conventional chemotherapy, breast cancer remains responsible for 15% of all cancer deaths in women.1 The basal-like/triple-negative (TNBC) subtype of breast cancer remains the most challenging, as there are currently few treatment options beyond conventional chemotherapy.Citation1Citation2,Citation3
In recent years, the importance of the immune microenvironment surrounding cancer cells has gained increasing interest with the advent of immunotherapy and the discovery of the significance of an activated immune system for the prognosis of cancer.Citation4–6 In breast cancer, most research has been centered around basal-like/TNBC or HER2 positive subtypes, as these are often characterized by a pronounced inflammatory infiltrate that has shown clear positive prognostic significance.Citation7,Citation8 In this context, it has been shown that tumor infiltrating lymphocytes and particularly the CD8 positive cytotoxic T-cell subset are important for immune-mediated tumor cell death.Citation9
PD-L1 inhibitor immune therapy in combination with nab-paclitaxel for advanced TNBC has recently been approved.Citation10,Citation11 However, as breast carcinoma is not one of the most immunogenic forms of cancer, the search continues for effective approaches to augment the immune response by priming tumors for immune therapy.Citation2,Citation12 Conventional chemotherapy may play a role in this, as several agents have shown relevant off-target effects on both the innate and adaptive immune systems.Citation2,Citation13,Citation14 Potentiating effects can be mediated through immunogenic tumor cell death, changes in IFN-γ and interleukin release, novel antigen presentation, and post-treatment replenishment of the lymphocyte pool with potential replacement of regulatory and exhausted phenotype T-cells.Citation2,Citation13,Citation14 Multiple clinical trials have investigated or are investigating the advantages of adding conventional backbone chemotherapy to immunotherapy; several of these have shown promising improvements in survival of patients receiving both chemotherapy and immunotherapy compared to patients receiving only monotherapyCitation2,Citation15. Thesignificance of which chemotherapy drug should be combined with immune therapy has recently been discussed in the context of the Impassion 130 trial combining the PD-L1 inhibitor atezolizumab with nab-paclitaxel,Citation11 and the subsequent Impassion 131 trial combining atezolizumab with paclitaxel.Citation16 Where the first study led to the approval of atezolizumab for TNBC, the second trial has not yet shown significant results. It is a matter for discussion whether this could be due to a greater use of steroids in the latter trial as a prerequisite for use with paclitaxel, differences in the delivery of nab-paclitaxel and paclitaxel in the tumor microenvironment or even due to TNBC heterogeneity.Citation17 Within TNBC as a category, the immune activated subtype represents a composition of microenvironments defined by factors beyond PD-L1 expression that could explain the differences in immunotherapy response between the two trials. In view of the negative results of the IMpassion-131 trial, it is currently recommended to use the combination of nab-paclitaxel with atezolizumab in metastatic TNBC.Citation18
Docetaxel and gemcitabine are among the forms of conventional cytotoxic chemotherapy that have effects on the tumor immune microenvironment (TME). In preclinical models, both drugs induce changes in the TME by modulating innate and adaptive immune responses, leading to an increased susceptibility to immune mediated cell death.Citation13,Citation19–27 Gemcitabine in particular seems to enhance T-cell activation in pre-clinical studies. Changes in the TME following gemcitabine treatment include inhibition of myeloid-derived suppressor cells, leading to more activated cytotoxic T-cells, naïve T-cell activation, and upregulation of natural killer cells along with changes in macrophage polarization.Citation22,Citation24,Citation28 Clinically, studies have suggested that an immune-active environment may predict chemotherapy efficacy including regimes containing taxanes or gemcitabine.Citation29 However, the predictive capacity of the TME for benefit from gemcitabine treatment, alone or in combination with docetaxel, has not yet been investigated in a setting involving randomized clinical trials of breast cancer.
For this study, we used the Danish SBG0102 phase III clinical trial, which randomized patients with advanced breast cancer to receive either docetaxel or gemcitabine + docetaxel (GD). The original trial did not find a survival benefit of GD over docetaxel alone.Citation30 However, subsequent post-hoc analysis using the PAM50 assay showed a large survival advantage in the GD group amongst patients with basal-like breast cancer.Citation31 As the basal-like subtype is the most immune-active intrinsic subtype of breast cancer,Citation32 we investigated whether the added benefit of gemcitabine, an immunostimulatory form of chemotherapy, correlated with more active immune responses in the primary tumor. As biomarkers for an activated immune response, we assessed CD8 as our primary T-cell marker; stromal tumor infiltrating lymphocytes (TILs); and immunosuppressive biomarkers including PD-1 (programmed cell death protein 1), PD-L1 (programmed death ligand 1), LAG3 (lymphocyte activation gene 33),and FOXP3 (forkhead box protein 3) were also investigated. Additionally, as a marker for the innate immune system, we investigated the predictive capacity of CD163 expressing tumor associated macrophages.
Material and methods
Study population and design
The present study is based on surgical material from patients enrolled in the Danish Breast Cancer Group (DBCG) randomized clinical trial SBG0102. In this trial, patients with locally advanced or metastatic breast cancer were randomized to receive either docetaxel or docetaxel + gemcitabine. A total of 337 women were included in the trial. Patients received either docetaxel (100 mg/m2) on day 1 every 21 days, or gemcitabine (1000 mg/m2) on days 1 and 8 and docetaxel (75 mg/m2) on day 8 every 21 days. A detailed description of the study including eligibility criteria and study results has been published previously.Citation30 No patients had received neoadjuvant chemotherapy prior to inclusion.
