ABSTRACT
Interferons (IFNs) are a large family of pleiotropic cytokines that regulate both innate and adaptive immunity and show anti-cancer effects in various cancer types. Moreover, it was revealed that IFN signaling plays critical roles in the success of cancer therapy strategies, thereby enhancing their therapeutic effects. However, IFNs have minimal or even adverse effects on cancer eradication, and mediate cancer immune escape in some instances. Thus, IFNs have a double-edged effect on the cancer immune response. Recent studies suggest that IFNs regulate each step of the cancer immunity-cycle, consisting of cancer antigen release, presentation of antigens and activation of T cells, trafficking and infiltration of effector T cells into the tumor microenvironment, and recognition and killing of cancer cells, which contributes to our understanding of the mechanisms of IFNs in regulating cancer immunity. In this review, we focus on IFNs and cancer immunity and elaborate on the roles of IFNs in regulating the cancer-immunity cycle.
Introduction
The fact that inactive viruses interfere with the amplification of live viruses was established by the end of the 1940s, although the mechanism underlying this phenomenon was unknown.Citation1 In 1957, Isaacs and Lindenmann found that incubation of heat-inactivated influenza virus with the chorioallantoic membrane of chick embryos induced the production of a new factor interfering with the amplification of live influenza virus in the membrane. They named this new factor interferon (IFN).Citation2 At the beginning of the 1960s, chicken interferon (IFN-β) was purified.Citation3 Soon after purification of IFN-β, Wheelock discovered a novel virus-inhibitor (IFN-γ) produced by human leukocytes similar as chick embryo interferon in 1965.Citation4 It is now known that IFNs are a large family of cytokines.
Although IFNs were originally identified as potent anti-viral factors, they were also recognized to regulate immune responses and inhibit cancers. Before IFNs were purified, scientists employed unpurified interferon to treat various types of cancer in mice and patients.Citation5 Since interferon possesses anti-viral effects and virus infection is associated with some malignancies (for example, Rous sarcoma virus cause Rous sarcoma), researchers began to use IFNs to treat virus-induced tumors in animal models in the mid-1960s and observed therapeutic effects on these tumors.Citation3,Citation5 The first clinical trial using IFN to treat cancer was initiated in 1971 in osteosarcoma,Citation6 and now IFNs have been used to treat various types of cancer in the clinic, including melanoma, hairy cell leukemia, and renal cell carcinoma.Citation7 However, IFN treatment has minimal or even adverse effects in some instances,Citation8 which suggests that IFNs play a complicated role in the cancer immune response.
Recently, immune checkpoint blockage (ICB) has been demonstrated to be a promising strategy to treat cancer,Citation9 and IFN signaling seems to be critical to successful ICB therapy.Citation10–12 Moreover, IFNs enhance the therapeutic sensitivity of ICBs in various cancer types.Citation13–17 These studies suggest that IFN signaling plays an important role in cancer immunotherapy. In this review, we focus on IFNs and cancer immunity, and elaborate on the roles of IFNs in regulating the cancer-immunity cycle.
IFNs and IFN-induced signaling
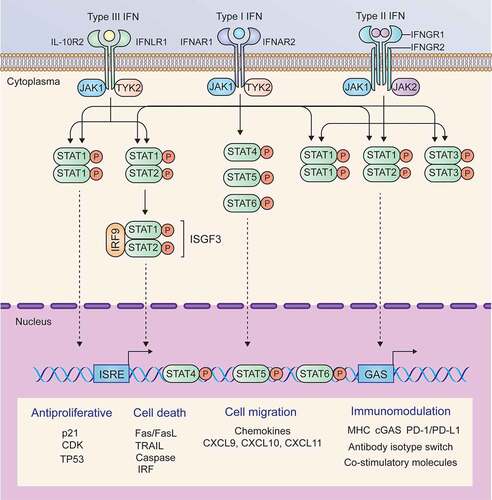
IFNs are divided into three subtypes based on their cognate receptors and sequence identity. IFN-α, IFN-β, IFN-ε, IFN-ĸ, and IFN-ω belong to type I IFNs that bind to the IFNα/β receptor composed of IFNAR1 and IFNAR2. Type I IFN receptors are expressed in most cell types in the body. IFNAR1 is absolutely necessary for type I IFN signaling,Citation18 whereas IFNAR2 has various isoforms with different effects on this signaling pathway. In humans, the longest IFNAR2c isoform and the soluble IFNAR2a isoform (lacking the transmembrane domain) activate this signaling.Citation19–22 The shorter IFNAR2b isoform inhibits this signaling by acting as a dominant-negative regulator.Citation23 IFN-γ is the only member of type II IFN and binds to the IFN-γ receptor composed of IFNGR1 and IFNGR2. IFNGR1 recognizes and binds to IFN-γ, and IFNGR2 is responsible for signal transduction. Both subunits of IFNGR are ubiquitously expressed in all mammalian cells.Citation24,Citation25 The type III IFNs consist of IFN-λ1 (IL-29), IFN-λ2 (IL-28A), IFN-λ3 (IL-28B), and IFN-λ4, and this type of IFN binds to the heterodimeric receptor composed of IFNLR1 (also known as IL28RA) and IL10RB.Citation19 Although the expression of IL10RB is widely expressed in many cell types, the expression of IFNLR1 is usually restricted in epithelial cells and absent in some immune cells, such as human NK cells; thus, the actions of type III IFNs may be restricted spatially.Citation26,Citation27
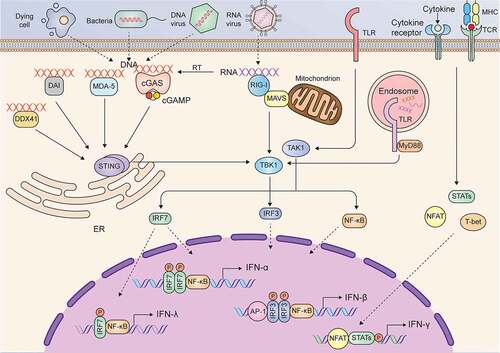
There are many intracellular and extracellular stimuli that trigger the production of type I IFNs. Typically, upon infection with microbes or exposure to damaged cellular components, danger-associated molecular patterns (DAMPs) can be recognized by pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), cyclic GMP-AMP synthase (cGAS), MDA-5, DAI, RIG-I like receptors, and DDX41in the cell membrane or cytoplasm. After recognizing and binding with DAMPs, these PRRs are activated and interact with adaptor proteins and activate kinases to phosphorylate NF-ĸB, IFN regulatory factor 3 (IRF3), and activating protein 1 (AP-1), which translocate into the nucleus and induce the expression of IFN-β in most cell types in the bodyCitation28 (). The production of IFN-α requires the transcription factor IRF7 rather than IRF3, and IFN-α is primarily expressed by plasmacytoid dendritic cells (DCs) because of the constitutive expression of IRF7.Citation29 The less-studied members of type I IFNs (IFN-ε, IFN-ĸ and IFN-ω) seem to be secreted in a tissue-specific manner in response to various stimuli.Citation19 Similar to type I IFNs, the stimuli and source of type III IFNs are broad, and most cell types in the body produce IFN-λ.Citation26 In contrast to type I and type III IFNs, the resource of IFN-γ is restricted in immune cells, such as T cells, B cells, natural killer (NK) cells, natural killer T (NKT) cells, DCs, and macrophages.Citation30–37 Many cytokines, such as IL-1, IL-2, IL-12, IL-15, IL-18, IL-21, IL-23, IL-27, IFN-α/β and TNF-α, can induce IFN-γ secretion in various types of immune cells.Citation38 For example, IL-12 alone or combined with other cytokines (such as IL-18) or combined with T cell receptor (TCR) and MHCII-Ag peptide complexes induce IFN-γ secretion in lymphoid cells mediated by signal transducer and activator of transcription 4 (STAT4) or nuclear factor of activated T cells (NFAT).Citation31,Citation39–41 In addition to cytokines, bacterial infection (such as mycobacteria or Legionella) or stimulation with the components of bacteria (such as lipopolysaccharide) induces IFN-γ production in macrophages and DCs via unclear mechanisms.Citation34
The functions of the three types of IFNs seem to be redundant, and the canonical signaling induced by different IFNs is also similar; in particular, type I and type III IFNs induce the same signaling. Upon binding to their ligands, IFNAR or IFNLR activates the constitutively interacting kinases JAK1 and TYK2. Activated JAK1 and TYK2 phosphorylate STAT1 and STAT2 and induce heterodimerization of STAT1 and STAT2 or homodimerization of STAT1, and then form a trimeric complex known as IFN-stimulated gene factor 3 (ISGF3) by interacting with IRF9. ISGF3 enters the nucleus and binds IFN-stimulated response elements (ISREs) to induce the expression of type I and type III IFN target genes. Unlike IFNAR and IFNLR, IFNGR binds to IFN-γ and activates JAK1 and JAK2. Activated JAK1 and JAK2 cause phosphorylation and homodimerization of STAT1, which translocates into the nucleus and binds IFN-γ-activating sites (GASs) to induce the transcription of IFN-γ target genes.Citation25 The signaling pathways and major target genes induced by the three types of IFNs are summarized in .
The implication of IFNs in cancer therapy
Given that the induction of IFNs is largely triggered by multiple DAMPs and the activation of IFN signaling pathway exhibits cell intrinsic (anti-proliferation and inducing cell death) and extrinsic (immunomodulation) anti-cancer activity, it seems reasonable to conclude that IFNs play a critical role in the successes of conventional cancer therapeutic strategies. Indeed, IFNs alone or strategies of stimulating IFN production, and combined using IFNs and other cancer therapies have been demonstrated to be effective to treat various malignancies. Additionally, it has also been revealed that deficiency of IFN signaling is one of the most important reasons for the resistance or failure of common cancer therapeutic strategies.
Firstly, the efficient type I IFN signaling was recognized as a footstone closely related to the success of conventional cancer therapeutic strategies, such as chemotherapy, radiotherapy and immunotherapy.Citation42 Sistigu et al. demonstrated that cancericidal effects of anthracyclines rely on cancer cell autonomously producing type I IFN induced by the activation of toll-like receptor 3 (TLR3).Citation43 Chemotherapeutic drug cyclophosphamide was revealed to modulate the transcriptional prolife of peripheral blood mononuclear cells (PBMCs) in patients with hematologic malignancies and induce a type I IFN associated sterile inflammation, which contributes to cancer cell elimination.Citation44–46 Apart from chemotherapy, the efficacy of radiotherapy was also highly entwined with the activation of type I IFN signaling.Citation47,Citation48 Both studies in mouse models of melanoma and colorectal carcinoma indicated that radiotherapy induces the production of type I IFN in myeloid cells and thus attributes to the generation of tumor infiltrating DC with enhanced ability to prime T cells.Citation47,Citation48 Additionally, both type I IFN (IFN-α and IFN-β) and type II IFN have been reported to enhance the efficacy of anti-PD1 or anti-PD-L1 in various cancer types, such as melanoma and pancreatic cancer.Citation13–17
Secondly, various IFN stimulating strategies based on targeting PRRs have been developed to treat cancer, and accumulating evidence indicate that PRR agonists synergize with other therapy approaches and attribute to a better therapeutic efficacy. Deng et al. found that the administration of STING agonist (2ʹ3’ cGAMP, 10μg) synergized with radiation (20 Gy) and significantly boost anti-cancer immune response in murine colon cancer bearing mouse models.Citation48 Ghaffari et al. also showed that STING agonist (2′3′-c-di-AM, 4 mg/kg i.p.) combined with anti-PD-1 antibody, greatly promotes IFN response and the expression of MHC class II genes and subsequently amplifies the therapeutic efficacy of carboplatin in the murine model of high-grade serous ovarian cancer.Citation49 Recently, Márquez-Rodaet al. found that intratumoral injection of a nanoplexed form of polyinosinic:polycytidylic acid (poly I:C), a TLR3 agonist called BO-112, in combination with PD-1 blockade therapy, significantly promotes infiltration of CD8+ T cells and increases the expression of genes associated with T cell cytotoxic activity.Citation50 Moreover, BO-112 is also found to restore the efficacy of T cell-based adoptive cell therapy (ACT) through increasing MHC class I expression of type I and type II IFN deficient melanoma cells in an IFN- and Nlrc5-independent manner.Citation51 Beside, a very recent study indicated that STING agonist (DMXAA or cGAMP) helps to subvert the immunosuppressive TME, thereby promotes CAR T cell trafficking and persistence in breast cancer.Citation52 In general, the PRRs in cancer cells or surrounding non-cancer cells (including infiltrated immune cells) senses DAMPs or directly activated by their agonists to induce the production of IFNs, which subsequently boost anti-cancer immune response to eradicate cancer cells. Therefore, positive feedback between cancericidal strategies and IFN-based anti-cancer immunity exists in the process of killing cancer cells.
