ABSTRACT
Background: The incidence of renal immune-related adverse events (irAEs) is reported to be 3.8%, with varied definitions of acute kidney injury (AKI). This study reports a 10-year experience at MD Anderson Cancer Center of patients diagnosed with melanoma and treated with immune checkpoint inhibitors (ICIs) and evaluated the incidence of AKI, associated factors, and its association with overall survival.
Methods: A retrospective chart review (2010–2019) of all patients with melanoma treated with ipilimumab, nivolumab, pembrolizumab, or atezolizumab was performed. All available serum creatinine data were extracted and used to calculate the estimated GFR (eGFR) using the CKD Epi equation, and to diagnose AKI using the two KDIGO (Kidney Disease: Improving Global Outcomes) criteria for defining stage I AKI in 1664 unique patients. Cumulative incidence rates of AKI after initiation of ICIs were calculated in the presence of death as a competing risk. The effects of covariates on the cumulative incidence function of AKI were evaluated in a univariant and multivariable analysis. Overall survival was estimated by Kaplan–Meier method in accordance to the occurrence of AKI.
Results: The incidence of AKI by definitions 1a and 1b were 3.49% and 3.33%, respectively. After adjudication, AKI attributable to ICI was 58% and 65% of the overall incidence of AKI in each definition respectively. Increasing age was associated with decreased risk of AKI. Asian race was associated with a higher risk of AKI. Comorbidities were not associated with increased risk of AKI while use of proton pump inhibitors (PPI), ipilimumab or ICI combinations were significantly associated with AKI. AKI was not significantly associated with overall survival.
Immune-related adverse events (irAEs) occurred in 30% of patients with AKI but their incidence was not different in patients with AKI attributable to ICI versus other AKI.
Conclusions: In a large population of patients with melanoma treated with ICIs, an accurate documentation of AKI in setting of ICI use and predictors associated is presented. AKI following ICI use is infrequent, not associated with mortality, and associated with the use of ipilimumab, ICI combinations and PPIs.
Introduction
Immune checkpoint inhibition (ICI) has had major success in cancer treatment and has changed the treatment paradigm in many cancers. ICIs act by releasing the natural regulators of the immune system thereby leading to overall immune activation and specifically activation of the immune system against antigens in tumors.Citation1 ICIs targets several immune checkpoint inhibitors, such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L1).Citation2 With antitumor effects come side effects. The challenge is to maximize the antitumor effects while avoiding deleterious off-target effects,Citation3 although some adverse off-target effects have been associated with improved survivalCitation4,Citation5 or tumor response.Citation6 Skin, gut, endocrine, lung, and musculoskeletal systems are the most commonly involved in immune-related adverse events (irAE’s), whereas cardiovascular, hematologic, renal, neurologic, and ophthalmologic effects are less common.Citation7
With increasing use of ICIs, there have been increased reports of adverse renal effects. Understanding the mechanism of these ICI-related adverse renal effects is lacking. Other drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs) have been shown to be associated with an increased risk of acute interstitial nephritis, which appears to be the predominant ICI – related form of renal injury.Citation8–11The incidence of ICI associated AKI is uncertain and has been cited as occurring in 1.4% to 16.5%.Citation11–14 However, in two recent studies where the attribution of AKI to ICI therapy was carefully adjudicated the overall incidence of AKI in one study was 17% (with only 3% attributed to ICI therapy)Citation13 and in the other study the overall incidence of AKI was 17.4% with only 4.2% attributable to ICIs.Citation12 Predictors of AKI have been reported as lower baseline estimated glomerular filtration rate (eGFR), combination ICI therapy, other irAEs, and PPI use.Citation11–13,Citation15 In addition, it has not been demonstrated that a specific type of malignancy is more frequently associated with AKI.
AKI associated with the use of ICIs was not always associated with an increased risk of mortality.Citation12,Citation14,Citation15 However, failure to recover from AKI was demonstrated to be an independent predictor of increased mortality.Citation11 Many of the referenced studies describing the incidence of ICI associated AKI or the risk factors predictive of AKI with ICI treatment have had heterogenous populations and have defined AKI either as a 50% increase from baseline creatinine, a twofold or more increase in serum creatinine or the need for dialysisCitation11–14 Recent work has demonstrated that patients with AKI defined as an absolute increase in serum creatinine of 0.3 mg/dl within 48 hours and patients with AKI defined as a 50% relative increase in serum creatinine within 7 days had statistically significant differences in length of stay and mortality.Citation16
In the current study, we sought to evaluate the incidence of AKI in a large, more uniform population of patients with melanoma, using the two definitions of Stage I AKI, with a focus on predictors of AKI and its association with survival.