The original study and subsequent supplementary biomarker studies were approved by the Danish National Committee on Biomedical Research Ethics [KF12-315,632 (August 2006)/H-KF-02-045-01 (June 2007)/H-190131109 (June 2019) with additional approval 77987 (March 2021)].
The study was designed in accordance with the reporting recommendations for tumor marker prognostic studies (REMARK).Citation33 Based on this, our group performed a test of the prespecified hypothesis that a preexisting immune response, as defined by the expression of specific immune response biomarkers in primary breast cancer tumor tissue, predicts superior time to progression and overall survival in advanced breast cancer patients, when randomized to a treatment regime containing both gemcitabine and docetaxel as compared to (higher dose) docetaxel alone.
As our primary biomarker, we selected CD8 positive intratumoral tumor infiltrating lymphocytes (TILs), as this biomarker mainly identifies the cytotoxic T-cell subset, shown to be a major effector of antitumor immune interactions and to carry prognostic significance in several studies.Citation34–37 For secondary analyses, we also selected well-described immune checkpoint molecules including PD-1, PD-L1, and LAG3. FOXP3 was assessed, as a marker for regulatory T-cells associated with dampening immune responses.Citation38 Supplementary, CD163, a macrophage marker, was assessed for predictive capacity.Citation39
Analyses were carried out on the whole cohort, and as a preselected subgroup analysis on the non-luminal breast cancers, a category combining the basal-like and HER2-enriched (HER2E) PAM50 subtypes based on published research showing these two subtypes to be similar in their tumor-immune microenvironment.Citation7,Citation32 Tests for heterogeneity of prognostic significance in luminal subtypes (luminal A and luminal B) versus non-luminal subtypes were also performed.
Study material and immunohistochemical staining
In this study, we utilized 11 previously constructed tissue microarray (TMA) blocks containing formalin fixed, paraffin-embedded primary tumor tissue from 276 patients enrolled in the SBG0102 study, provided by the DBCG Tumor Tissue Data Repository. The TMAs contain two 2 mm cores of primary breast cancer tissue per patient, as previously described.Citation40 Immunohistochemical (IHC) staining for CD8 (clone C8/144B, Dako), PD-1 (clone NAT105, CellMarque), LAG3 (clone 17B4, Abcam), FOXP3 (clone 236A/E7, Abcam) and CD163 (clone 10D6, Leica Biosystems) was performed as per manufacturer’s protocol using the Ventana Discovery Ultra staining platform at the Genetic Pathology Evaluation Center (Vancouver, Canada). PD-L1 staining using the SP142 clone (Roche) was performed following manufacturer’s instructions at the Victoria Deeley Research Center (Victoria, Canada).
Staining and scoring of the standard breast cancer biomarkers ER, PR, and HER2 had been performed in connection with previous publications following published methods at the Genetic Pathology Evaluation Center, Vancouver, Canada.Citation40–43
Slides were scanned and digitized on the Aperio AT2 scanner at 20X magnification. Representative photomicrographs are shown in supplementary Figure S1.
Biomarker assessment
Biomarker assessment was performed by two experienced pathologists, DG (CD8, PD-1, FOXP3, LAG3 and CD163) and ES (TILs and PD-L1). Difficult cases were discussed between the two pathologists and consensus was reached. The two pathologists did not have access to clinical data when scoring the biomarkers.
For CD8, LAG3, PD-1 and FOXP3, scoring data were captured for stromal TILs (sTILs) and intratumoral TILs (iTILS). As previously published, sTILs were defined as TILs present in the peritumoral stroma, not in direct contact with carcinoma cells. iTILs were defined as TILs in direct contact with carcinoma cells. This strategy was chosen as TILs in the intratumoral and stromal compartment have been shown to exhibit different prognostic associations.Citation44,Citation45
For CD163, membranous or cytoplasmic expression on tumor-associated macrophages was scored as previously described.Citation46
CD8, LAG3, FOXP3 and PD-1 iTILs and sTILs and CD163 positive macrophages were reported as an absolute count in one TMA core, or in cases where two interpretable cores were available, the mean of the absolute counts in the two TMA cores corresponding to each patient. Cutpoints for biomarker positivity were locked down prior to clinical data analysis. For CD8, cutpoints that had been previously established and validated on a large cohort were used.Citation44 As the previous cohort used 0.6 mm cores, cutoffs were expanded by a factor 10 to account for the increased area of the 2 mm cores used in this study. For CD8 iTILs, the adjusted cutoffs were ≤10 vs >10; for CD8 sTILs cutoffs were ≤30 vs >30. For LAG3 and PD-1, we followed our previous publications on lymphocyte biomarkers and set cutoffs to dichotomize between no expression and any expression (0 vs > 0) both for iTILs and sTILs.Citation47,Citation48 FOXP3 iTILs cutoff was set to <2 vs ≥2 and cutoff for FOXP3 sTILs was <3 vs ≥3.Citation48 The cutoffs for CD163 expressing tumor associated macrophages were also adjusted from studies in our previous cohort and were set to <56 vs ≥56.Citation39
PD-L1 scoring was performed according to manufacturer’s guidelines as area of PD-L1 positive TILs out of total tumor area.Citation49 A cutoff of ≥1% for positive expression was used when relevant, as this is the commonly used cutoff for clinical trials and treatment indications.Citation10 When relevant, PD-L1 was also tested as a continuous variable.