Thirdly, accumulating evidence indicated that deficiency of IFN signaling is one of the most important reasons for the immune dysfunction and even the resistance or failure of common cancer therapeutic strategies. For example, the efficacy of immune checkpoint blockade therapy was significantly reduced on STING knockout mice bearing B16-SIY melanoma, because the loss of STING signaling impaired the tumor-cell-derived DNA triggered production of type I IFN and thus failed to activate DCs.Citation53 Ghosh et al. showed that mutant p53 mediates apoptosis resistance and immune evasion of cancer cells through interacting with TBK1 and then preventing the formation of TBK1/STING/IRF3 complex and finally impairing the expression of IFN-β.Citation54 An early study indicated that the expression of interferon-stimulated genes (ISGs) was impaired in the lymphocytes from patients with breast cancer, melanoma, and gastrointestinal cancer, which indicates that defect in IFN signaling in lymphocyte may represent a common cancer-associated mechanism of immune dysfunction.Citation55 Similarly, it was showen that the downstream targets of IFN-γ were downregulated in different melanoma cell lines with the disappointing response to immunotherapies, suggesting downregulation of IFN-γ signaling is common in melanoma and potentially predicts the response to immunotherapy.Citation56 It has been elucidated recently that JAK1 defeated melanoma B16 cells were insensitive to T cell-based adoptive cell therapy (ACT) due to the incompetence in both type I and II IFN signaling.Citation51 Similarly, loss-of-function mutations in JAK1/2 has also been revealed to be responsible for the primary and acquired resistance to anti-PD-1 blockage in melanoma and colon cancer carcinoma.Citation12 Christopher et al. demonstrated that effective antitumor responses to anti-PD-1 blockage required DCs to produce IL-12 upon sensing IFN-γ released from T cells, in turn DC derived IL-12 activates T effector cells, whereas IFN-γ deficiency impaired the anti-PD-1 efficiency.Citation10
In summary, it is a promising strategy to use IFNs alone or combined with therapeutic strategies to treat cancer. Preclinical studies have indicated that IFNs are competent in provoking cancer immunity in different cancer types (). Consistent with the results of preclinical studies, a number of clinical studies have also confirmed the efficiency of IFNs in the management of various types of cancer (). However, it should be mentioned that different types of IFNs may be suitable for the treatment of different types of cancer.Citation25
Table 1. Pre-clinical studies of IFNs in cancer treatment
Table 2. Clinical trials of IFNs in cancer treatment
Although the positive roles of IFNs in cancer therapy have been well recognized, IFNs occasionally have been noticed to induce the acquisition of therapy resistance through mediating cancer immune escape by affecting both immune cells and nonimmune cells in the tumor microvironment.Citation91 For instance, Jacquelot et al. reported that sustained type I IFN activation induce the up-regulation of programmed cell death ligand 1 (PD-L1) in both tumor and DCs and then enhance the expression of nitric oxide synthase 2 (NOS2), which is related to the accumulation of Treg and myeloid cells in the TME, finally lead to the resistance to programmed cell death 1 (PD-1) blockade.Citation92 Consistently, type I IFN also upregulates the expression of NOS2 and PD-L1 gene in PBMCs from melanoma patients.Citation92 Type I IFN singling also induce radiation resistance by promoting the recruitment of immunosuppressive myeloid cells via the CCR2 pathway.Citation93 In addition, several studies indicated that the overexpression of a subset of ISGs known as interferon-related DNA damage resistance signature (IRDS) reduced the sensitivity of tumor cells to genotoxic therapy strategies in vitro.Citation94,Citation95Another similar study showed that tumor cells taken up the stroma-cell-derived exosomes containing non-coding RNA and repeat/transposable elements, enhanced STAT1-drived expression of IRDS and the activation of NOTH3. And the cooperation of STAT1 and NOTH3 triggered the accumulation of therapy resistant tumor-initiating cells and tumor recurrence.Citation96
Conclusively, more effort should be paid to further understand the mechanisms by which IFNs regulate cancer immunity and how they are involved in other cancer therapies in order to conceive optimal and efficient therapeutic strategies for cancer management, and it may reduce therapeutic resistance of IFN-based therapy by adjusting the dosage and administration duration or combination with other therapeutic approaches.
IFNs regulate the cancer-immunity cycle
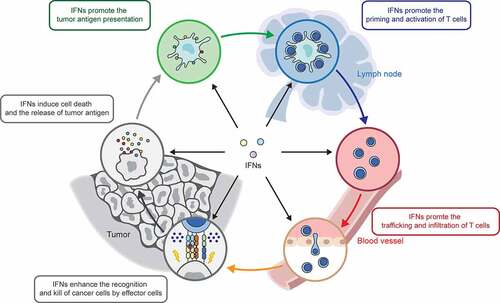
Cancer growth is determined by the balance between cell proliferation and cell death. Neoantigens resulting from gene mutation or overexpression of oncogenes in cancer cells are released after cell death, captured, and then presented to T cells by antigen-presenting cells (APCs), such as DCs and macrophages, which cause the activation of effector T cells. The activated effector T cells traffic and infiltrate into tumor tissues, where they recognize and kill cancer cells with the same tumor antigens, resulting in the release of more antigens. This cyclic process is defined as the cancer immunity cycle.Citation97 Current studies suggest that IFNs regulate each step of the cancer immunity cycle (), contributing to our understanding of the mechanisms by which IFNs regulate cancer immunity.
IFNs directly induce cell death and promote release of tumor antigens
It has long been believed that only IFN-γ directly induces malignant cell death, whereas the other types of IFNs exert their anti-cancer effects by regulating the activity of host immune cells, such as DCs, NK cells and cytotoxic T lymphocytes (CTLs).Citation25,Citation98 Recent studies have shown that all types of IFNs have direct pro-apoptotic effects.
Both IFN-α and IFN-β regulate intrinsic and extrinsic apoptotic pathways.Citation99,Citation100 Mechanistically, IFN-α and IFN-β induce the transcription of the TP53 gene, inhibit proliferation, and induce apoptosis of cancer cells.Citation101 Moreover, they also directly induce the production of pro-apoptotic factors, such as TRAIL and FASCitation102,Citation103 and enhance their pro-apoptotic effects in various malignant cell types.Citation104,Citation105 Additionally, IRF family members are well-known ISGs induced by type I IFNs.Citation25 Most members of the IRF family, such as IRF1, IRF3, and IRF5, have been documented to induce cell death in various malignant tumor types.Citation106–108 Although the pro-cytotoxic effects of type III and other members of type I IFNs are currently not well studied, considering that the ISGs induced by them are almost same, it is reasonable to deduce that most members of type I and type III IFNs could directly induce malignant cell death. As expected, several recent studies have shown that type III IFNs, such as IFN-λ1, IFN-λ2, and IFN-λ4, induce cell death in various malignant tumor types.Citation90,Citation109–112
The pro-apoptotic effects of IFN-γ have been well documented and intensively reviewed previously.Citation113–115 Like type I and type III IFNs, IFN-γ also induces the transcription of pro-apoptosis genes, such as TRAIL, FAS, FAS ligand, and caspase-8.Citation116–119 Moreover, it can promote apoptosis of malignant cells by inducing endoplasmic reticulum stress and reactive oxygen species (ROS).Citation120 Apart from apoptosis, IFN-γ also induces ferroptosis in cancer cells. It was recently reported that IFN-γ secreted by activated CD8+ T cells enhanced the ferroptosis of cancer cells by inhibiting the expression of SLC3A2 and SLC7A11, which disturbed the uptake of cysteine and thus resulted in lipid peroxidation and consequent ferroptosis of cancer cells.Citation121 Additionally, IFN-γ has been reported to induce ETosis in lung cancer cells.Citation122,Citation123 ETosis is a suicidal process in which the cell extrudes its intracellular DNA and histones to generate an extracellular reticular structure. This event is a special type of cell death that usually occurs in neutrophils and mast cells.Citation124 IFN-γ can induce oxidative stress and the upregulation of ROS, which promotes mimic ETosis in lung malignant cells.Citation122 Additionally, IFN-γ treatment was also found to induce caspase-mediated DNA damage and further activate ATR/ATM-regulated peptidyl arginine deiminase 4 (PAD4) mediated histone 3 citrullination, triggering mimic ETosis in A549 human lung cancer cells.Citation123
IFNs promote the tumor-antigen presentation
DCs are typically antigen-presenting cells that process and present antigens to T cells. Generally, endogenous antigens (such as synthesized virus antigens) are presented to CD8+ T cells in a class I MHC-dependent manner, whereas the exogenous antigens are presented to CD4+ T cells in a class II MHC dependent manner by DCs. Tumor-antigens are acquired and processed by DCs and presented to CD8+ T cells with the help of Th1 CD4+ T cells.Citation125 It has been demonstrated that all types of IFNs promote the tumor-antigen presentation process of DCs.
The effects of IFNs on the differentiation and maturation of DCs have been investigated as early as 1998 when the type I IFNs, such as IFN-α and IFN-β, were first identified to be not only necessary for the differentiation,Citation126,Citation127 but also facilitate the maturation and activation of DCs.Citation128,Citation129 Based on these findings, IFN-α or IFN-β has been developed as one of the standard components of cytokine cocktails inducing maturation of DCs.Citation130 In 2011, Diamond et al. revealed that type I IFNs were essential for tumor-specific antigen presentation of DCs, because the lack of IFNAR1 in DCs resulted in defects of antigen cross-presentation to CD8+ T cells.Citation131 Moreover, DCs treated with IFN-α2b or IFN-α5 showed enhanced adhesion to cultured lymphatic endothelial cells, indicating that IFN-α is favor of the adhesion and transmigration of DCs.Citation132 Various studies have consistently demonstrated that the activation of IFN-β-producing signaling pathways also facilitate the process of tumor-antigen presentation of DCs. For example, TLR agonists, such as lipopolysaccharide (LPS) and polyinosinic: polycytidylic acid (poly I:C), are known to stimulate the maturation of DCs via activation of the TLR signal transduction pathway.Citation133,Citation134 Additionally, STING agonists have been reported to promote the infiltration of DCs into the TME and enhance the antigen-presentation ability of DCs through the STING-TBK1 signaling pathway.Citation97,Citation135,Citation136
It has been long believed that the major function of NK, NKT, and γδ T cells was to lyse virus-infected or transformed cells through the cytolytic effect of IFN-γ. However, recent studies have shown that these IFN-γ-producing innate lymphocytes also facilitate the antigen-presentation of DCs.Citation137 A study comparing the efficiency of several clinical grade DC maturation cocktails demonstrated that LPS plus IFN-γ is more potent in inducing the maturation of DCs compared with the gold standard cocktails based on IFN-α and other cytokines.Citation138 Moreover, it has been demonstrated that IFN-γ produced by CD4+ T cells in the TME induces the expression of class I and class II MHC molecules and stimulates the production of antigen processing machinery by APCs, which enhances the antigen-presentation to T cells in a class I or class II restricted manner.Citation139–141 One recent study also confirmed that short-term (less than 48 h) exposure to LPS and IFN-γ promotes the maturation of DCs; however, long-term exposure to LPS and IFN-γ inhibits the functions of DCs and even induces apoptosis of DCs.Citation142 This study suggests that long-term exposure to inflammation may result in the exhaustion of DCs in the cancer tissue microenvironment.
Some type III IFN members have also been reported to regulate the maturation of DCs. For example, IFN-λ1 has been shown to induce the maturation of DCs, break immune tolerance and potentially contribute to clearance of hepatitis B virus (HBV) by the immune system.Citation143,Citation144 However, no study has investigated and compared the effect and efficiency of type III IFNs on promoting maturation of DCs in the TME.