Materials & methods
Study design and patient population
This retrospective study was approved by the institutional review board at the University of Texas MD Anderson Cancer Center, and the procedures followed were in accordance with the principles of the Declaration of Helsinki. Patients were identified by querying the MD Anderson pharmacy data from January 1, 2010, to November 12, 2019, to identify all patients with melanoma (metastatic or adjuvant) treated with ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab. From 1920 unique patients, 256 patients were excluded for: only one record, missing start and end drug data, or AKI occurring prior to ICI exposure-leaving 1664 evaluable patients.
Data collection and definitions
Detailed demographic information for each patient, including age, sex, and race/ethnicity (Black, Hispanic, Asian, and White) were attained from MD Anderson Epic medical record system. In addition, exposure to NSAIDS and/or PPI collected during the study period. Comorbidities were congestive heart failure (CHF), coronary artery disease (CAD), peripheral vascular disease (PVD), hypertension (HTN), and liver disease. All available creatinine values and survival data were collected. Creatinine values were recalculated for eGFR using the CKD-EPI Creatinine Equation. Baseline eGFR was the first available eGFR prior to starting ICI and categorized as eGFR greater than or equal to 60cc/min/1.73 m2vs Stage III–V (eGFR less than 60cc/min/1.73m2) based on KDIGO guidelines.Citation17
AKI was identified by the two following definitions from the Kidney Disease: Improving Global Outcomes guidelines:Citation17,Citation18 1a) an absolute increase in serum creatinine of ≥0.3 mg/dl within 48 hours, and 1b) a relative increase in serum creatinine of ≥50% within 7 days. By either of the 2 AKI definitions, if 2 consecutive AKIs occurred within 3 weeks, the second episode was counted as the same episode as the previous one. All cases of AKI were reviewed by two nephrologists (A.A. and OM.). Attribution of AKI to ICI required that there was no antecedent hypotension, sepsis, volume depletion or use of nephrotoxic drugs. Over the entire study period, of the 1664 patients, 1453 (87%) received one period of ICI therapy, 210 received two periods and one patient received 3 periods of ICI therapy. Only the records for the first treatment period of each patient were used for the analyses related to AKI.
Statistical analysis
Time to first AKI was defined as time from ICI treatment initiation to time of first AKI. Cumulative incidence rates of AKI after initiation of ICIs were calculated in the presence of death as a competing risk. The effects of covariates on the cumulative incidence function of AKI were evaluated in the univariable setting using Gray’s test.Citation19 In the multivariable setting, Fine and Gray’s method was used to model the probability of sub-distribution function of AKI by applying decreasing weights to patients who died before experiencing AKI.Citation20,Citation21 Validity of the proportional cause-specific hazards and sub-distribution hazards assumptions were assessed using the proportionality test on time-varying covariates.
Overall survival (OS) was defined as from the time of initiation of ICI therapy to the time of death. OS time for the surviving patients was right censored at the time of the end of the first treatment period. The distribution of OS was estimated by the Kaplan–Meier method in accordance to the occurrence of AKI using landmark analysis.Citation22 Log-rank test was performed to test the difference in survival between patients with and without AKI 6 months post ICI initiation.Citation23 Regression analyses of survival data based on the Cox proportional hazards modelCitation24 were conducted. Since AKI is not an exogenous time-dependent covariate to OS as it is also the output of a stochastic process generated by the same patient, which is directly related to the failure mechanism, a joint modeling approach is required.Citation25 We used the joint models built in an R package, JMbayes, developed by Dimitris RizopoulosCitation25 to assess the association of the longitudinally measured AKI with OS. The JMbayes package was developed to fit shared parameter models for the joint modeling of longitudinal responses and event times under a Bayesian approach.Citation26 We first fitted a mixed effect model of the longitudinal measured AKI, and a Cox proportional hazards model of OS adjusting for patient age at the initiation of ICI therapy, sex, race/ethnicity, and type of ICIs. We then fitted a joint model of AKI and OS. SAS version 9.4, R version 3.5.3 (R Foundation for Statistical Computing), and S-Plus version 8.2 were used to carry out the computations for all analyses.