TILs were assessed on hematoxylin-eosin (HE) stained slides, according to the extensively validated guidelines published by the International Immuno-Oncology Biomarker working group. Briefly, TILs were scored as the percentage of intratumoral stroma occupied by lymphocytes.Citation9 The median prevalence of TILs in our cohort was used as cutoff (≤1% vs >1%). When relevant, TILs were also tested as a continuous variable.
PAM50 intrinsic subtyping
PAM50 intrinsic subtyping using the Nanostring nCounter system on the SBG0102 material has been published previously, including a detailed description of RNA extraction, Nanostring nCounter processing and data analysis.Citation31
Statistical analysis
Statistical analysis was performed by the DBCG according to a written prespecified plan. Descriptive statistics were utilized to summarize patient characteristics. Differences in patient characteristics between biomarker groups were assessed with chi-squared test or Fishers exact test, excluding unknowns.
As in the original trial, the primary endpoint was time to progression (TTP) and secondary endpoint overall survival (OS). TTP was defined as the time from random assignment to date of progression with censoring at last visit date or death. Complete follow-up on vital status data until July 1st, 2020 was ensured through linkage to the Danish Civil Registration System (CPR). OS was defined as the time elapsed from random assignment until death from any cause.
TTP and OS rates were estimated according to the Kaplan–Meier method. The effects of the biomarker groups (low vs. high) and biomarkers as continuous variables per 10%-point increase on TTP and OS were quantified in terms of hazard ratios (HRs) with 95% confidence intervals (CI) and estimated unadjusted and adjusted using Cox proportional hazard models. Biomarkers were assessed individually, i.e. in separate models.
In multivariate analysis, the following variables were considered: PAM50 subtype, ECOG performance status (0–1 vs 2), visceral disease (yes vs no), age (<50 vs ≥ 50), stage of disease (locally advanced vs metastatic), number of metastatic sites (<3 vs ≥ 3) and treatment regime. Variables with statistical significance were included in the final multivariate models. Tests for proportional hazards were assessed by Schoenfeld residuals models. The Wald test for interaction between biomarker expression groups and subtype (luminal vs non-luminal) or treatment arm was used to evaluate differences between subgroups and predictive effects, respectively.
All tests were two-sided with a significance level of 0.05, unadjusted for number of comparisons.
All statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Clinicopathological characteristics and biomarker expression
Biomarker assessment was available in 237 out of the 276 patients with TMA available. In 39 cases, biomarker assessment was not possible due to insufficient tissue remaining in the core, or too few invasive breast cancer cells present. Out of the 237 patients, IHC assessment of CD8 was possible in 234 cases, 233 cases for FOXP3, 230 cases for LAG3, 231 cases for PD-1, 219 cases for PD-L1 and 224 cases for CD163. TIL evaluation on HE slides was possible in 216 cases. Out of the 237 patients with IHC scores, 5 patients did not have PAM50 information available. A flow diagram of the study cohort is presented in Supplementary Figure S2.
The group of patients available for biomarker assessment differed from the excluded group wwith regard toprior chemotherapy regimens (anthracyclines vs non-anthracyclines) and radiotherapy (P < .05) (data not shown). This is most likely due to patients with locally advanced disease, as these patients more often only had a needle biopsy available for testing, resulting in tissue being unavailable or with too few tumor cells for testing. The excluded group did not differ from the included group in other parameters. Patient characteristics according to treatment allocation have been previously described for the whole cohortCitation30 and can be seen in Supplementary Table S1 for the cohort used in this study.
We found high CD8 iTIL expression in a total of 80 patients (34%); the median absolute count was 7 per 2 mm tissue core. High CD8 sTIL expression was seen in 182 patients (78%) with a median of 100. Patient characteristics according to CD8 iTILs can be seen in Supplementary Table S3. Neither CD8 iTIL nor sTIL expression were significantly associated with the subtype () or hormone receptor status (Supplementary Table S3) in this data set of primary tumor tissue samples from patients who developed advanced disease. CD8 positivity was also not associated with any other relevant clinicopathological characteristics.
Table 1. Correlations of investigated biomarkers with PAM50 subtypes (n = 232)
Median absolute counts per core were 1 for FOXP3 iTILs, 12 for FOXP3 sTILs, and 0 for LAG3 and PD-1 (iTILs or sTILs). Median PD-L1 expression was 0, and median HE TILs were 1%. Median CD163 positive cell count (macrophages) was 46. Associations of biomarkers with PAM50 subtypes are presented in . Expression of FOXP3, LAG3, PD-1, PD-L1, and HE TIL counts were all associated with PAM50 subtypes (P < .05). For all of them, a higher percentage of positive expression was seen in the basal-like and HER2 enriched subtypes. PD-1, LAG3 and FOXP3 iTILs were also associated with ER negativity (data not shown).
For the whole cohort, total number of events were 231 for OS and 176 for TTP. For the non-luminal subtypes (basal-like + HER2E), there were 74 events for OS and 59 events for TTP.
In multivariate analysis of the study, PAM50 subtype, ECOG performance status (0–1 vs 2) and visceral disease (yes vs. no) were found to be significant for OS. Treatment with combination therapy (GD) was associated with significantly longer TTP compared to monotherapy with docetaxel. Likewise, PAM50 subtypes were significantly associated with TTP. Hazard ratios for all significant variables are presented in Supplementary Table S2.