Apart from the modulation of DCs, IFN signaling also plays a critical role in the crosstalks between innate immune cells and DCs. In the TME, tumor derived cGAMP was taken up or transferred to the endothelial cells, DCs or macrophages via some specific transporters,Citation145–147 which triggers the production of type I IFN. Afterward, secreted type I IFN promotes the infiltration of innate cytolytic cells, such as NK cells. And activated NK cells by the ligands on cancer cells will perform their cytotoxic function to promote the release of tumor antigen and concomitantly secrete several chemoattractants, such as XCL1, CCL5Citation148 or FLT3LG,Citation149 which mediate the recruitment of conventional dendritic cells (cDC), a type of DCs specialized in cross-presentation. IFNs are also important in NK-dependent DC maturation. An early research found that the interaction between NK cells and DCs lead to the engagement of NKp30, which further induces the production of TNF-α and IFN-γ from NK cells, and these cytokines will promote the maturation of DCs.Citation150
IFNs promote the priming and activation of t cells
DCs not only process and present antigens to T cells, but also prime and activate T cells by providing cytokines that are essential for the activation of naïve T cells; thus, DCs also play important roles in their priming and activation. As early as 2002, it was demonstrated that IFN-α was essential for DCs to stimulate naïve T-cell proliferation based on the observation that the lack of IFNAR1 in DCs or blocking IFN-α with neutralizing antibody impaired the ability of DCs from bone marrow to stimulate T cell priming.Citation129 Similarly, Longhi et al. also revealed that the systemic type I IFN signaling pathway is required for DCs to induce a CD4+ Th1 immune response in vivo.Citation133 Additionally, IFN-γ is one of the most important cytokines for inducing Th1 polarization. NK cells interact with DCs and then assist the polarization of Th1 cells in an IFN-γ-dependent manner.Citation151 Moreover, Type III IFNs also facilitate Th1 polarization. A study indicated that naïve and memory human CD4+ T cells express IL-28AR (IFNLR1) and preclude the expression of Th2 cytokines (IL-4 and IL-13) in these cells.Citation152 Consistent with this study, IFN-λ1 was also found to reduce IL-13 secretion but enhance IFN-γ secretion in human PBMCs following mitogen stimulation (Con-A).Citation153 Hence, IFN-λ could modulate Th1/Th2 balance by elevating Th1 cytokines but restricting the production of Th2 cytokines,Citation154,Citation155 which contributes to T cell priming and cancer elimination.
Type I IFNs not only promote the priming of T cells, but also prolong the survival and augment the proliferation of activated T cells through the cell-intrinsic type I IFN signaling pathway. Marrack et al. first reported in 1999 that IFN-α/β plays an important role in maintaining the vitality of T cells in vitro.Citation156 It was then revealed that type I IFN directly stimulated the clonal expansion and effector differentiation of CD8+ T cells in vitro and in vivo, because IFNAR expression by T cells was necessary for this process.Citation157,Citation158 In addition to type I IFN, Zimmerman et al. demonstrated that IFN-γ also promoted the survival and proliferation of tumor-specific T cells by upregulating the expression of survivin and Ifi202.Citation159
IFNs promote trafficking and infiltration of t cells
Trafficking and infiltration of T cells is one of the key steps in the anti-cancer response of T cells. T cells must traffic toward the tumor site and undergo extravasation before they recognize and eliminate tumor cells. This process is largely dependent on multiple chemokines, including CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10. These chemokines are important signaling molecules that recruit T cells in the TME.Citation160 IFNs are pleiotropic cytokines that promote multiple types of cells to produce chemokines that attract and recruit T cells in the TME. Padovan et al. found that IFN-α stimulates the secretion of CXCL9 and CXCL10 in monocyte-derived DCs and consequently promotes the infiltration of CD8+ T cells.Citation161 IFN-β promotes the expression of CCL5 and CXCR3 in melanoma cells and augments CD8+ T cell recruitment into the tumor.Citation14 IFN-γ has been reported to promote the production of CXCL10 in melanoma cells,Citation162 which enhances the production of CCL5 in fibroblasts, a common component cell type in the TME.Citation163
Intact endothelial cells are also very important for T cell infiltration by providing an adhesion face. Moreover, endothelial cells were recently identified as the main source of type I IFNs in the TME to stimulate the infiltration of CD8+ T cells into the TME.Citation164 IFNs can also affect the functions of endothelial cells and promote T cell infiltration. For example, type I IFN is found to promote the synthesis of CCL5 in endothelial cells.Citation165 In addition, treatment of endothelial cells with IFN-γ can selectively augment the migration of Th1 cells, the subtype of T cells that promotes cellular immunity.Citation166
Apart from endothelial cells, tumor vasculature also plays a crucial role in the process of trafficking and infiltration of T cells. However, IFNs, as anti-angiogenic cytokines,Citation167 may restrict the construction of vessels in the TME and thus make it difficult for T cell trafficking. Hence, it is necessary to evaluate the net effect of IFN treatment on the trafficking and infiltration of T cells during cancer management.
IFNs enhance the recognition and killing of cancer cells by effector cells
The coordination between antigen peptide-class I MHC molecules and TCR provides initial and essential signals for T cell-mediated elimination of cancer cells.Citation168 IFNs have been shown to enhance the recognition of cancer cells by T cells by boosting these crucial signals. An early study indicated that IFN-α increased the surface expression of tumor-associated antigens in breast cancer and colon cancer cells.Citation169 Moreover, IFNs can promote the expression of MHC class I molecules on cancer cells, which contributes to tumor antigen peptide presentation and recognition by T cells.Citation66,Citation76,Citation86,Citation170,Citation171 Additionally, IFNs also elevate the expression of adhesion molecules to stabilize the interaction between T cells and target cells. It was demonstrated that IFN-γ not only induces the expression of MHC, but also stimulates the expression of adhesion molecule ICAM-1 in human bladder carcinoma cells.Citation172
Activated T cells kill target cancer cells either through the release of perforin and granzyme or by enhancing the expression of tumor necrosis factor (TNF) family proteins, including FasL (CD95L), TRIAL, and mTNF, to induce apoptosis of target cells.Citation173–175 IFNs promote the expression of these molecules involved in the cytotoxic effects of T cells. Type I IFN was found to promote the expression of the activation marker CD69 and contribute to increase the cytotoxicity of γδ T cells against leukemia cells.Citation176 Several studies have demonstrated that IFN-γ increases the expression of perforin, granzyme B, CD95, CD95 ligand, and TRAIL in effector T cells, thereby promotes cancer cell death.Citation177–179 Type III IFNs also increase the cytotoxic effect of T cells. It has been reported that IFN-λ3 stimulation significantly enhanced the co-expression of CD107a and granzyme B, and increased the release of perforin in CTLs of macaques.Citation180
In addition to T cells, NK cells and NKT cells are also significant effector cells killing cancer cells, and IFNs enhance the cytotoxicity of these effector cells. A study has demonstrated that IFNR2−/-NK cells showed significantly compromised cytotoxicity against RMA-S MSCV compared with WT NK cells, suggesting type I IFNs play a pivotal role in the killing of cancer cells by NK cells.Citation181 Moreover, combined with perforin, IFN-γ is also important for the successful rejection of MHC class I-deficient RMA-S-CD80 tumor cells by NK cells.Citation182 Additionally, IFN-α and TLR ligands are reported to directly modulate the function of NKT cells by promoting the secretion of cytotoxic cytokines, such as TNF-α and IFN-γ.Citation183 A recent study found that IFN-α treatment (1000 IU/mL, 18h) also induce the elevated expression of CD69 and perforin of NK cells and NKT cells from melanoma patients.Citation184
Both NK cells and macrophages also kill cancer cells through the mechanism of antibody-dependent cell-mediated cytotoxicity (ADCC), which mainly depends on the binding of the Fc fragment of IgG antibodies and their coordinated receptor FcγR located on effector cells.Citation185 IFN-α has been reported to induce ADCC against B16 melanoma cells in vivo.Citation186 IFN-β also contributes to enhancing the sensitivity of lung cancer cells to ADCC.Citation187 IFN-γ is a predominant activator of macrophages,Citation188 and thus promotes killing of cancer cells via ADCC.Citation187 Mechanistically, type I IFNs and IFN-γ could promote antibody isotype switching into IgG.Citation189,Citation190 The regulation of class switch recombination is largely dependent on germline (GL) transcription, which means that distinct cytokines determine the isotypes of antibodies synthesized by B cells by inducing different transcription factors targeting various cytokine-responsive elements accompanied by GL promoters.Citation191 STAT1 and T-bet, induced by IFN signaling,Citation192,Citation193 are both important transcriptional activators for IgG germline transcription.Citation194–196 Additionally, IFN-γ has also been found to enhance the transcription of the Fc receptor for IgG,Citation197,Citation198 which may also contribute to ADCC.
IFNs negatively regulate anti-cancer immunity
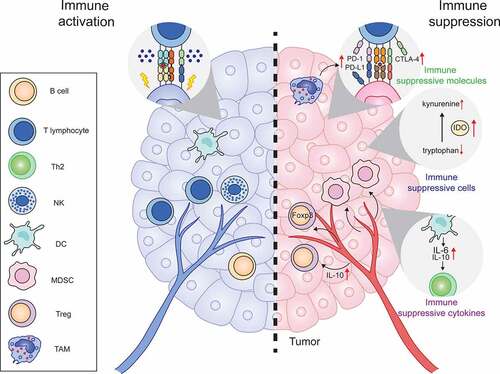
Activation of the immune response usually triggers negative feedback mechanisms and suppresses immune responses to maintain immune homeostasis. As expected, although most studies suggest that IFNs competently facilitate anti-tumor immune response, increasing evidence indicates that IFNs also negatively regulate anti-tumor immunity by either stimulating the expression of immunosuppressive molecules or recruiting the infiltration of immune-suppressive cells into the TME ().
Numerous studies have indicated that IFN-γ induces the expression of PD-1 and PD-L1 in cancer cells. For example, IFN-γ promoted the expression of PD-L1 in pancreatic cancer.Citation199 IFN-γ secreted by tumor-infiltrating lymphocytes has been found to stimulate the expression of PD-L1 in human melanocytic lesions.Citation200 Mechanistically, IFN-γ secreted by tumor-associated macrophages was reported to induce PD-L1 elevation through the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway and the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway.Citation201 IFN-γ is also reported to stimulate the expression of PD-L1 in melanoma in a P53 related JAK2 dependent manner.Citation202 Apart from PD-1 and PD-L1, the expression of CTLA-4 was also found to be upregulated by IFN-γ signaling in melanoma cells and melanocytes.Citation203 Moreover, tryptophan-metabolizing enzyme indoleamine-2,3-dioxygenase (IDO), a potent negative regulator of anti-cancer immunity, was also reported to be induced by IFN-γ secreted by CD8+ T cells in the TME, which suppresses anti-cancer immunity in a melanoma model.Citation204
Additionally, IFNs also induce the production of immunosuppressive cytokines. For example, IFN-β has been found to induce the expression of IL-10 and IL-6 in DCs, which results in Th2-biased immune suppression.Citation205 Moreover, type I and type III IFNs enhance the expression of IL-10R on APCs and sensitize them to IL-10 stimulation, which negatively regulates the activity of IFNs on APCs through inhibiting TLR-induced IL-12 production.Citation206
In addition to immune suppressive molecules and cytokines, IFNs also promote the infiltration of immune suppressive cells. Type I IFN has been found to be associated with myeloid-derived suppressor cell (MDSC) mobilization via the CCR2 pathway and leads to the radiation resistance in a mouse cancer model.Citation93 Besides, type I IFN contributes to induction of the infiltration of regulatory T cell (Treg) through upregulation of IL-10.Citation207 Whereas IFN-λ has also been reported to trigger the proliferation of FOXP3-expressing suppressor T cells by inducing tolerogenic DCs.Citation208 Additionally, the loss of E74-like transcription factor (Elf5) in a triple-negative breast cancer tumor model reduces the expression of an ubiquitin ligase named FBXW7, leads to the stabilization of IFN-γ receptor 1 (IFNGR1).Citation209 Then, the enhanced IFN-γ signaling promotes the infiltration of immunosuppressive neutrophils and the upregulation of PD-L1 expression.Citation209
Finally, IFNs have an anti-angiogenic ability to constrain tumor growth. It is noteworthy that the diminishment of tumor vessels may also make it difficult for the trafficking and infiltration of T cells, and thus compromise anti-tumor immunity. However, this point needs to be validated by further experiments.
Nonimmune effects of IFNs
IFNs, as cytokines, share several common characteristics of most cytokines. The receptors of one cytokine may be distributed on a variety of cell types. In addition, even a slight dose of cytokines may induce various biological effects due to the affinity between the cytokine and its corresponding receptor. This means that IFN administration in cancer treatment may contribute to unexpected severe side effects when the dosage is slightly higher than it should be. In fact, many nonimmune effects of IFN administration in patients have been reported, such as skin rash, flu-like symptoms, nephropathy, gastrointestinal discomfort, endocrine disorders, autoimmune diseases, and mental disorders.Citation210–215 Additionally, it has been revealed that IFNs participate in the regulation of cell cycle, cell differentiation, angiogenesis, and cancer development and progression.