Results
Data for 1664 patients diagnosed with melanoma and treated with the selected ICIs over the period studied were analyzed. Patient characteristics are summarized in . The median follow-up time was 9 months and duration of treatment was 88 days. Of the cohort, 66% were male and 99% were White, with median age of 63 years. Single-agent usage was the most common regimen: Ipilimumab (28.49%), pembrolizumab (26.20%), nivolumab (19.89%) atezolizumab (1.32%); with the following combinations accounting for the remaining regimens: ipilimumab/pembrolizumab (14.54%); ipilimumab/nivolumab (9.56%). Due to small numbers CAD, CHF, and PVD were not evaluated. Immune-related adverse events (irAEs) occurred in 30% (22 out of 72 patients) of patients with AKI but their incidence was not different in patients with AKI attributable to ICI versus other AKI most common being dermatitis, transaminitis, thyroiditis, and colitis. Among patients with AKI, 25 cases had undergone a kidney biopsy with acute tubulointerstitial nephritis (ATIN) as the predominate diagnosis. Other pathologies were IgA nephropathy, vasculitis, acute tubular necrosis and hypertension-related changes.
Table 1. Distribution of the patients (N = 1664) by demographic and clinical characteristics and AKI definition
Incidence and predictors of AKI according by definition 1a (≥0.3 mg/dl increase in creatinine over 48 hours)
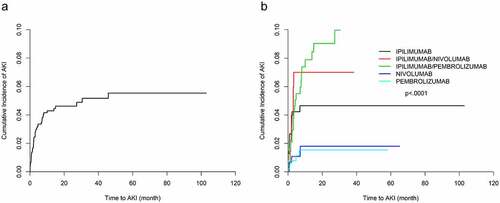
Using the definition 1a, AKI was identified in 58 (3.5%) patients in the study period. Of the 58 patients with AKI, 40 (2.4%) had 1 episode of AKI, 17 (1.0%) had 2 episodes of AKI, and 1 (0.06%) had 3 episodes of AKI with a median time to first AKI of 2 months. Overall cumulative incidence rate of AKI 12 months posttreatment initiation was 4.21%. The corresponding 12-month cumulative incidence rates were 4.62%, 6.82%, 7.8% and 1.8% for ipilimumab-, ipilimumab/nivolumab-, ipilimumab/pembrolizumab- and nivolumab-treated patients, respectively (). The median increase in creatinine in the AKI cases was 0.42 mg/dl over 48 hours with a minimum increase of 0.33 mg/dl and a maximum of 2.22 mg/dl with only one patient experiencing the maximum change in AKI. Of the episodes of AKI 58.6% were adjudicated as attributable to ICI. In a univariable analysis (), patients receiving ipilimumab were at increased risk of having AKI compared to those receiving pembrolizumab (hazard ratio [HR], 3.402; 95% confidence interval [95% CI], 1.260–9.186; P = .0002). In addition, patients receiving combinations of ipilimumab/nivolumab (HR, 4.713; 95% CI, 1.542–14.404; P = .0065) and ipilimumab/pembrolizumab (HR, 6.281; 95% CI, 2.413–16.349; P = .0002) were at increased risk of AKI when compared to pembrolizumab. Neither hypertension nor liver disease was associated with an increased risk of AKI in a univariable analysis. Based on baseline eGFR, less than or equal to 60cc/min when compared to greater than 60cc/min were not significantly associated with AKI. In a multivariable analysis (), use of ipilimumab and use of ICI combinations were independent predictors of AKI (ipilimumab: HR, 3.281; 95% CI, 1.213–8.873; P < .0001; ipilimumab/nivolumab: HR, 3.725; 95% CI, 1.144–12.134; P = .0291; and ipilimumab/pembrolizumab: HR, 6.305; 95% CI, 2.436–16.318; P = .0001). Compared to White patients, Asian patients had higher risk of experiencing AKI (HR, 4.182; 95% CI, 1.090–16.043; P = .0370). In addition, PPI use was a significant independent predictor of AKI (HR, 2.387; 95% CI, 1.328–4.291; P = .0036). NSAID use was not a significant predictor of AKI in either univariable or multivariable analysis.