Prognostic value of immune biomarkers in SBG0102 advanced breast cancers
In the whole cohort, CD8 iTILs were not prognostic for either TTP or OS in uni- or multivariate analysis (, and b, Supplementary Figure S3, A-B and Supplementary Table S4). In a test of heterogeneity for differential effect in the luminal and non-luminal subtypes (basal like and HER2E together, 76 patients), there was a lower HR for OS for the CD8 iTILs high group in univariate analysis (HR 0.69 [95%CI 0.43–1.11], vs HR 1.28 [0.91–1.81] for luminal cases, Pinteraction = 0.04). This was not preserved in multivariate analysis (0.70 [0.43–1.13] vs HR 1.21 [0.84–1.74], Pinteraction = 0.08) (Supplementary Table S5).
Table 2. Prognostic hazard ratios in the overall cohort for high vs low levels of immune biomarkers in univariate analysis
Figure 1. Time to progression (TTP) and associations with CD8 levels in the SBG0102 trial population. A. CD8 iTILs and TTP in the overall cohort, B. CD8 iTILs and TTP in the non-luminal (BL and HER2E) subtypes C. TTP for the CD8 iTILs low group, stratified by treatment with docetaxel or docetaxel + gemcitabine (DG) D. TTP for the CD8 iTILs high group, stratified by treatment with docetaxel or DG. Corresponding figures for overall survival can be seen in Supplementary Figure S3. iTILs: intratumoral tumor infiltrating lymphocytes
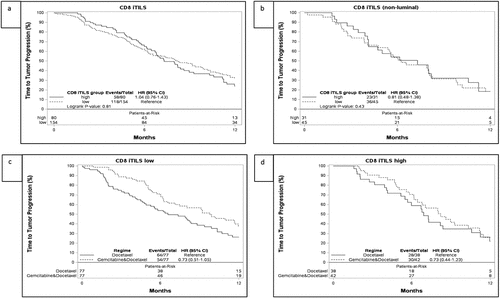
While we had chosen intratumoral TILs as our primary mode of assessment of CD8 as we had in other studies,Citation44,Citation50 stromal CD8+ TILs were also assessed. CD8 sTILs did not show prognostic significance for the whole cohort. However, in a test of heterogeneity of effect in the luminal and nonluminal subtypes, there was also a trend toward a lower HR for TTP in the CD8 sTILs high non-luminal subtypes than in the CD8 sTILs high luminal subtypes (HR 0.46 [0.24–0.86] for non-luminal subtypes vs HR 1.00 [0.63–1.58] for luminal subtypes, Pinteraction = 0.06) in univariate analysis. The same was seen in multivariate analysis (Supplementary Table S5). CD8 sTILs also showed a trend for improved OS in the non-luminal subtypes in univariate analysis, although this was not significant (HR 0.65 [0.38–1.12]) (Supplementary Figure S4) .
FOXP3 iTILs, PD-1, LAG3 iTILs and HE TILs also did not show prognostic significance when looking at the whole cohort in univariate analysis (). LAG3 sTILs did show prognostic significance for OS in univariate analysis (HR 1.46 [1.11–1.93]), and PD-L1 high expression was significant for TTP in univariate analysis (HR 1.59 [1.03–2.44]); however, these effects were not retained in multivariate analysis (Supplementary Table S4). FOXP3 sTILs were not significant for TTP in univariate analysis; however, in multivariate analysis including PAM50 subtypes and treatment regime, high FOXP3 sTILs were significant for TTP (HR 0.67 [0.46–0.98], P = .04). This was mainly driven by adjustment for PAM50 subtypes (TTP for LAG3 iTILs when only adjusted for PAM50 subtypes: HR: 0.63 [0.44–0.93]; TTP when only adjusted for treatment HR 0.93 [0.66–1.31]).
In the planned subgroup analysis of the non-luminal and luminal subtypes, FOXP3, PD-1, PD-L1, LAG3 and HE TILs did not impact survival in either uni- or multivariate analysis, and there was no differential effect in luminal and non-luminal subtypes (Supplementary Table S5).
Predictive value of immune biomarkers for gemcitabine + docetaxel vs. docetaxel monotherapy
There were no significant differences in treatment effect according to CD8 iTIL status for patients treated with docetaxel or GD for the primary endpoint of TTP or the secondary endpoint of OS ( and d, ).
Table 3. Predictive hazard ratios for treatment effect for TTP in patients treated with docetaxel + gemcitabine vs docetaxel alone. Values shown for multivariate analysis for the whole subset, and for the focused set of analyses performed on the non-luminal subset a Basal like + HER2E
For CD163, there were no significant differences for TTP (). However, patients with high expression benefitted significantly more from GD than from docetaxel in univariate analysis of OS (HR for low expression 1.16 [CI:0.81–1.65], HR for high expression 0.63 [0.41–0.96], Pinteraction = 0.03). However, this effect was not significant in multivariate analysis (HR for low expression 1.09 [CI:0.76–1.58], HR for high expression 0.62 [0.40–0.96], Pinteraction = 0.051)
None of the other biomarkers investigated were predictive of treatment effect when looking at the whole cohort (see and and b).