IFNs can regulate the cell cycle by targeting cell cycle regulatory proteins or pathways related to the cell cycle. It has been reported that IFN-α restricts the cell cycle from G0 to S phase in prostate cancer cell lines by upregulating the expression of the cyclin-dependent kinase inhibitor p21.Citation216,Citation217 Sangfelt et al. also found that IFN-α treatment caused the induction of a group of cyclin-dependent kinase inhibitors (CKIs), including p21, p15, and p27.Citation217 IFN-α also stalls the cell cycle by inhibiting cyclin D3 and cdc25ACitation218 or inhibiting cyclin E- and cyclin D1-dependent CDK2 kinase activity.Citation219 Additionally, Lu et al. proved that IFN-α constrains the growth of hematopoietic progenitor cells by activating the p38 mitogen-activated protein kinase pathway.Citation220 In addition to IFN-α, IFN-γ also induce the expression of p21WAF1 and thus contribute to cell cycle arrest in the prostate cancer cell line DU145.Citation221 Moreover, IFN-λ was also reported to induce G1 phase arrest in esophageal carcinoma cells.Citation222
It has been known that IFNs promote the differentiation of some naïve cells, such as hematopoietic progenitor cells.Citation223,Citation224 Recent studies showed that IFNs also promote the differentiation of various types of malignant cells. A study has elucidated that differentiation of mouse myeloid leukemic cells can be induced by IFN treatment.Citation225 IFN-β has been shown to induce terminal cellular differentiation or programmed cell death in non-small-cell lung cancer.Citation226 In addition, IFN-α alone or in combination with retinoic acid contribute to the differentiation of cervical carcinoma cell lines.Citation227 IFNs (IFN-α, IFN-β, and IFN-γ) also show anti-angiogenic effects,Citation166,Citation228,Citation229 and inhibition of angiogenesis is one of the important mechanisms involved in the anti-cancer effects of IFN. Mechanistically, IFNs inhibit the expression of pro-angiogenic factors, such as vascular endothelial growth factor and basic fibroblast growth factor.Citation230,Citation231
Finally, IFNs are involved in carcinogenesis and cancer progression by inducing inflammation, which is one of the hallmarks of cancer and closely intertwined with cancer development. IFN-γ is a pro-inflammatory cytokine and is associated with a group of inflammation-related diseases of the digestive tract, such as inflammatory bowel disease and ulcerative colitis,Citation232 which are important risk factors for colorectal cancer (CRC), a typical inflammation-related cancer. Kobelt et al. also demonstrated that IFN-γ, accompanied with TNF-α, promote the growth and metastasis of colon cancer cells (HCT 116) by enhancing the expression of the MACC1 gene, a crucial oncogene involved in CRC metastasis.Citation233 In addition to CRC, IFN-γ also promotes metastasis of pancreatic cancer, another type of inflammation-related cancer. It has been reported that IFN-γ administration promotes epithelial-mesenchymal transition (EMT) of pancreatic cancer cells by enhancing the expression of vimentin and reducing the expression of E-cadherin in a dose-dependent manner.Citation199 However, in other studies, IFN-β and IFN-γ have been reported to suppress metastasis of human astroglioma and fibrosarcoma cell lines by suppressing the expression of matrix metalloproteinase 9 (MMP-9), the enzyme undermining ECM promoting malignant cell spreading.Citation234 These paradoxical results suggest that the effects of IFN-γ on cancer progression may be diverse in different cancer types.
Conclusions and perspectives
Since IFNs play a critical role in the immune responses, they have attracted great interest in the cancer immunotherapy. In this review, we elaborated on their effects at each step of the cancer-immunity cycle. Conclusively, IFNs potently regulate the cancer immunity and function at each step of the cancer-immunity cycle. However, the anti-cancer immune suppressive roles of IFNs are emerging and worthy of attention. Especially, it has been noticed that IFNs promote cancer progression in some cases by inducing cancer-associated inflammation.
It was believed that immune system both restricts and promotes cancer development and progression.Citation235 The double-edged roles of IFNs in the cancer immunity may be in accordance with the theory of cancer immunoediting, which consists of elimination, equilibrium, and escape.Citation236 IFNs play important roles in each phase of cancer immunoediting.Citation25 Since IFNs positively regulate each step of the cancer-immunity cycle, there is no doubt that IFNs contribute to the process of cancer elimination. IFNs may also be involved in immune equilibrium. It has been reported that IFN-γ can stimulate the expression of IL-7 in the gut epithelium,Citation237 which is an important cytokine that maintains memory CD8+ T cells. In addition, tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in the skin.Citation238 Thus, it can be postulated that IFN-γ may be associated with immune equilibrium in the anti-cancer immune response. In the last phase, IFNs facilitate the immune escape of cancer cells by upregulating immune suppressive molecules and promoting the infiltration of immune suppressive cells. For example, continuous exposure of leukemia cells to IFN-α caused a decrease in IFN-α-induced apoptosis due to the loss of STAT2.Citation239 Clarifying the phase-specific roles of IFNs in cancer immunity may be helpful to optimize stage-specific immunotherapy based on IFNs.
Conclusively, the double-edged effects of IFNs on the regulation of anti-cancer immune response embody the important philosophical tenet of traditional Chinese medicine: the theory of yin (negative regulation) and yang (positive regulation).Citation240 There are several factors influencing the yin and the yang of IFNs in cancer immunosurveillance and cancer immune escape. One is the duration of the IFN signaling in the TME. Generally, rapid activation of IFN signaling induces the acute inflammation and is beneficial to mobilize the immune system and eradiate cancer cells. However, the sustained or prolonged stimulation by IFN signaling causes the chronic inflammation, which is associated with immune agingCitation241 and leads to inflammation-associated cancers.Citation228 Another is the effects of IFNs on different types of immune cells in the TME. As a family of pleiotropic cytokines, IFNs modulate the behaviors of both immune-activating cells (e.g. CTL, γ/δ T cell, DC, B cell) and immunosuppressive cells (e.g. MDSC, Treg, M2) ().Citation19 In addition, the nature of ISGs also should be taken into account for evaluating the yin and yang effect of IFNs on cancer immunity. ISGs are diverse, and some ISGs encode molecules involved in the regulation of cell death, danger signal sensing, and positively promoting immune response. Whereas some ISGs encode immune checkpoint blockade molecules and thus have the opposite effects and cause immune suppression.
Taken together, the activation of IFNs signaling has double-edged effects on anti-cancer immunity. Considering that IFNs induce the expression of immune suppressive molecules, such as PD1, PD-L1, CTLA4 and IDO, combining them with immune checkpoint blockage therapy is a promising strategy to enhance the therapeutic effect of IFNs in the clinic, and such translational studies combining use of IFNs with anti-PD-L1 or anti-PD-1 antibodies are emerging.Citation13–17
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Henle W. 1950. Interference phenomena between animal viruses; a review. Journal of Immunology (Baltimore, Md: 1950). 64:203–20.
- Isaacs A, Lindenmann J, Virus Interference. I. 1957. The interferon. proceedings of the royal society of London series B. Biological Sciences. 147(927):258–267. doi:10.1098/rspb.1957.0048.
- Lampson GP, Tytell AA, Nemes MM, Hilleman MR 1963. Purification and characterization of chick embryo interferon. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 112:468–478. doi:10.3181/00379727-112-28080
- Wheelock EF. 1965. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 149(3681):310–311. doi:10.1126/science.149.3681.310.
- Gresser I, Tovey MG. 1978. Antitumor effects of interferon. Biochim Biophys Acta. 516(2):231–247. doi:10.1016/0304-419x(78)90009-4.
- Billiau A. 1981. The clinical value of interferons as antitumor agents. Eur J Cancer Clin Oncol. 17(9):949–967. doi:10.1016/s0277-5379(81)80001-6.
- Vidal P. 2020. Interferon α in cancer immunoediting: from elimination to escape. Scand J Immunol. 91(5):e12863. doi:10.1111/sji.12863.
- Ö S, Thorén FB. 2016. Opposing effects of immunotherapy in melanoma using multisubtype interferon-alpha - can tumor immune escape after immunotherapy accelerate disease progression?. Oncoimmunology. 5(3):e1091147. doi:10.1080/2162402x.2015.1091147.
- Nishino M, Ramaiya NH, Hatabu H, Hodi FS. 2017. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 14(11):655–668. doi:10.1038/nrclinonc.2017.88.
- Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, et al. 2018. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity. 49(6):1148–1161. e1147. doi:10.1016/j.immuni.2018.09.024.
- Pai CS, Huang JT, Lu X, Simons DM, Park C, Chang A, Tamaki W, Liu E, Roybal KT, Seagal J, et al. 2017. Clonal Deletion of Tumor-Specific T Cells by Interferon-γ Confers Therapeutic Resistance to Combination Immune Checkpoint Blockade. Immunity 2019; 50(2):477–492.e478. doi:10.1016/j.immuni.2019.01.006.
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. 2017. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 7(2):188–201. doi:10.1158/2159-8290.Cd-16-1223.
- Liang Y, Tang H, Guo J, Qiu X, Yang Z, Ren Z, Sun Z, Bian Y, Xu L, Xu H, et al. 2018. Targeting IFNα to tumor by anti-PD-L1 creates feedforward antitumor responses to overcome checkpoint blockade resistance. Nat Commun. 9(1):4586. doi:10.1038/s41467-018-06890-y.
- Uehara J, Ohkuri T, Kosaka A, Ishibashi K, Hirata Y, Ohara K, Nagato T, Oikawa K, Aoki N, Harabuchi Y, et al. 2017. Intratumoral injection of IFN-β induces chemokine production in melanoma and augments the therapeutic efficacy of anti-PD-L1 mAb. Biochem Biophys Res Commun. 490(2):521–527. doi:10.1016/j.bbrc.2017.06.072.
- Guo J, Xiao Y, Iyer R, Lu X, Lake M, Ladror U, Harlan J, Samanta T, Tomlinson M, Bukofzer G, et al. 2019. Empowering therapeutic antibodies with IFN-α for cancer immunotherapy. PloS One. 14(8):e0219829. doi:10.1371/journal.pone.0219829.
- Rahimi Kalateh Shah Mohammad G, Ghahremanloo A, Soltani A, Fathi E, Hashemy SI. 2020. Cytokines as potential combination agents with PD-1/PD-L1 blockade for cancer treatment. J Cell Physiol. 235(7–8):5449–5460. doi:10.1002/jcp.29491.
- Zhang M, Huang L, Ding G, Huang H, Cao G, Sun X, Lou N, Wei Q, Shen T, Xu X, et al. 2020. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. Journal for Immunotherapy of Cancer. 8(1):1. doi:10.1136/jitc-2019-000308.
- Domanski P, Fish E, Nadeau OW, Witte M, Platanias LC, Yan H, Krolewski J, Pitha P, Colamonici OR. 1997. A region of the beta subunit of the interferon alpha receptor different from box 1 interacts with Jak1 and is sufficient to activate the Jak-Stat pathway and induce an antiviral state. J Biol Chem. 272(42):26388–26393. doi:10.1074/jbc.272.42.26388.
- Parker BS, Rautela J, Hertzog PJ. 2016. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 16(3):131–144. doi:10.1038/nrc.2016.14.
- Samarajiwa SA, Mangan NE, Hardy MP, Najdovska M, Dubach D, Braniff SJ, Owczarek CM, Hertzog PJ. 2014. Soluble IFN receptor potentiates in vivo type I IFN signaling and exacerbates TLR4-mediated septic shock. Journal of Immunology Baltimore, Md: 1950. 192(9):4425–4435. doi:10.4049/jimmunol.1302388.
- Hardy MP, Owczarek CM, Trajanovska S, Liu X, Kola I, Hertzog PJ. 2001. The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood. 97(2):473–482. doi:10.1182/blood.v97.2.473.
- De Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, Zaker-Tabrizi L, Fung KY, Forster SC, Beddoe T, et al. 2013. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat Immunol. 14(9):901–907. doi:10.1038/ni.2667.
- Gazziola C, Cordani N, Carta S, De Lorenzo E, Colombatti A, Perris R. 2005. The relative endogenous expression levels of the IFNAR2 isoforms influence the cytostatic and pro-apoptotic effect of IFNalpha on pleomorphic sarcoma cells. Int J Oncol. 26:129–140.
- Van De Vosse E, Van Dissel JTIFN. 2017. γR1 defects: mutation update and description of the IFNGR1 variation database. Hum Mutat. 38(10):1286–1296. doi:10.1002/humu.23302.
- Dunn GP, Koebel CM, Schreiber RD. 2006. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 6(11):836–848. doi:10.1038/nri1961.