Table 2. Univariable analysis of association between time to first AKI by patient characteristics and ICI regimens
Table 3. Multivariable analysis of association of AKI by patient characteristics and ICI regimens
Incidence and predictors of AKI definition 1b (≥50% increase in creatinine over 7 days)
Using definition 1b, AKI was identified in 72 (4.33%) patients in the study period. Of the 72 patients with AKI (3.49%), had 1 episode of AKI; and 14 (0.84%), had 2 episodes of AKI with a median time to first AKI of 1.7 months. Overall cumulative incidence rate of AKI 12 months posttreatment initiation was 5.48%. The corresponding 12-month cumulative incidence rates were 5.03%, 8.72%, 11.06% and 3.21% for ipilimumab-, ipilimumab/nivolumab-, ipilimumab/pembrolizumab- and nivolumab-treated patients, respectively (). The median increase in creatinine in the AKI cases was 1.59 mg/dl over 7 days with a minimum increase of 1.50 mg/dl and a maximum of 3.50 mg/dl with only one patient experiencing the maximum change in AKI. Of these cases of AKI, 65% were adjudicated as attributable to ICI. In a univariable analysis (), increased risk of AKI was seen in patients treated with ipilimumab when compared with those treated with pembrolizumab (HR, 4.932; 95% CI 1.695–14.346; P = .0034). In addition, patients receiving combinations of ipilimumab/nivolumab (HR, 7.384; 95% CI, 2.328–23.417; P = .0007), ipilimumab/pembrolizumab (HR, 9.559; 95% CI, 3.37–37.112; P < .0001) and nivolumab alone (HR, 3.242; 95% CI, 1.002–10.49; P = .0496) were at increased risk of AKI when compared to pembrolizumab. Use of NSAIDS and use of PPIs were associated with increased risk of AKI (NSAIDs: HR, 2.730; 95% CI, 1.436–5.194; P = .0022; PPIs: HR, 2.359; 95% CI, 1.424–3.905; P = .0009). Increased age (per year increase) was associated with AKI, while Asian race was more likely to experience AKI (HR, 5.970; 95% CI, 2.164–16.470; P = .0006). As for the definition 1a of AKI, liver disease, hypertension, and baseline eGFR were not significantly associated with AKI.
Figure 2. Cumulative incidence plots of AKI (Definition 1b: (≥50% increase in creatinine over 7 days). (a) Whole cohort. (b) Subgroups by type of ICI
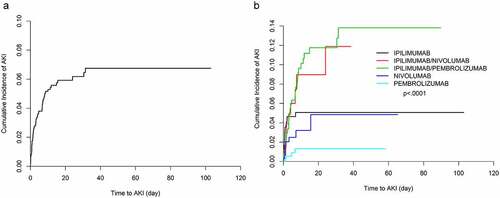
In a multivariable analysis (), again the use of ipilimumab and combinations were independent predictors of AKI (ipilimumab: HR, 4.096; 95% CI, 1.415–11.856; P = .0093; ipilimumab/nivolumab: HR, 5.101; 95% CI, 1.554–16.745; P= .0072; and ipilimumab/pembrolizumab: HR, 9.041; 95% CI, 3.246–25.177; P < .0001). Increased age (per year increase) was negatively associated with AKI (HR, 0.981; 95% CI, 0.966–0.996; P = .0152). As with the definition 1a, Asian patients were more likely than White patients to develop AKI after ICI exposure (HR, 4.387; 95% CI, 1.519–12.664; P = .0063). PPI exposure was associated with increased risk of AKI using definition 1b (HR, 2.355; 95% CI, 1.393–3.983; P = .0014), but use of NSAIDs was not.
AKI and overall survival
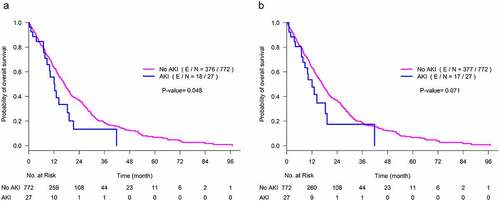
Median OS of the entire study cohort was 19 months with a 95% confidence interval of (17.3, 20.3) months. Using either definition of AKI while adjusting for the other covariates in a joint model no association of AKI with overall survival was seen (, P = .14; and, P = .12 definitions 1a and 1b, respectively) (, & b). In addition, age, race, sex, ICI type, and use of PPI were not associated with overall survival.