Figure 2. Time to progression (TTP) and associations with FOXP-3 iTILs levels and treatment arm in the SBG0102 trial population (A+B) and in the nonluminal subtypes (C+D). A. TTP for the FOXP3 iTILs low group, stratified by treatment with docetaxel or docetaxel + gemcitabine (DG), B. TTP for the FOXP3 iTILs high group, stratified by treatment with docetaxel or docetaxel + gemcitabine, C. TTP for the FOXP3 iTILs low non-luminal group, stratified by treatment with docetaxel or docetaxel + gemcitabine. D. TTP for the FOXP3 iTILs high non-luminal group, stratified by treatment with docetaxel or DG. Corresponding figures for overall survival can be seen in Supplementary Figure S4. iTILs: intratumoral tumor infiltrating lymphocytes
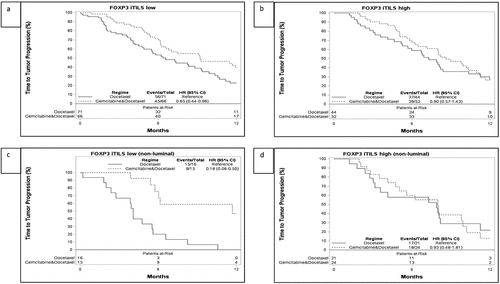
We also performed subgroup analyses of CD8 iTILs and sTILs, FOXP3 iTILs and CD163 in the non-luminal subtypes (basal + HER2E). Low FOXP3 iTILs were found to benefit significantly more from GD than from docetaxel alone with aanHR of 0.22 (CI: 0.09–0.52) for low FOXP3 iTILs compared to HR 0.92 (CI: 0.47–1.80) for high FOXP3 iTILs (Pinteraction = 0.01) for TTP ( and d, Supplementary Figure S5). CD8 status and CD163 were not predictive in the non-luminal subgroup analysis.
Discussion
In the SBG0102 clinical trial randomizing patients with advanced breast cancer to receive either docetaxel or docetaxel + gemcitabine, we found that CD8 positive TILs were not prognostic for the overall cohort but did show some prognostic significance in the non-luminal subtypes. These results align well with what we expected, as TILs in ER positive subtypes do not carry prognostic significance to the same degree as they do in the ER negative subset.Citation51 CD8 TILs and our other biomarkers of adaptive immunity also did not have predictive value for benefit from gemcitabine in the whole cohort. The macrophage marker CD163 did give some indication of a possible significant predictive effect for OS. Studies have shown that docetaxel and gemcitabine modulate the innate immune system including macrophages and myeloid-derived suppressor cells in both stimulatory and inhibitory ways.Citation19,Citation20,Citation24 Taken together with results of our study, further research on the predictive capacity of markers of the innate immune system is warranted in prospective cohorts treated with immune modulating chemotherapies.
In the subgroup analysis of the non-luminal subtypes, where the degree of immune response should be most significant for outcome, we also found that CD8 TILs were not predictive for benefit from gemcitabine. In this group, we did, however, find that patients with low FOXP3 levels benefitted more from GD than from docetaxel alone. The FOXP3 positive subset of T-lymphocytes are also known as regulatory T-cells, and function as negative regulators of CD8 positive cytotoxic T-cells, thereby contributing to a dampening of the immune response to tumor cells. Some chemotherapeutic drugs have been shown to specifically deplete regulatory T-cells,Citation28,Citation52 so in patients with already low levels of FOXP3 regulatory T-cells, this might mean that the inhibitory effect of these cells could become almost totally removed. Our results could signify that patients with low levels of regulatory T-cells do benefit from the addition of another chemotherapeutic drug that contributes to a net immunostimulatory effect. However, events were few in this exploratory analysis and our results should be tested in a larger cohort. Although most studies on the effect of docetaxel and gemcitabine on the TME have shown a stimulating effect, some pre-clinical studies have instead shown a detrimental effect on the efficacy of immune eradication of cancer cells with treatment with either docetaxel or gemcitabine. Debangshu eet al.showed that treatment with taxanes induced the T-cell inhibitory biomarkers PD-L1 and CD73 and macrophage inhibiting CD47 on breast cancer cells.Citation53 An inhibitory effect on natural killer cells by docetaxel has also been shown.Citation54 One possible explanation for our inability to identify a benefit from gemcitabine in tumors with CD8 high expression is that co-treatment with docetaxel may have canceled out any immune stimulatory effects.
Nevertheless, giving taxanes together with immunotherapy has already been shown to benefit the TNBC subset of patients and seems to be more beneficial than giving immunotherapy alone. The Impassion 130 trial, which led to FDA-approval of the PD-L1 inhibitor atezolizumab, combined nab-paclitaxel with atezolizumab, for first-line treatment of unresectable, locally advanced or metastatic breast cancer patients. This study has reported improved survival in the PD-L1 positive group of patients. There are several ongoing trials combining docetaxel or paclitaxel with other immune therapies and in combination with one or more other chemotherapy options (clinicaltrials.gov: NCT03639948, NCT00179309, NCT03894007)).[Citation2,Citation55,Citation56] It is, however, important to note that the Impassion 131 trial, treating metastatic TNBC with atezolizumab and paclitaxel as first-line treatment, has not yet shown a benefit of adding atezolizumab. The Impassion 131 trial was done in a patient population quite similar to that in Impassion 130, but with a different study design and using paclitaxel instead of nab-paclitaxel.Citation16
There are also several trials underway using regimens containing gemcitabine in combination with checkpoint inhibitors and other chemotherapy treatments.Citation2,Citation55 However, our results suggest that a addition of a second chemotherapy drug may not translate into clinical benefit despite presence of a demonstrable tumor immune reaction. This may be an important consideration for planning future trials, as patients may be spared from treatment with several different chemotherapies given in addition to immune therapy.