- Donnelly RP, Kotenko SV. 2010. Interferon-lambda: a new addition to an old family. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 30(8):555–564. doi:10.1089/jir.2010.0078.
- Syedbasha M, Egli A. 2017. Interferon Lambda: modulating Immunity in Infectious Diseases. Front Immunol. 8:119. doi:10.3389/fimmu.2017.00119.
- Iwanaszko M, Kimmel M. 2015. NF- κB and IRF pathways: cross-regulation on target genes promoter level. BMC Genomics. 16(1):307. doi:10.1186/s12864-015-1511-7.
- Solis M, Goubau D, Romieu-Mourez R, Genin P, Civas A, Hiscott J. 2006. Distinct functions of IRF-3 and IRF-7 in IFN-alpha gene regulation and control of anti-tumor activity in primary macrophages. Biochem Pharmacol. 72(11):1469–1476. doi:10.1016/j.bcp.2006.06.002.
- Bach EA, Aguet M, Schreiber RD. 1997. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 15(1):563–591. doi:10.1146/annurev.immunol.15.1.563.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 75(2):163–189. doi:10.1189/jlb.0603252.
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. Journal of Immunology (Baltimore, Md: 1950). 163:4647–4650.
- Santos LS, Sgnotto FDR, Inoue AHS, Padreca AF, Menghini RP, Duarte A, Victor JR. 2019. IgG from Non-atopic Individuals Induces In Vitro IFN-γ and IL-10 Production by Human Intra-thymic γδT Cells: a Comparison with Atopic IgG and IVIg. Arch Immunol Ther Exp (Warsz). 67(4):263–270. doi:10.1007/s00005-019-00545-6.
- Frucht DM, Fukao T, Bogdan C, Schindler H, O’Shea JJ, Koyasu S. 2001. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 22(10):556–560. doi:10.1016/s1471-4906(01)02005-1.
- Gessani S, Belardelli F. 1998. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 9(2):117–123. doi:10.1016/s1359-6101(98)00007-0.
- Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. 1998. IL- 12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. Journal of Immunology (Baltimore, Md: 1950). 161:3400–3407.
- Flaishon L, Hershkoviz R, Lantner F, Lider O, Alon R, Levo Y, Flavell RA, Shachar I. 2000. Autocrine secretion of interferon gamma negatively regulates homing of immature B cells. J Exp Med. 192(9):1381–1388. doi:10.1084/jem.192.9.1381.
- Malmgaard L. 2004. Induction and regulation of IFNs during viral infections. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 24(8):439–454. doi:10.1089/1079990041689665.
- Vignali DA, Kuchroo VKIL. 2012. 12 family cytokines: immunological playmakers. Nat Immunol. 13(8):722–728. doi:10.1038/ni.2366.
- Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. 2002. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. Journal of Immunology (Baltimore, Md: 1950). 168(3):1146–1153. doi:10.4049/jimmunol.168.3.1146.
- Mavropoulos A, Sully G, Cope AP, Clark AR. 2005. Stabilization of IFN-gamma mRNA by MAPK p38 in IL-12- and IL-18-stimulated human NK cells. Blood. 105(1):282–288. doi:10.1182/blood-2004-07-2782.
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Type KG. 2015. I interferons in anticancer immunity. Nat Rev Immunol. 15(7):405–414. doi:10.1038/nri3845.
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. 2014. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 20(11):1301–1309. doi:10.1038/nm.3708.
- Moschella F, Torelli GF, Valentini M, Urbani F, Buccione C, Petrucci MT, Natalino F, Belardelli F, Foà R, Proietti E. 2013. Cyclophosphamide induces a type I interferon-associated sterile inflammatory response signature in cancer patients’ blood cells: implications for cancer chemoimmunotherapy. Clin Cancer Res. 19(15):4249–4261. doi:10.1158/1078-0432.Ccr-12-3666.
- Ziccheddu G, Proietti E, Moschella F. 2013. The Janus face of cyclophosphamide: a sterile inflammatory response that potentiates cancer immunotherapy. Oncoimmunology. 2(9):e25789. doi:10.4161/onci.25789.
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. 2013. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 39(1):74–88. doi:10.1016/j.immuni.2013.06.014.
- Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. 2011. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 71(7):2488–2496. doi:10.1158/0008-5472.Can-10-2820.
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. 2014. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 41(5):843–852. doi:10.1016/j.immuni.2014.10.019.
- Ghaffari A, Peterson N, Khalaj K, Vitkin N, Robinson A, Francis JA, Koti M. 2018. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer. 119(4):440–449. doi:10.1038/s41416-018-0188-5.
- Márquez-Rodas I, Longo F, Rodriguez-Ruiz ME, Calles A, Ponce S, Jove M, Rubio-Viqueira B, Perez-Gracia JL, Gómez-Rueda A, López-Tarruella S, et al. 2020. Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci Transl Med. 12(565):565. doi:10.1126/scitranslmed.abb0391.
- Kalbasi A, Tariveranmoshabad M, Hakimi K, Kremer S, Campbell KM, Funes JM, Vega-Crespo A, Parisi G, Champekar A, Nguyen C, et al. 2020. Uncoupling interferon signaling and antigen presentation to overcome immunotherapy resistance due to JAK1 loss in melanoma. Sci Transl Med. 12(565):565. doi:10.1126/scitranslmed.abb0152.
- Xu N, Palmer DC, Robeson AC, Shou P, Bommiasamy H, Laurie SJ, Willis C, Dotti G, Vincent BG, Restifo NP, et al. 2021. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 218(2):2. doi:10.1084/jem.20200844.
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. 2014. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 41(5):830–842. doi:10.1016/j.immuni.2014.10.017.
- Ghosh M, Saha S, Bettke J, Nagar R, Parrales A, Iwakuma T, Van Der Velden AWM, Martinez LA. 2021. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell. 39(4):494–508.e5. doi:10.1016/j.ccell.2021.01.003.
- Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, Swetter SM, Carlson RW, Fisher GA, Koong A, et al. 2009. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci U S A. 106(22):9010–9015. doi:10.1073/pnas.0901329106.
- Alavi S, Stewart AJ, Kefford RF, Lim SY, Shklovskaya E, Rizos H. 2018. Interferon Signaling Is Frequently Downregulated in Melanoma. Front Immunol. 9:1414. doi:10.3389/fimmu.2018.01414.
- Gordy JT, Luo K, Kapoor A, Kim ES, Ayeh SK, Karakousis PC, Markham RB. 2020. Treatment with an immature dendritic cell-targeting vaccine supplemented with IFN-α and an inhibitor of DNA methylation markedly enhances survival in a murine melanoma model. Cancer Immunol Immunother. 69(4):569–580. doi:10.1007/s00262-019-02471-0.
- Krepler C, Certa U, Wacheck V, Jansen B, Wolff K, Pehamberger H. 2004.Pegylated and conventional interferon-alpha induce comparable transcriptional responses and inhibition of tumor growth in a human melanoma SCID mouse xenotransplantation model. J Invest Dermatol. 123(4):664–669. doi:10.1111/j.0022-202X.2004.23433.x.
- Liu P, Zhang C, Chen J, Zhang R, Ren J, Huang Y, Zhu F, Li Z, Wu G. 2011. Combinational therapy of interferon-α and chemotherapy normalizes tumor vasculature by regulating pericytes including the novel marker RGS5 in melanoma. J Immunother. 34(3):320–326. doi:10.1097/CJI.0b013e318213cd12.
- Bauer JA, Morrison BH, Grane RW, Jacobs BS, Borden EC, Lindner DJ. 2003. IFN-alpha2b and thalidomide synergistically inhibit tumor-induced angiogenesis. J Interferon Cytokine Res. 23(1):3–10. doi:10.1089/10799900360520397.
- Hirata A, Hashimoto H, Shibasaki C, Narumi K, Aoki K. 2019. Intratumoral IFN-α gene delivery reduces tumor-infiltrating regulatory T cells through the downregulation of tumor CCL17 expression. Cancer Gene Ther. 26(9–10):334–343. doi:10.1038/s41417-018-0059-5.
- Ishii S, Hiroishi K, Eguchi J, Hiraide A, Imawari M. 2006. Dendritic cell therapy with interferon-alpha synergistically suppresses outgrowth of established tumors in a murine colorectal cancer model. Gene Ther. 13(1):78–87. doi:10.1038/sj.gt.3302608.
- Ueda K, Akiba J, Ogasawara S, Todoroki K, Nakayama M, Sumi A, Kusano H, Sanada S, Suekane S, Xu K, et al. 2016. Growth inhibitory effect of an injectable hyaluronic acid-tyramine hydrogels incorporating human natural interferon-α and sorafenib on renal cell carcinoma cells. Acta Biomater. 29:103–111. doi:10.1016/j.actbio.2015.10.024.
- Moriya F, Ogasawara S, Basaki Y, Akiba J, Kojiro S, Fukahori S, Ishizaki H, Nishida N, Matsuoka K, Kojiro M, et al. 2008. Growth inhibitory effects of pegylated IFN-alpha2b and 5-fluorouracil in combination on renal cell carcinoma cell lines in vitro and in vivo. Int J Oncol. 33(4):647–655. doi:10.1016/j.ijrobp.2008.06.320.
- Bielefeldt-Ohmann H, Fitzpatrick DR, Marzo AL, Jarnicki AG, Musk AW, Robinson BW. 1995.Potential for interferon-alpha-based therapy in mesothelioma: assessment in a murine model. J Interferon Cytokine Res. 15(3):213–223. doi:10.1089/jir.1995.15.213.
- Wang WJ, Qin SH, Zhang JW, Jiang YY, Zhang JN, Zhao L. 2014. Combination doxorubicin and interferon-α therapy stimulates immunogenicity of murine pancreatic cancer Panc02 cells via up-regulation of NKG2D ligands and MHC class I. Asian Pac J Cancer Prev. 15(22):9667–9672. doi:10.7314/apjcp.2014.15.22.9667.
- Huang SF, Kim SJ, Lee AT, Karashima T, Bucana C, Kedar D, Sweeney P, Mian B, Fan D, Shepherd D, et al. 2002. Inhibition of growth and metastasis of orthotopic human prostate cancer in athymic mice by combination therapy with pegylated interferon-alpha-2b and docetaxel. Cancer Res. 62(20):5720–5726.
- Umemoto S, Haruta M, Sakisaka M, Ikeda T, Tsukamoto H, Komohara Y, Takeya M, Nishimura Y, Senju S. 2019. Cancer therapy with major histocompatibility complex-deficient and interferon β-producing myeloid cells derived from allogeneic embryonic stem cells. Cancer Sci. 110(10):3027–3037. doi:10.1111/cas.14144.
- Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, Von Köckritz-blickwede M, Schilling B, Brandau S, Weiss S, Jablonska J. 2016. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 138(8):1982–1993. doi:10.1002/ijc.29945.
- GuhaSarkar D, Neiswender J, Su Q, Gao G, Sena-Esteves M. 2017.Intracranial AAV-IFN-β gene therapy eliminates invasive xenograft glioblastoma and improves survival in orthotopic syngeneic murine model. Mol Oncol. 11(2):180–193. doi:10.1002/1878-0261.12020.
- Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, Ponnazhagan S. 2008.Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 15(21):1446–1453. doi:10.1038/gt.2008.101.
- Dickson PV, Hagedorn NL, Hamner JB, Fraga CH, Ng CY, Stewart CF, Davidoff AM. 2007. Interferon beta-mediated vessel stabilization improves delivery and efficacy of systemically administered topotecan in a murine neuroblastoma model. J Pediatr Surg. 42(1):160–165. discussion 165 doi:10.1016/j.jpedsurg.2006.09.052.
- Dickson PV, Hamner JB, Burger RA, Garcia E, Ouma AA, Kim SU, Ng CY, Gray JT, Aboody KS, Danks MK, et al. 2007. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-beta and restricts tumor growth in a murine model of disseminated neuroblastoma. J Pediatr Surg. 42(1):48–53. doi:10.1016/j.jpedsurg.2006.09.050.
- Kaido T, Maury C, Schirrmacher V, Gresser I. 1994. Successful immunotherapy of the highly metastatic murine ESb lymphoma with sensitized CD8+ T cells and IFN-alpha/beta. Int J Cancer. 57(4):538–543. doi:10.1002/ijc.2910570417.
- Yoon W, Park YC, Kim J, Chae YS, Byeon JH, Min SH, Park S, Yoo Y, Park YK, Kim BM. 2017. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma. Eur J Cancer. 70:48–61. doi:10.1016/j.ejca.2016.10.010.