Table 4. Association of AKI with OS adjusting for the other covariates in a joint model
Discussion
Immune-related adverse events have been noted with the use of immunotherapies and especially ICIs, such as anti-CTLA-4, anti-PD-L1 and anti-PD-1. There has been increasing research on ICI toxicity since it can affect any organ in the body and at times leads to cessation of ICI use, thereby limiting the patient’s cancer treatment, or leading to fatal outcomes.Citation27,Citation28 ICIs have proven effective in advanced melanoma treatment and have become a standard of care.Citation29 The associated irAEs and improved response in melanoma have been noted in several studies.Citation30,Citation31 In this retrospective analysis of 10 years of experience at MD Anderson Cancer Center, reports the incidence, risk factors and outcomes of AKI in the largest cohort of a single malignancy treated with ICIs. Unlike previous reports, the definition of AKI was standardized according to the KDIGO criteria and cases were adjudicated for attribution of AKI to ICI therapy. The overall incidence of AKI according to KDIGO definition 1a (an increase in the serum creatinine by >0.3 mg/dl above baseline within 48 h) was 3.48%, and by definition 1b (a 50% increase in the serum creatinine from baseline within 7 days) was 4.32%. AKI adjudicated to be attributable to ICI’s in the larger of the two groups (1b) was only 2.82%, thus both the overall incidence of AKI and AKI attributable to ICI encountered in the present series were lower than in prior reports.Citation11–13 The onset of AKI in this cohort (2 months definition 1a; 1.7 months definition 1b) is consistent with prior reports.1112,Citation13
Among the risk factors for AKI that were considered, comorbidities including hypertension, liver disease, and CVD were not significantly associated with AKI by multivariable analysis. In previous studies, hypertension, diabetes mellitus, congestive heart failure (CHF), chronic obstructive pulmonary disease, cirrhosis, and coronary artery disease (CAD) have been considered as possible risk factors for AKI and in only one of these studies was one of these risk factors, hypertension, identified as an independent predictor of ICI-induced AKIs.12,Citation13,Citation32 In agreement with other published data, the present study demonstrated no association between sex and AKI after ICI exposure.Citation12,Citation13,Citation32 A recent multicenter study demonstrated that lower baseline eGFR is an independent predictor of AKI after ICI exposure.Citation11 In contrast, a recent retrospective cohort, reported that baseline eGFR was not an independent predictor of AKI.Citation12 In agreement with the latter study, in the present study, preexisting CKD III–V was not associated with increased risk of AKI, and therefore the use of ICI should not be withheld in patients with impaired kidney function, particularly given the very low incidence of AKI in these patients.
An interesting observation in the present study was the reduced risk of AKI with increasing age, and the increased risk of AKI in Asian patients, both of which require further validation. The latter finding should be interpreted with caution, however, since the reported cohort lacked racial diversity: 1642 patients were White, 10 patients were Black, and only 12 patients were Asian. As far as increase in age and impact of AKI after ICI there have been limited data about irAEs associated with older age, but in a study of 858 melanoma patients aged 65 years and older (mean age at ICI treatment, 74.8 years), 60% of the patients experienced irAEs, with 20.7% experiencing severe irAEs such as colitis, hypothyroidism, dermatitis, and hypophysitis. Patients in that study who developed non-severe irAEs had improved OS compared to those with severe irAEs; the latter were 28% more likely to die than patients with no irAEs.Citation33 However, there was no mention of renal-associated irAEs; therefore, renal function may be unique as far as age and ICI exposure and needs to be further investigated and the findings in the present report confirmed.