Strengths and limitations
Strengths of our study include use of material from a randomized clinical trial, a prespecified statistical plan, and validated biomarkers with prespecified cut-points in a formal prospective-retrospective design. However, our study also has important limitations. Our tumor material originated from the primary surgery of the patients, but the study intervention happened in the advanced setting, after patients had received systemic treatment and radiotherapy. Our study did not include tissue from the recurrences or metastases. Studies have shown that hormone receptors and HER2 status may change between primary tumors and their subsequent metastases.Citation57 The biological and clinical significance of receptor conversion in the context of the TME is not known. Heterogeneity may exist between the immune profiles of the primary and metastatic lesions and characterization of metastatic lesions may provide insights for guiding the course of systemic therapies.Citation58 However, studies including biopsies of primary and matched metastatic sites have shown that though the absolute lymphocyte counts may decline in the latter lesions, the distribution of T and B cell subsets in metastatic sites closely approximate that of the primary tumor. These observations suggest that the primary tumor may in fact drive the immune cell niche in metastatic sites.Citation59–62 From a clinical perspective, it is important to note that the biomarker study on IMpassion-130 trial samples demonstrated that despite lower prevalence of PD-L1 in metastatic samples, there was no difference in the treatment efficacy with respect to biomarker expression in the primary or metastatic sites.Citation62 However, further prospective, validation studies with clinical outcome are warranted.
Contrary to the original trial from which the material used in this study was taken, there was a significant benefit of GD vs D for the overall cohort (Supplementary Table S2). However, the difference in treatment effect is most likely random, and survival estimates differ only slightly from the original study.Citation30
Our study included all intrinsic breast cancer subtypes and was not powered to investigate the basal-like + CD8 high group alone, the group that would probably be the most likely to show prognostic significance of immune-related biomarkers. This was further supported by our finding that FOXP3 sTILs did have prognostic significance when adjusted for all four PAM50 subtypes, but not when looking at the combined non-luminal subtypes HER2E and basal-like together.
For evaluation of biomarkers, we chose to distinguish between lymphocytes in the peritumoral stroma and intratumoral lymphocytes. Evaluation of stromal TILs is standard when evaluating TILs on HHE-stainedslides, mainly because (blue) lymphocytes are easier to identify against a (pink) stromal background than against a (blue) carcinoma cell background, leading to increased interobserver reproducibility.Citation63 We chose CD8 iTILs as our primary biomarker as, from a biological standpoint, TILs that are in direct contact with tumor cells should be the most significant in the tumor-cytotoxic immune cell interaction, and immunohistochemically brown-stained intratumoral TILs are readily identifiable within carcinoma cell nests. However, we found in our study that CD8 sTILs, present in greater numbers and in a greater fraction of cases than iTILs, were significantly prognostic for our primary endpoint of TTP, whereas CD8 iTILs were not. The best method for evaluating TILs immunohistochemically remains controversial, and it is possible that we made a suboptimal choice of primary biomarker.
Finally, we used TMAs in our study. Advantages of TMAs are that a large number of tumor samples can be stained for biomarkers under very similar conditions, ensuring a uniform technical result. Also, evaluation can be more standardized when looking at smaller sections of tumor, than when looking at a whole tumor slide. Disadvantages of TMAs relate mainly to only looking at a very small section of tumor, which can be an issue if biomarkers are heterogeneously expressed. In our sstudy,we used duplicate 2 mm cores, which increase the area investigated somewhat from the more commonly used 0.6 mm or 1 mm cores.
Conclusion
Evidence suggests that effects of conventional chemotherapy are, in part, mediated through modulation of the tumor microenvironment. Gemcitabine in particular has been shown to affect specific immune cell types in the tumor microenvironment, raising the hypothesis that the degree and type of inflammation could be predictive of treatment effect. Using a formal prospective-retrospective study design, we tested this in material from a clinical trial that randomized patients to docetaxel or docetaxel + gemcitabine. For our biomarkers tied to cytotoxic T-cell immune response, we did not find that levels of our primary biomarker (CD8+ intratumoral T-cells) were predictive of treatment response. Only in a subgroup analysis of FOXP3+ lymphocytes in non-luminal breast cancers was a significant predictive effect observed. CD163, a marker of the innate immune system showed borderline significance, indicating that further studies investigating predictive capacity of the innate immune system are warranted. Overall, our results imply that adding several types of immune stimulating conventional chemotherapy may not enhance immune benefits. These results may be significant for future immunotherapy trials combining one or more conventional types of chemotherapy with immune therapy.
Disclosure
ES Stovgaard, K Asleh, D Nielsen, E Balslev, N Riaz, S Leung, A-V Lænkholm, D Gao, LB Nielsen, declare no conflicts of interest. TO Nielsen declares a proprietary interest in the Prosigna assay for PAM50 intrinsic subtyping. M-B Jensen has received institutional funding from Nanostring Technologies within the past two years.
Supplemental Material
Download ()Acknowledgments
This study was supported by the Canadian Cancer Society (grant number #705463), Kristian and Margrethe Kjaer Foundation, Else and Mogens Wedell-Wedellsborg Foundation and Emil and Inger Hertz Foundation. K. Asleh is supported by the Vanier Canada Graduate Scholarship – Canadian institutes of Health Research.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- WHO. Breast cancer [Internet]. [accessed 2020 Oct 20]. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/.
- Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. JNCCN J Natl Compr Cancer Netw. 2020;18(4):479–11. doi:10.6004/jnccn.2020.7554.
- Pouptsis A, Swafe L, Patwardhan M. Surgical and systemic treatment of hereditary breast cancer: a mini-review with a focus on BRCA1 and BRCA2 mutations. Front Oncol. 2020;10:553080. doi:10.3389/fonc.2020.553080.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013.