- Sikorski M, Bobek M, Zrubek H, Marcinkiewicz J. 2004. Dynamics of selected MHC class I and II molecule expression in the course of HPV positive CIN treatment with the use of human recombinant IFN-gamma. Acta Obstet Gynecol Scand. 83:299–307.
- Liu LL, Smith MJ, Sun BS, Wang GJ, Redmond HP, Wang JH. 2009. Combined IF N-gamma-endostatin gene therapy and radiotherapy attenuates primary breast tumor growth and lung metastases via enhanced CTL and NK cell activation and attenuated tumor angiogenesis in a murine model. Ann Surg Oncol. 16(5):1403–1411. doi:10.1245/s10434-009-0343-6.
- Green DS, Husain SR, Johnson CL, Sato Y, Han J, Joshi B, Hewitt SM, Puri RK, Zoon KC. 2019. Combination immunotherapy with IL-4 Pseudomonas exotoxin and IFN-α and IFN-γ mediate antitumor effects in vitro and in a mouse model of human ovarian cancer. Immunotherapy. 11(6):483–496. doi:10.2217/imt-2018-0158.
- George J, Banik NL, Ray SK. 2009. Combination of hTERT knockdown and IFN-gamma treatment inhibited angiogenesis and tumor progression in glioblastoma. Clin Cancer Res. 15(23):7186–7195. doi:10.1158/1078-0432.Ccr-09-1425.
- Oba M, Yano S, Shuto T, Suico MA, Eguma A, Kai H. 2008. IFN-gamma down-regulates Hsp27 and enhances hyperthermia-induced tumor cell death in vitro and tumor suppression in vivo. Int J Oncol. 32(6):1317–1324. doi:10.3892/ijo_32_6_1317.
- Wang L, Wang Y, Lu Y, Zhang Q, Qu X. 2015. Heterozygous deletion of ATG5 in Apc(Min/+) mice promotes intestinal adenoma growth and enhances the antitumor efficacy of interferon-gamma. Cancer Biol Ther. 16(3):383–391. doi:10.1080/15384047.2014.1002331.
- Yoon SJ, Heo DS, Kang JO, Lee SG, Kim CD, Sung MW, Kim NK. 1998. Synergistic anti-tumor effects with co-expression of GM-CSF and IFN-gamma in murine tumors. Int J Cancer. 77(6):907–912. doi:10.1002/(sici)1097-0215(19980911)77:6<907::aid-ijc18>3.0.co;2-y.
- Lasfar A, De Latorre A, Abushahba W, Cohen-Solal KA, Castaneda I, Yuan Y, Reuhl K, Zloza A, Raveche E, Laskin DL, et al. 2016. Concerted action of IFN-α and IFN-λ induces local NK cell immunity and halts cancer growth. Oncotarget. 7(31):49259–49267. doi:10.18632/oncotarget.10272.
- Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, De La Torre A, Lasfar A, Kotenko SV. 2010. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 59(7):1059–1071. doi:10.1007/s00262-010-0831-3.
- Tian S, Hui X, Fan Z, Li Q, Zhang J, Yang X, Ma X, Huang B, Chen D, Chen H. 2014. Suppression of hepatocellular carcinoma proliferation and hepatitis B surface antigen secretion with interferon-λ1 or PEG-interferon-λ1. Faseb J. 28(8):3528–3539. doi:10.1096/fj.14-250704.
- Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. 2006. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 176(12):7686–7694. doi:10.4049/jimmunol.176.12.7686.
- Hasegawa K, Tagawa M, Takagi K, Tsukamoto H, Tomioka Y, Suzuki T, Nishioka Y, Ohrui T, Numasaki M. 2016. Anti-tumor immunity elicited by direct intratumoral administration of a recombinant adenovirus expressing either IL-28A/IFN-λ2 or IL-29/IFN-λ1. Cancer Gene Ther. 23(8):266–277. doi:10.1038/cgt.2016.29.
- Hui X, Chen H, Zhang S, Ma X, Wang X, Huang B. 2011. Antitumor activities of recombinant human interferon (IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer. Cancer Lett. 311(2):141–151. doi:10.1016/j.canlet.2011.07.004.
- Yan Y, Zhang J, Liu Y, Zhu T, Yuan L, Ge Y, Ding H, Bu X. 2013. Inhibition of lung adenocarcinoma transfected with interleukin 28A recombinant adenovirus (Ad-mIFN-λ2) in vivo. Cancer Biother Radiopharm. 28(2):124–130. doi:10.1089/cbr.2012.1247.
- Tezuka Y, Endo S, Matsui A, Sato A, Saito K, Semba K, Takahashi M, Murakami T. 2012. Potential anti-tumor effect of IFN-λ2 (IL-28A) against human lung cancer cells. Lung Cancer. 78(3):185–192. doi:10.1016/j.lungcan.2012.09.005.
- Minn AJ. 2015. Interferons and the Immunogenic Effects of Cancer Therapy. Trends Immunol. 36(11):725–737. doi:10.1016/j.it.2015.09.007.
- Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, Ferrere G, Becharef S, Vétizou M, Daillère R, et al. 2019. Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 29(10):846–861. doi:10.1038/s41422-019-0224-x.
- Liang H, Deng L, Hou Y, Meng X, Huang X, Rao E, Zheng W, Mauceri H, Mack M, Xu M, et al. 2017. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 8(1):1736. doi:10.1038/s41467-017-01566-5.
- Khodarev NN, Minn AJ, Efimova EV, Darga TE, Labay E, Beckett M, Mauceri HJ, Roizman B, Weichselbaum RR. 2007. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 67(19):9214–9220. doi:10.1158/0008-5472.Can-07-1019.
- Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, et al. 2008. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 105(47):18490–18495. doi:10.1073/pnas.0809242105.
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et al. 2014. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 159(3):499–513. doi:10.1016/j.cell.2014.09.051.
- Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. 2019. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 18(1):152. doi:10.1186/s12943-019-1087-y.
- Kim R, Emi M, Tanabe K. 2007. Cancer immunoediting from immune surveillance to immune escape. Immunology. 121(1):1–14. doi:10.1111/j.1365-2567.2007.02587.x.
- Choi EA, Lei H, Maron DJ, Wilson JM, Barsoum J, Fraker DL, El-Deiry WS, Spitz FR. 2003. Stat1-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand and the cell-surface death signaling pathway by interferon beta in human cancer cells. Cancer Res. 63:5299–5307.
- Thyrell L, Erickson S, Zhivotovsky B, Pokrovskaja K, Sangfelt O, Castro J, Einhorn S, Grandér D. 2002. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 21(8):1251–1262. doi:10.1038/sj.onc.1205179.
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, et al. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 424(6948):516–523. doi:10.1038/nature01850.
- Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Type YH. 1999. I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 189(9):1451–1460. doi:10.1084/jem.189.9.1451.
- Buechner SA, Wernli M, Harr T, Hahn S, Itin P, Erb P. 1997. Regression of basal cell carcinoma by intralesional interferon-alpha treatment is mediated by CD95 (Apo-1/Fas)-CD95 ligand-induced suicide. J Clin Invest. 100(11):2691–2696. doi:10.1172/jci119814.
- Kazaana A, Sano E, Yoshimura S, Makita K, Hara H, Yoshino A, Ueda T. 2019. Promotion of TRAIL/Apo2L-induced apoptosis by low-dose interferon-β in human malignant melanoma cells. J Cell Physiol. 234(8):13510–13524. doi:10.1002/jcp.28029.
- Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. 2013. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 110(33):E3109–3118. doi:10.1073/pnas.1301218110.
- Manzella L, Tirrò E, Pennisi MS, Massimino M, Stella S, Romano C, Vitale SR, Vigneri P. 2016. Roles of Interferon Regulatory Factors in Chronic Myeloid Leukemia. Curr Cancer Drug Targets. 16(7):594–605. doi:10.2174/1568009616666160105105857.
- Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. 2003. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 63:6424–6431.
- Hu G, Barnes BJ. 2009. IRF- 5 is a mediator of the death receptor-induced apoptotic signaling pathway. J Biol Chem. 284(5):2767–2777. doi:10.1074/jbc.M804744200.
- Guenterberg KD, Grignol VP, Raig ET, Zimmerer JM, Chan AN, Blaskovits FM, Young GS, Nuovo GJ, Mundy BL, Lesinski GB, et al. 2010. Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol Cancer Ther. 9(2):510–520. doi:10.1158/1535-7163.Mct-09-0461.
- Li Q, Kawamura K, Tada Y, Shimada H, Hiroshima K, Tagawa M. 2013. Novel type III interferons produce anti-tumor effects through multiple functions. Frontiers in Bioscience (Landmark Edition). 18(3):909–918. doi:10.2741/4152.
- Balabanov D, Zhao L, Zhu Z, Hunzeker ZE, Tonner HM, Ding VA, Wakefield MR, Bai Q, Fang Y. 2019. IL- 29 Exhibits Anti-Tumor Effect on Pan-48 Pancreatic Cancer Cells by Up-regulation of P21 and Bax. Anticancer Res. 39(7):3493–3498. doi:10.21873/anticanres.13495.
- Onabajo OO, Porter-Gill P, Paquin A, Rao N, Liu L, Tang W, Brand N, Prokunina-Olsson L. 2015.Expression of Interferon Lambda 4 Is Associated with Reduced Proliferation and Increased Cell Death in Human Hepatic Cells. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 35(11):888–900. doi:10.1089/jir.2014.0161.
- Tura BJ, Bunyan KE, Harrison DJ. 2001. The effect of IFNgamma on the hepatocyte: cell cycle and apoptosis. Int J Exp Pathol. 82(6):317–326. doi:10.1046/j.1365-2613.2001.00207.x.
- Shen J, Xiao Z, Zhao Q, Li M, Wu X, Zhang L, Hu W, Cho CH. 2018. Anti-cancer therapy with TNFα and IFNγ: a comprehensive review. Cell Prolif. 51(4):e12441. doi:10.1111/cpr.12441.
- Silva-Santos B, Serre K, Norell H. 2015.γδ T cells in cancer. Nat Rev Immunol. 15(11):683–691. doi:10.1038/nri3904.
- Kim KB, Choi YH, Kim IK, Chung CW, Kim BJ, Park YM, Jung YK. 2002. Potentiation of Fas- and TRAIL-mediated apoptosis by IFN-gamma in A549 lung epithelial cells: enhancement of caspase-8 expression through IFN-response element. Cytokine. 20(6):283–288. doi:10.1006/cyto.2003.2008.
- Ahn EY, Pan G, Vickers SM, McDonald JM. 2002. IFN-gammaupregulates apoptosis-related molecules and enhances Fas-mediated apoptosis in human cholangiocarcinoma. International Journal of Cancer. 100(4):445–451. doi:10.1002/ijc.10516.
- Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, Kim CH, Kim SJ, Kim JD, Choi IS, et al. 1999. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. Journal of Immunology (Baltimore, Md: 1950). 162(4):1889–1895.
- Shin EC, Ahn JM, Kim CH, Choi Y, Ahn YS, Kim H, Kim SJ, Park JH. 2001. IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. International Journal of Cancer. 93(2):262–268. doi:10.1002/ijc.1310.
- Watanabe Y, Suzuki O, Haruyama T, Akaike T. 2003. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem. 89(2):244–253. doi:10.1002/jcb.10501.
- Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. 2019. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 569(7755):270–274. doi:10.1038/s41586-019-1170-y.
- Lin CF, Chen CL, Chien SY, Tseng PC, Wang YC, Tsai TT. 2016. Oxidative Stress Facilitates IFN-γ-Induced Mimic Extracellular Trap Cell Death in A549 Lung Epithelial Cancer Cells. PloS One. 11(8):e0162157. doi:10.1371/journal.pone.0162157.
- Lin CF, Chien SY, Chen CL, Hsieh CY, Tseng PC, Wang YC. 2016.IFN- γ Induces Mimic Extracellular Trap Cell Death in Lung Epithelial Cells Through Autophagy-Regulated DNA Damage. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 36(2):100–112. doi:10.1089/jir.2015.0011.
- Wartha F, Henriques-Normark B. 2008. ETosis: a novel cell death pathway. Sci Signal. 1(21):25. doi:10.1126/stke.121pe25.
- Santana MA, Esquivel-Guadarrama F. 2006. Cell biology of T cell activation and differentiation. Int Rev Cytol. 250:217–274. doi:10.1016/s0074-7696(06)50006-3.
- Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. Journal of Immunology (Baltimore, Md: 1950). 161:1947–1953.
- Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. 1998. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 64(3):358–367. doi:10.1002/jlb.64.3.358.