In this multivariable model, looking at both definitions of AKI, the use of PPIs, CTLA-4 antibody ipilimumab, and ICIs in combination were associated with an increased risk of AKI, as has been previously published.Citation11–13 A possible hypothesis of PPI induced AIN is that PPI’s work by activating effector T cells and this effect becomes additive when T cells become primed by use of ICI medications thereby increasing the risk of AKI.Citation34 This explanation suggests that AKI attributable to ICI may itself be considered an irAE. The pathogenesis of ICI induced AKI either by anti-PD1 or anti-CTLA4 is poorly understood. It is more commonly associated with AIN and rarely induction of autoimmune disease such as vasculitis and glomerulonephritis.Citation10,Citation35–39 In the present study, however, the incidence of irAEs was not significantly different between those with AKI due to other causes versus those with ICI-attributable AKI. Several papers have evaluated irAEs, and further studies of genetic predisposition and perturbations in microbiota environment are under way.Citation40,Citation41 However, in relation to comorbidities associated with other irAEs, autoimmune disease has been well recognized as a risk factor for irAEs;Citation35
The lower incidence of AKI herein reported, compared to previous reports may be attributable to the fact that this study population was uniform in cancer type (melanoma only); therefore, with less likely exposure to other nephrotoxic agents such as platinum drugs or tyrosine kinase inhibitors (lung cancers and renal cell cancer). The impetus for using the two different definitions for KDIGO stage 1 AKI was the report that these two definitions identified patients with AKI with different outcomes (length of stay and mortality).Citation16 In the present study, however, a joint model analysis identified that AKI was not associated with worse overall survival with either definition. These are consistent with the findings of another recent study involving 309 patients which included majority melanoma (84%) patients.Citation12 Recently published data, however, have demonstrated that patients without kidney recovery from AKI do have a higher mortality rate than those with complete or partial kidney recovery.Citation11 In addition, a recent study of 821 patients exposed to ICI concluded that a single episode of AKI was an independently associated with increased mortality where only 10% of their study population were melanoma patients.Citation14
Our study has several limitations: being a retrospective study in a large population, it is possible that the use of nephrotoxic agents, other irAEs, or conditions other than ICI exposure that would have led to AKI may have been missed. In addition, our study is a single center study and some of our findings will need further validation in a multicenter cohort. We also lack data on American Joint Committee on Cancer (AJCC) staging and further details on melanoma subtypes evaluated. However, the present results are in line with what has been published as far as incidence, predictors, and lack of impact on overall survival, which confirms the value of the present data in a uniform population of patients with melanoma irrespective of how AKI is defined.
AKI in patients with melanoma treated with ICIs is infrequent, and infrequently directly attributable to ICI. Traditional risk factors for AKI did not appear to be associated with AKI in this homogeneous cancer population. Consistent with prior reports, PPI usage, ipilimumab, and combination ICI therapy were significantly associated with AKI by either definition of KDIGO stage 1 AKI. IrAEs occurred in 30% of patients with AKI but did not occur more frequently in patients with ICI-attributable AKI. Unlike other forms of AKI, there was no association of AKI associated with ICI therapy and mortality in a multivariable analysis. The present study adds to and confirms published data about the incidence, risk factors, and survival associated with AKI and ICI exposure. More evidence-based guidelines and biomarkers, early detection, or predictors to optimize patient treatments while undergoing immunotherapy to accomplish effective cancer care and preserve renal function would be the ultimate goal.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Disclosure of potential conflicts of interest
The author(s) declare that they have no competing interests.
Authors’ Contributions
M.A: Concept/design, interpretation, drafting article, Critical revision of article, Approval of article.
A.A: Concept/design, interpretation, drafting article, Critical revision of article, Approval of article, Statistics, Data collection, and Secured funding.
H.L: Concept/design, Data analysis/interpretation, drafting article, Critical revision of article, Approval of article, and Statistics
O.M, J.L, V.P, W.A, J.S, U.S, C.Y, A.D, W.S: Data analysis/interpretation, Drafting article, Critical revision of article, Approval of article
Acknowledgments
Editorial support was provided by Sunita Patterson in Scientific Publications Services, Research Medical Library at The University of Texas MD Anderson Cancer Center.
Additional information
Funding
References
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–11. doi:10.1056/NEJMoa1406498.
- Haslam A, Prasad V. Estimation of the Percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535.
- Konstantina T, Konstantinos R, Anastasios K, Anastasia M, Eleni L, Ioannis S, Sofia A, Dimitris M. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer. 2019;135:29–32. doi:10.1016/j.lungcan.2019.06.015.
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with Anti–Cytotoxic T-Lymphocyte Antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi:10.1200/JCO.2005.06.205.
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681–6688. doi:10.1158/1078-0432.CCR-07-0187.
- Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning Y-M, Singh H, Suzman D, Xu J, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death Ligand 1 Antibody. J Clin Oncol. 2019;37(30):2730–2737.
- Postow MA, Sidlow R, Hellmann MD, Longo DL. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481.
- Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–647. doi:10.1016/j.kint.2016.04.008.
- Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68(2):287–291. doi:10.1053/j.ajkd.2016.02.057.
- Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7(1):2. doi:10.1186/s40425-018-0478-8.
- Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: a Multicenter Study. J Am Soc Nephrol. 2020;31(2):435–446.
- Meraz-Munoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, Kim J, Wald R, Kitchlu A. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. 2020;8(1):8. doi:10.1136/jitc-2019-000467.
- Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, Cortazar FB, Leaf DE, Mooradian MJ, Villani A-C, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14(12):1692–1700. doi:10.2215/CJN.00990119.
- Ga M, Agraz I, Serón D, José SM. Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol Dial Transplant. 2021. Pubmed: PMID 33547795.
- Garcia-Carro C, Bolufer M, Bury R, Catañeda Z, Muñoz E, Felip E, Lorente D, Josep Carreras M, Gabaldon A, Agraz I, et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol Dial Transplant. 2021. doi:10.1093/ndt/gfab034.
- Sparrow HG, Swan JT, Moore LW, Gaber AO, Suki WN. Disparate outcomes observed within Kidney Disease: improving Global Outcomes (KDIGO) acute kidney injury stage 1. Kidney Int. 2019;95(4):905–913. doi:10.1016/j.kint.2018.11.030.
- Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, Kane-Gill SL, Liu KD, Prowle JR, Shaw AD, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98(2):294–309. doi:10.1016/j.kint.2020.04.020.
- Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, Inker LA, Levin A, Mehrotra R, Palevsky PM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117–1129. doi:10.1016/j.kint.2020.02.010.
- Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. doi:10.1214/aos/1176350951.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144.
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi:10.1002/sim.2712.
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. doi:10.1080/01621459.1958.10501452.
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170.
- Cox DR. Regression Models and Life-Tables. J R Stat Soc B. 1972;34:187.
- Rizopoulos D. The R package jmbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J Stat Softw. 2016;72(7):1–46. doi:10.18637/jss.v072.i07.
- Cox D. Citation classic - regression-models and life-tables. Cc/Art Human. 1986;16(42):16-16.
- Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi:10.1200/JCO.2014.60.0379.
- Abdel-Wahab N, Shah M, Suarez-Almazor ME, Nishikawa H. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: a Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221.
- Ugurel S, Rohmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies–update 2017. Eur J Cancer. 2017;83:247–257. doi:10.1016/j.ejca.2017.06.028.
- Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning Y-M, Singh H, Suzman D, Xu J, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death Ligand 1 Antibody. Journal of Clinical Oncology. 2019;37(30):2730.
- Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Khattak A, Carlino MS, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage iii melanoma randomized to receive pembrolizumab or placebo a secondary analysis of a randomized clinical trial. Jama Oncol. 2020;6(4):519–527. doi:10.1001/jamaoncol.2019.5570.
- Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: a Multicenter Study. J Am Soc Nephrol. 2020;31(2):435–446.
- Mian I, Yang M, Zhao H, Shah M, Diab A, Shannon V, Patel A, Amaria RN, Giordano SH, Suarez-Almazor ME, et al. Immune-related adverse events and survival in elderly patients with melanoma treated with ipilimumab. Journal of Clinical Oncology. 2016;34(15_suppl):3047. doi:10.1200/JCO.2016.34.15_suppl.3047.
- Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29(8):2039–2052. doi:10.1681/ASN.2018050488.
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med. 2018;169(2):133–134. doi:10.7326/L18-0209.
- Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17(6):610–616. doi:10.1016/j.autrev.2018.01.010.
- Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology (Oxford). 2019;58(Supplement_7):vii40–vii8. doi:10.1093/rheumatology/kez297.
- Shah M, Tayar JH, Abdel-Wahab/ N, Suarez-Almazor ME. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum. 2019;48(4):736–740. doi:10.1016/j.semarthrit.2018.05.006.
- Safa H, Johnson DH, Trinh VA, Rodgers TE, Lin H, Suarez-Almazor ME, Fa’ak F, Saberian C, Yee C, Davies MA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7(1):319. doi:10.1186/s40425-019-0774-y.
- Dubin K, Callahan MK, Ren BY, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:7. doi:10.1038/ncomms10391.
- Wolchok JD, Weber JS, Hamid O, Lebbe C, Maio M, Schadendorf D, de Pril V, Heller K, Chen TT, Ibrahim R, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9.