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi:10.1038/nm.3909.
- Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018;24(3):511–520. doi:10.1158/1078-0432.CCR-16-3001.
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen P-L, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi:10.1093/annonc/mdu112.
- Gao G, Wang Z, Qu X. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):179. doi:10.1186/s12885-020-6668-z.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van Den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol. 2015;26(2):259–271.
- Gonzalez-Ericsson PI, Stovgaard ES, Sua LF, Reisenbichler E, Kos Z, Carter JM, Michiels S, Le Quesne J, Nielsen TO, Lænkholm A-V, et al. The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J Pathol. 2020;250(5):667–684. doi:10.1002/path.5406.
- Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi:10.1016/S1470-2045(19)30689-8.
- Hamilton G. Avelumab: search for combinations of immune checkpoint inhibition with chemotherapy. Expert Opin Biol Ther. 2020;21:1–12.
- Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang K-Y, Ferrone S, Gameiro SR. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133(3):624–636. doi:10.1002/ijc.28070.
- Voorwerk L, Slagter M, Horlings HM, Sikorska K, Van De Vijver KK, De Maaker M, Nederlof I, Kluin RJC, Warren S, Ong S, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920–928. doi:10.1038/s41591-019-0432-4.
- Shaikh SS, Emens LA. Current and emerging biologic therapies for triple negative breast cancer. Expert Opin Biol Ther. 2020;1–12. doi:10.1080/14712598.2020.1801627.
- Primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC) ± atezolizumab (atez … | oncologyPRO. [accessed 2020 Dec 10]. https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/primary-results-from-impassion131-a-double-blind-placebo-controlled-randomised-phase-iii-trial-of-first-line-paclitaxel-pac-atezolizumab-atez.
- Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SAW, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–1698. doi:10.1158/1078-0432.CCR-14-0432.
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–1649. doi:10.1016/j.annonc.2020.09.010.
- Mu XY, Wang RJ, Yao ZX, Zheng Z, Jiang J-T, Tan M-Y, Sun F, Fan J, Wang X, Zheng J-H, et al. RS 504393 inhibits M-MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol Immunol. 2019;109:140–148. doi:10.1016/j.molimm.2019.02.014.
- Zhang Y, Bush X, Yan B. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials 2019;189:48–59. doi:10.1016/j.biomaterials.2018.10.022.
- Gravett AM, Trautwein N, Stevanović S, Copier J. Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. Oncoimmunology. 2018;7(6):e1438107. doi:10.1080/2162402X.2018.1438107.
- Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong J, Zheng X, Xu L. Low-dose gemcitabine treatment enhances immunogenicity and natural killer cell-driven tumor immunity in lung cancer. Front Immunol. 2020;11:331. doi:10.3389/fimmu.2020.00331.
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi:10.1158/1078-0432.CCR-10-0733.
- Millrud CR, Mehmeti M, Leandersson K. Docetaxel promotes the generation of anti-tumorigenic human macrophages. Exp Cell Res. 2018;362(2):525–531. doi:10.1016/j.yexcr.2017.12.018.
- Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–3544. doi:10.1158/1078-0432.CCR-07-4025.
- D’Costa Z, Jones K, Azad A, Van Stiphout R, Lim SY, Gomes AL, Kinchesh P, Smart SC, Gillies Mckenna W, Buffa FM, et al. Gemcitabine-induced TIMP1 attenuates therapy response and promotes tumor growth and liver metastasis in pancreatic cancer. Cancer Res. 2017;77(21):5952–5962. doi:10.1158/0008-5472.CAN-16-2833.
- Bulle A, Dekervel J, Deschuttere L, Nittner D, Libbrecht L, Janky R, Plaisance S, Topal B, Coosemans A, Lambrechts D, et al. Gemcitabine recruits M2-type tumor-associated macrophages into the stroma of pancreatic cancer. Transl Oncol. 2020;13(3):100743. doi:10.1016/j.tranon.2020.01.004.
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi:10.1158/0008-5472.CAN-09-3690.
- Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. 2019;10:1654. doi:10.3389/fimmu.2019.01654.
- Nielsen DL, Bjerre KD, Jakobsen EH, Cold S, Stenbygaard L, Sørensen PG, Kamby C, Møller S, Jørgensen CLT, Andersson M, et al. Gemcitabine plus docetaxel versus docetaxel in patients with predominantly human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: a randomized, phase III study by the Danish breast cancer cooperative group. J Clin Oncol. 2011;29(36):4748–4754. doi:10.1200/JCO.2010.33.9507.
- Jorgensen CLT, Nielsen TO, Bjerre KD, Liu S, Wallden B, Balslev E, Nielsen DL, Ejlertsen B. PAM50 breast cancer intrinsic subtypes and effect of gemcitabine in advanced breast cancer patients. Acta Oncol. 2014;53(6):776–787. doi:10.3109/0284186X.2013.865076.
- Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer. 2017;24(1):3–15. doi:10.1007/s12282-016-0698-z.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97(16):1180–1184. doi:10.1093/jnci/dji237.
- Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(1):59. doi:10.1186/s40425-016-0165-6.
- Dieci MV, Tsvetkova V, Orvieto E, Piacentini F, Ficarra G, Griguolo G, Miglietta F, Giarratano T, Omarini C, Bonaguro S, et al. Immune characterization of breast cancer metastases: prognostic implications. Breast Cancer Res. 2018;20(1):62. doi:10.1186/s13058-018-1003-1.
- Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, et al. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108(5):853–858. doi:10.1111/cas.13229.
- Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol. 2014;25(8):1536–1543. doi:10.1093/annonc/mdu191.
- Shou J, Zhang Z, Lai Y, Chen Z, Huang J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs: a systematic review and meta-analysis. BMC Cancer. 2016;16(1):687. doi:10.1186/s12885-016-2732-0.
- Chafe SC, Riaz N, Burugu S, Gao D, Leung SCY, Lee AF, Lee C-H, Dedhar S, Nielsen TO. Granulocyte colony stimulating factor expression in breast cancer and its association with carbonic anhydrase ix and immune checkpoints. Cancers 2021;13(5):1–21. doi:10.3390/cancers13051022.
- Asleh K, Lyck Carstensen S, Tykjær Jørgensen CL, Burugu S, Gao D, Won JR, Jensen M-B, Balslev E, Lænkholm A-V, Nielsen DL, et al. Basal biomarkers nestin and INPP4B predict gemcitabine benefit in metastatic breast cancer: samples from the phase III SBG0102 clinical trial. Int J Cancer. 2019;144(10):2578–2586. doi:10.1002/ijc.31969.
- Cheang MCU, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–1376. doi:10.1158/1078-0432.CCR-07-1658.
- Cheang MCU, Treaba DO, Speers CH, Olivotto IA, Bajdik CD, Chia SK, Goldstein LC, Gelmon KA, Huntsman D, Gilks CB, et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24(36):5637–5644. doi:10.1200/JCO.2005.05.4155.
- Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, Huntsman D, Olivotto IA, Nielsen TO, Gelmon K, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26(35):5697–5704. doi:10.1200/JCO.2007.15.8659.
- Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8 +lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi:10.1186/bcr3148.
- Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, Bago-Horvath Z, Bane A, Bedri S, Brock J, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–1164. doi:10.1038/modpathol.2016.109.
- Lee CH, Espinosa I, Vrijaldenhoven S, Subramanian S, Montgomery KD, Zhu S, Marinelli RJ, Peterse JL, Poulin N, Nielsen TO, et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin Cancer Res. 2008;14(5):1423–1430. doi:10.1158/1078-0432.CCR-07-1712.
- Burugu S, Gao D, Leung S, Chia SK, Nielsen TO. LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Ann Oncol. 2017;28(12):2977–2984. doi:10.1093/annonc/mdx557.
- Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16(5):432. doi:10.1186/s13058-014-0432-8.
- VENTANA PD-L1 (SP142) assay interpretation guide for triple-negative breast carcinoma (TNBC). [accessed 20 Oct 2020. https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp142-assay1.html.
- Liu S, Chen B, Burugu S, Leung S, Gao D, Virk S, Kos Z, Parulekar WR, Shepherd L, Gelmon KA, et al. Role of cytotoxic tumor-infiltrating lymphocytes in predicting outcomes in metastatic HER2-positive breast cancer a secondary analysis of a randomized clinical trial. JAMA Oncol. 2017;3(11):e172085. doi:10.1001/jamaoncol.2017.2085.
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi:10.1200/JCO.2011.41.0902.
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. doi:10.1002/eji.200324181.
- Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL. Chemotherapy induces enrichment of CD47+ /CD73 + /PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci. 2018;115(6):E1239–E1248. doi:10.1073/pnas.1718197115.
- Tang M, Gao S, Zhang L, Liu B, Li J, Wang Z, Zhang W. Docetaxel suppresses immunotherapy efficacy of natural killer cells toward castration-resistant prostate cancer cells via altering androgen receptor-lectin-like transcript 1 signals. Prostate 2020;80(10):742–752. doi:10.1002/pros.23988.
- Home - ClinicalTrials.gov. [accessed 2020 Oct 20]. https://clinicaltrials.gov/.
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi:10.1056/NEJMoa1910549.
- Schrijver WAME, Suijkerbuijk KPM, Van Gils CH, Van Der Wall E, Moelans CB, Van Diest PJ. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(6):568–580. doi:10.1093/jnci/djx273.
- Foukakis T, Lövrot J, Matikas A, Zerdes I, Lorent J, Tobin N, Suzuki C, Brage SE, Carlsson L, Einbeigi Z, et al. Immune gene expression and response to chemotherapy in advanced breast cancer. Br J Cancer. 2018;118(4):480–488. doi:10.1038/bjc.2017.446.
- Szekely B, Bossuyt V, Li X, Wali VB, Patwardhan GA, Frederick C, Silber A, Park T, Harigopal M, Pelekanou V, et al. Immunological differences between primary and metastatic breast cancer. . Annals of Oncology. 2018;29(11):2232–2239. doi:10.1093/annonc/mdy399.
- Rozenblit M, Huang R, Danziger N, Hegde P, Alexander B, Ramkissoon S, Blenman K, Ross JS, Rimm DL, Pusztai L, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020;8(2):e001558. doi:10.1136/jitc-2020-001558.
- Zhu L, Narloch JL, Onkar S, Joy M, Broadwater G, Luedke C, Hall A, Kim R, Pogue-Geile K, Sammons S, et al. Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J Immunother Cancer. 2019;7(1):265. doi:10.1186/s40425-019-0755-1.
- Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, Iwata H, Barrios CH, Nechaeva M, Nguyen-Duc A, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. JNCI J Natl Cancer Inst. 2021:djab004. doi:10.1093/jnci/djab004.
- Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, Van De Vijver K, Estrada MV, Gonzalez-Ericsson PI, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, tils in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24(5):235–251. doi:10.1097/PAP.0000000000000162.