- Radvanyi LG, Banerjee A, Weir M, Messner H. 1999. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 50(5):499–509. doi:10.1046/j.1365-3083.1999.00625.x.
- Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 99(9):3263–3271. doi:10.1182/blood.v99.9.3263.
- Alfaro C, Perez-Gracia JL, Suarez N, Rodriguez J, Fernandez De Sanmamed M, Sangro B, Martin-Algarra S, Calvo A, Redrado M, Agliano A, et al. 2011. Pilot clinical trial of type 1 dendritic cells loaded with autologous tumor lysates combined with GM-CSF, pegylated IFN, and cyclophosphamide for metastatic cancer patients. Journal of Immunology (Baltimore, Md: 1950). 187(11):6130–6142. doi:10.4049/jimmunol.1102209.
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. 2011. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 208(10):1989–2003. doi:10.1084/jem.20101158.
- Rouzaut A, Garasa S, Teijeira A, González I, Martinez-Forero I, Suarez N, Larrea E, Alfaro C, Palazón A, Dubrot J, et al. 2010. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-α. Eur J Immunol. 40(11):3054–3063. doi:10.1002/eji.201040523.
- Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. 2009. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 206(7):1589–1602. doi:10.1084/jem.20090247.
- Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 1(4):311–316. doi:10.1038/79758.
- Škrnjug I, Guzmán CA, Cyclic RC. 2014. GMP-AMP displays mucosal adjuvant activity in mice. PloS One. 9(10):e110150. doi:10.1371/journal.pone.0110150.
- Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ. 2017. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A. 114(7):1637–1642. doi:10.1073/pnas.1621363114.
- Münz C, Steinman RM, Fujii S. 2005. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 202(2):203–207. doi:10.1084/jem.20050810.
- Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, Van Ham SM. 2007. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 25(41):7145–7152. doi:10.1016/j.vaccine.2007.07.031.
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. 2010. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 207(3):651–667. doi:10.1084/jem.20091921.
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. 2010. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 207(3):637–650. doi:10.1084/jem.20091918.
- Redondo M, Ruiz-Cabello F, Concha A, Hortas ML, Serrano A, Morell M, Garrido F. 1998. Differential expression of MHC class II genes in lung tumour cell lines. European Journal of Immunogenetics: Official Journal of the British Society for Histocompatibility and Immunogenetics. 25(6):385–391. doi:10.1046/j.1365-2370.1998.00116.x.
- Carstensen LS, Lie-Andersen O, Obers A, Crowther MD, Svane IM, Long-Term HM. 2019. Exposure to Inflammation Induces Differential Cytokine Patterns and Apoptosis in Dendritic Cells. Front Immunol. 10:2702. doi:10.3389/fimmu.2019.02702.
- Sun H, Bi L, Zhou J, Zhou D, Liu Y, Jin G, Yan W. 2015. Modulation of the function of dendritic cells in adolescents with chronic HBV infection by IFN-λ1. Int J Clin Exp Pathol. 8:1743–1751.
- Sun HH, Zhou DF, Zhou JY. 2016. The role of DCs in the immunopathogenesis of chronic HBV infection and the methods of inducing DCs maturation. J Med Virol. 88(1):13–20. doi:10.1002/jmv.24306.
- Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. 2019. SLC19A1 Is an Importer of the Immunotransmitter cGAMP. Mol Cell. 75(2):372–381. e375. doi:10.1016/j.molcel.2019.05.006.
- Zhou C, Chen X, Planells-Cases R, Chu J, Wang L, Cao L, Li Z, López-Cayuqueo KI, Xie Y, Ye S, et al. 2020. Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels Augments STING-Mediated Interferon Responses and Anti-viral Immunity. Immunity. 52(5):767–781. e766. doi:10.1016/j.immuni.2020.03.016.
- Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, Piskol R, Ridgway J, McNamara E, Huang H, et al. 2020. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity. 52(2):357–373. e359. doi:10.1016/j.immuni.2020.01.014.
- Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis E Sousa C. 2018. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 172(5):1022–1037. e1014 doi:10.1016/j.cell.2018.01.004.
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. 2018. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 24(8):1178–1191. doi:10.1038/s41591-018-0085-8.
- Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, Moretta A. 2005. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 106(2):566–571. doi:10.1182/blood-2004-10-4035.
- Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 5(12):1260–1265. doi:10.1038/ni1138.
- Dai J, Megjugorac NJ, Gallagher GE, Yu RY, Gallagher G. 2009. IFN-lambda1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood. 113(23):5829–5838. doi:10.1182/blood-2008-09-179507.
- Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, Gallagher G. 2007. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 8(3):254–261. doi:10.1038/sj.gene.6364382.
- Egli A, Santer DM, O’Shea D, Tyrrell DL, Houghton M. 2014. The impact of the interferon-lambda family on the innate and adaptive immune response to viral infections. Emerging Microbes & Infections. 3(1):e51. doi:10.1038/emi.2014.51.
- Lohoff M, Mak TW. 2005. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat Rev Immunol. 5(2):125–135. doi:10.1038/nri1552.
- Marrack P, Kappler J, Type MT. 1999. I interferons keep activated T cells alive. J Exp Med. 189(3):521–530. doi:10.1084/jem.189.3.521.
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. 2005. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. Journal of Immunology (Baltimore, Md: 1950). 174(8):4465–4469. doi:10.4049/jimmunol.174.8.4465.
- Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. 2006. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. Journal of Immunology (Baltimore, Md: 1950). 176(8):4682–4689. doi:10.4049/jimmunol.176.8.4682.
- Zimmerman M, Yang D, Hu X, Liu F, Singh N, Browning D, Ganapathy V, Chandler P, Choubey D, Abrams SI, et al. 2010. IFN-γ upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PloS One. 5(11):e14076. doi:10.1371/journal.pone.0014076.
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. 2009. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69(7):3077–3085. doi:10.1158/0008-5472.Can-08-2281.
- Padovan E, Spagnoli GC, Ferrantini M, Heberer M. 2002. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 71:669–676.
- Mauldin IS, Wang E, Deacon DH, Olson WC, Bao Y, Slingluff CL Jr. 2015. TLR2/6 agonists and interferon-gamma induce human melanoma cells to produce CXCL10. International Journal of Cancer. 137(6):1386–1396. doi:10.1002/ijc.29515.
- Kawka E, Witowski J, Fouqet N, Tayama H, Bender TO, Catar R, Dragun D, Jörres A. 2014. Regulation of chemokine CCL5 synthesis in human peritoneal fibroblasts: a key role of IFN-γ. Mediators Inflamm. 2014:590654. doi:10.1155/2014/590654.
- Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, et al. 2015. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 112(50):15408–15413. doi:10.1073/pnas.1512832112.
- Nakano M, Fujii T, Hashimoto M, Yukawa N, Yoshifuji H, Ohmura K, Nakaizumi A, Mimori T. 2012. Type I interferon induces CX3CL1 (fractalkine) and CCL5 (RANTES) production in human pulmonary vascular endothelial cells. Clin Exp Immunol. 170(1):94–100. doi:10.1111/j.1365-2249.2012.04638.x.
- Kawai T, Seki M, Hiromatsu K, Eastcott JW, Watts GF, Sugai M, Smith DJ, Porcelli SA, Taubman MA. 1999. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. Journal of Immunology (Baltimore, Md: 1950). 163:3269–3278.
- Yıldırım C, Nieuwenhuis S, Teunissen PF, Horrevoets AJ, Van Royen N. 2015. van der Pouw Kraan TC. Interferon-Beta, a Decisive Factor in Angiogenesis and Arteriogenesis. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 35(6):411–420. doi:10.1089/jir.2014.0184.
- Houghton AN, Guevara-Patiño JA. 2004. Immune recognition of self in immunity against cancer. J Clin Invest. 114(4):468–471. doi:10.1172/jci22685.
- Greiner JW, Hand PH, Noguchi P, Fisher PB, Pestka S, Schlom J. 1984. Enhanced expression of surface tumor-associated antigens on human breast and colon tumor cells after recombinant human leukocyte alpha-interferon treatment. Cancer Res. 44:3208–3214.
- Yadav D, Ngolab J, Lim RS, Krishnamurthy S, Bui JD. 2009. Cutting edge: down-regulation of MHC class I-related chain A on tumor cells by IFN-gamma-induced microRNA. Journal of Immunology (Baltimore, Md: 1950). 182(1):39–43. doi:10.4049/jimmunol.182.1.39.
- Propper DJ, Chao D, Braybrooke JP, Bahl P, Thavasu P, Balkwill F, Turley H, Dobbs N, Gatter K, Talbot DC, et al. 2003. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 9(1):84–92.
- Park DS, Park JH, Lee JL, Lee JM, Kim YH, Lee JM, Kim SJ. 1997. Induction of ICAM-1, HLA-DR molecules by IFN-gamma and oncogene expression in human bladder cancer cell lines. Urol Int. 59(2):72–80. doi:10.1159/000283027.
- Jenne DE, Tschopp J. 1988. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 103(1):53–71. doi:10.1111/j.1600-065x.1988.tb00749.x.
- Salek-Ardakani S, Croft M. 2010. Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 30(4):205–218. doi:10.1089/jir.2010.0026.
- Watts TH. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 23(1):23–68. doi:10.1146/annurev.immunol.23.021704.115839.
- Watanabe N, Narita M, Yokoyama A, Sekiguchi A, Saito A, Tochiki N, Furukawa T, Toba K, Aizawa Y, Takahashi TM. 2006. Type I IFN-mediated enhancement of anti-leukemic cytotoxicity of γδ T cells expanded from peripheral blood cells by stimulation with zoledronate. Cytotherapy. 8(2):118–129. doi:10.1080/14653240600620200.
- Lin CF, Lin CM, Lee KY, Wu SY, Feng PH, Chen KY, Chuang HC, Chen CL, Wang YC, Tseng PC, et al. 2017. Escape from IFN-γ-dependent immunosurveillance in tumorigenesis. J Biomed Sci. 24(1):10. doi:10.1186/s12929-017-0317-0.
- Kearney CJ, Vervoort SJ, Hogg SJ, Ramsbottom KM, Freeman AJ, Lalaoui N, Pijpers L, Michie J, Brown KK, Knight DA, et al. 2018. Tumor immune evasion arises through loss of TNF sensitivity. Science Immunology. 3(23):23. doi:10.1126/sciimmunol.aar3451.
- Prévost-Blondel A, Neuenhahn M, Rawiel M, Pircher H. 2000. Differential requirement of perforin and IFN-gamma in CD8 T cell-mediated immune responses against B16.F10 melanoma cells expressing a viral antigen. Eur J Immunol. 30(9):2507–2515. doi:10.1002/1521-4141(200009)30:9<2507::aid-immu2507>3.0.Co;2-v.
- Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. 2010. IL- 28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Molecular Therapy: The Journal of the American Society of Gene Therapy. 18(9):1714–1723. doi:10.1038/mt.2010.118.
- Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. 2007. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 178(12):7540–7549. doi:10.4049/jimmunol.178.12.7540.
- Kelly JM, Takeda K, Darcy PK, Yagita H, Smyth MJ. 2002. A role for IFN-gamma in primary and secondary immunity generated by NK cell-sensitive tumor-expressing CD80 in vivo. J Immunol. 168(9):4472–4479. doi:10.4049/jimmunol.168.9.4472.
- Villanueva AI, Haeryfar SM, Mallard BA, Kulkarni RR, Sharif S. 2015. Functions of invariant NK T cells are modulated by TLR ligands and IFN-α. Innate Immun. 21(3):275–288. doi:10.1177/1753425914527327.
- Liu Y, Ma J, Yang Y, Liu L, Zhu G, Wang X, Wang S, Guo W, Yue Q, Zhao T, et al. 2020. Impact of Interferon-alpha1b (IFN-α1b) on Antitumor Immune Response: an Interpretation of the Promising Therapeutic Effect of IFN-alpha1b on Melanoma. Med Sci Monit. 26:e922790. doi:10.12659/msm.922790.
- Iannello A, Ahmad A. 2005. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 24(4):487–499. doi:10.1007/s10555-005-6192-2.
- Eisenthal A, Cameron RB, Rosenberg SA. 1990. Induction of antibody-dependent cellular cytotoxicity in vivo by IFN-alpha and its antitumor efficacy against established B16 melanoma liver metastases when combined with specific anti-B16 monoclonal antibody. Journal of Immunology. 144(11):4463–4471. Baltimore, Md: 1950
- Wang W, Nishioka Y, Ozaki S, Jalili A, Verma VK, Hanibuchi M, Abe S, Minakuchi K, Matsumoto T, Sone S. 2009. Chimeric and humanized anti-HM1.24 antibodies mediate antibody-dependent cellular cytotoxicity against lung cancer cells. Lung Cancer (Amsterdam, Netherlands). 63(1):23–31. doi:10.1016/j.lungcan.2008.04.009.
- Virelizier JL, Arenzana-Seisdedos F. 1985. Immunological functions of macrophages and their regulation by interferons. Med Biol. 63:149–159.
- Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. 2010. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 207(7):1485–1500. doi:10.1084/jem.20092695.
- Snapper CM, Peschel C, Paul WE. 1988. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. Journal of Immunology. 140(7):2121–2127. Baltimore, Md: 1950
- Oudinet C, Braikia FZ, Dauba A, Khamlichi AA. 2020. Mechanism and regulation of class switch recombination by IgH transcriptional control elements. Adv Immunol. 147:89–137. doi:10.1016/bs.ai.2020.06.003.
- Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol. 14(1):36–49. doi:10.1038/nri3581.
- Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, et al. 2001. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 98(26):15137–15142. doi:10.1073/pnas.261570598.
- Xu W, Zhang JJ. 2005. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. Journal of Immunology Baltimore, Md: 1950. 175(11):7419–7424. doi:10.4049/jimmunol.175.11.7419.
- Nguyen HV, Mouly E, Chemin K, Luinaud R, Despres R, Fermand JP, Arnulf B, Bories JC. 2012. The Ets-1 transcription factor is required for Stat1-mediated T-bet expression and IgG2a class switching in mouse B cells. Blood. 119(18):4174–4181. doi:10.1182/blood-2011-09-378182.
- Peng SL, Szabo SJ, Glimcher LH. 2002.T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 99(8):5545–5550. doi:10.1073/pnas.082114899.
- Pearse RN, Feinman R, Shuai K, Darnell JE Jr., Ravetch JV. 1993.Interferon gamma-induced transcription of the high-affinity Fc receptor for IgG requires assembly of a complex that includes the 91-kDa subunit of transcription factor ISGF3. Proc Natl Acad Sci U S A. 90(9):4314–4318. doi:10.1073/pnas.90.9.4314.
- Paquette RL, Minosa MR, Verma MC, Nimer SD, Koeffler HP. 1995. An interferon-gamma activation sequence mediates the transcriptional regulation of the IgG Fc receptor type IC gene by interferon-gamma. Mol Immunol. 32(12):841–851. doi:10.1016/0161-5890(95)00056-k.
- Imai D, Yoshizumi T, Okano S, Itoh S, Ikegami T, Harada N, Aishima S, Oda Y, Maehara Y. 2019. IFN- γ Promotes Epithelial-Mesenchymal Transition and the Expression of PD-L1 in Pancreatic Cancer. J Surg Res. 240:115–123. doi:10.1016/j.jss.2019.02.038.
- Deng R, Zhang P, Liu W, Zeng X, Ma X, Shi L, Wang T, Yin Y, Chang W, Zhang P, et al. 2018. HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer. Clin Epigenetics. 10(1):153. doi:10.1186/s13148-018-0589-6.
- Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning W, Zeng H, Zhang N, Du W, Chen C, et al. 2017. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol. 22(6):1026–1033. doi:10.1007/s10147-017-1161-7.
- Thiem A, Hesbacher S, Kneitz H, Di Primio T, Heppt MV, Hermanns HM, Goebeler M, Meierjohann S, Houben R, Schrama D. 2019. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. Journal of Experimental & Clinical Cancer Research: CR. 38(1):397. doi:10.1186/s13046-019-1403-9.
- Mo X, Zhang H, Preston S, Martin K, Zhou B, Vadalia N, Gamero AM, Soboloff J, Tempera I, Zaidi MR. 2018. Interferon-γ Signaling in Melanocytes and Melanoma Cells Regulates Expression of CTLA-4. Cancer Res. 78(2):436–450. doi:10.1158/0008-5472.Can-17-1615.
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. 2013. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 5(200):200ra. doi:10.1126/scitranslmed.3006504.
- Huang YM, Adikari S, Båve U, Sanna A, Alm G. 2005. Multiple sclerosis: interferon-beta induces CD123(+)BDCA2- dendritic cells that produce IL-6 and IL-10 and have no enhanced type I interferon production. J Neuroimmunol. 158(1–2):204–212. doi:10.1016/j.jneuroim.2004.08.014.
- Liu BS, Janssen HL, Boonstra A. 2012. Type I and III interferons enhance IL-10R expression on human monocytes and macrophages, resulting in IL-10-mediated suppression of TLR-induced IL-12. Eur J Immunol. 42(9):2431–2440. doi:10.1002/eji.201142360.
- Mojic M, Takeda K, Hayakawa Y. 2017. The Dark Side of IFN-γ: its Role in Promoting Cancer Immunoevasion. Int J Mol Sci. 19(1):1. doi:10.3390/ijms19010089.
- Mennechet FJ, Uzé G. 2006.Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 107(11):4417–4423. doi:10.1182/blood-2005-10-4129.
- Singh S, Kumar S, Srivastava RK, Nandi A, Thacker G, Murali H, Kim S, Baldeon M, Tobias J, Blanco MA, et al. 2020. Loss of ELF5-FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-γ signalling. Nat Cell Biol. 22(5):591–602. doi:10.1038/s41556-020-0495-y.
- Raison CL, Demetrashvili M, Capuron L, Miller AH. 2005. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 19(2):105–123. doi:10.2165/00023210-200519020-00002.
- Loftis JM, Hauser P. 2004. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 82(2):175–190. doi:10.1016/j.jad.2004.04.002.
- Ozturk M, Basoglu F, Yilmaz M, Ozagari AA, Interferon BS. 2016. β associated nephropathy in a Multiple Sclerosis patient: a case and review. Mult Scler Relat Disord. 9:50–53. doi:10.1016/j.msard.2016.06.012.
- Kovacs D, Kovacs P, Eszlari N, Gonda X, Juhasz G. 2016. Psychological side effects of immune therapies: symptoms and pathomechanism. Curr Opin Pharmacol. 29:97–103. doi:10.1016/j.coph.2016.06.008.
- Van Gool AR, Kruit WH, Engels FK, Stoter G, Bannink M, Eggermont AM. 2003. Neuropsychiatric side effects of interferon-alfa therapy. Pharmacy World & Science: PWS. 25(1):11–20. doi:10.1023/a:1022449613907.
- Patten SB. 2006. Psychiatric side effects of interferon treatment. Curr Drug Saf. 1(2):143–150. doi:10.2174/157488606776930562.
- Hobeika AC, Subramaniam PS, Johnson HM. 1997.IFNalpha induces the expression of the cyclin-dependent kinase inhibitor p21 in human prostate cancer cells. Oncogene. 14(10):1165–1170. doi:10.1038/sj.onc.1200939.
- Sangfelt O, Erickson S, Castro J, Heiden T, Gustafsson A, Einhorn S, Grandér D. 1999. Molecular mechanisms underlying interferon-alpha-induced G0/G1 arrest: CKI-mediated regulation of G1 Cdk-complexes and activation of pocket proteins. Oncogene. 18(18):2798–2810. doi:10.1038/sj.onc.1202609.
- Tiefenbrun N, Melamed D, Levy N, Resnitzky D, Hoffman I, Reed SI, Kimchi A. 1996. Alpha interferon suppresses the cyclin D3 and cdc25A genes, leading to a reversible G0-like arrest. Mol Cell Biol. 16(7):3934–3944. doi:10.1128/mcb.16.7.3934.
- Zhang K, Kumar R. 1994. Interferon-alpha inhibits cyclin E- and cyclin D1-dependent CDK-2 kinase activity associated with RB protein and E2F in Daudi cells. Biochem Biophys Res Commun. 200(1):522–528. doi:10.1006/bbrc.1994.1479.
- Lu M, Zhang W, Li Y, Berenzon D, Wang X, Wang J, Mascarenhas J, Xu M, Hoffman R. 2010. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp Hematol. 38(6):472–480. doi:10.1016/j.exphem.2010.03.005.
- Hobeika AC, Etienne W, Cruz PE, Subramaniam PS, Johnson HM. 1998. IFNgamma induction of p21WAF1 in prostate cancer cells: role in cell cycle, alteration of phenotype and invasive potential. International Journal of Cancer. 77(1):138–145. doi:10.1002/(sici)1097-0215(19980703)77:1<38::aid-ijc21>3.0.co;2-9.
- Li Q, Kawamura K, Ma G, Iwata F, Numasaki M, Suzuki N, Shimada H, Tagawa M. 2010. Interferon-lambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anti-cancer agents. European Journal of Cancer (Oxford, England: 1990). 46(1):180–190. doi:10.1016/j.ejca.2009.10.002.
- Zhao X, Ren G, Liang L, Ai PZ, Zheng B, Tischfield JA, Shi Y, Shao C. 2010. Brief report: interferon-gamma induces expansion of Lin(-)Sca-1(+)C-Kit(+) Cells. Stem Cells (Dayton, Ohio). 28(1):122–126. doi:10.1002/stem.252.
- Schürch CM, Riether C, Ochsenbein AF. 2014. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 14(4):460–472. doi:10.1016/j.stem.2014.01.002.
- Yamamoto-Yamaguchi Y, Tomida M, Hozumi M. 1989. Contrasting effect of IFN-gamma and IFN-alpha/beta on differentiation of some clones of mouse myeloid leukemic cells. Leuk Res. 13(3):253–257. doi:10.1016/0145-2126(89)90020-9.
- Lokshin A, Mayotte JE, Levitt ML. 1995. Mechanism of interferon beta-induced squamous differentiation and programmed cell death in human non-small-cell lung cancer cell lines. J Natl Cancer Inst. 87(3):206–212. doi:10.1093/jnci/87.3.206.
- Yokoyama M, Nakao Y, Iwasaka T, Pater A, Sugimori H. 2001. Retinoic acid and interferon-alpha effects on cell growth and differentiation in cervical carcinoma cell lines. Obstet Gynecol. 98(2):332–340. doi:10.1016/s0029-7844(01)01449-1.
- Shao X, Liu C. 2006.Influence of IFN- alpha and IFN- gamma on lymphangiogenesis. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 26(8):568–574. doi:10.1089/jir.2006.26.568.
- McCarty MF, Bielenberg D, Donawho C, Bucana CD, Fidler IJ. 2002. Evidence for the causal role of endogenous interferon-alpha/beta in the regulation of angiogenesis, tumorigenicity, and metastasis of cutaneous neoplasms. Clin Exp Metastasis. 19(7):609–615. doi:10.1023/a:1020923326441.
- Kommineni VK, Nagineni CN, William A, Detrick B, Hooks JJ. 2008. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem Biophys Res Commun. 374(3):479–484. doi:10.1016/j.bbrc.2008.07.042.
- Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ. 1998. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 58:808–814.
- Ikewaki N. 1988. Production of human B cell hybridomas with interleukin 2 receptors selected by a new selective medium, HAT-neo. Kitasato Arch Exp Med. 61:225–235.
- Kobelt D, Zhang C, Clayton-Lucey IA, Glauben R, Voss C, Siegmund B, Stein U. 2020. Pro-inflammatory TNF-α and IFN-γ Promote Tumor Growth and Metastasis via Induction of MACC1. Front Immunol. 11:980. doi:10.3389/fimmu.2020.00980.
- Ma Z, Qin H, Benveniste EN. 2001. Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-gamma and IFN-beta: critical role of STAT-1alpha. Journal of Immunology (Baltimore, Md: 1950). 167(9):5150–5159. doi:10.4049/jimmunol.167.9.5150.
- Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY). 331(6024):1565–1570. doi:10.1126/science.1203486.
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 3(11):991–998. doi:10.1038/ni1102-991.
- Barata JT, Durum SK, Seddon B. 2019. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. 20(12):1584–1593. doi:10.1038/s41590-019-0479-x.
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, et al. 2019. Tissue-resident memory CD8(+) T cells promote melanoma-immune equilibrium in skin. Nature. 565(7739):366–371. doi:10.1038/s41586-018-0812-9.
- Romero-Weaver AL, Wang HW, Steen HC, Scarzello AJ, Hall VL, Sheikh F, Donnelly RP, Gamero AM. 2010. Resistance to IFN-alpha-induced apoptosis is linked to a loss of STAT2. Molecular Cancer Research: MCR. 8(1):80–92. doi:10.1158/1541-7786.Mcr-08-0344.
- Cao X. 2008. Immunology in China: the past, present and future. Nat Immunol. 9(4):339–342. doi:10.1038/ni0408-339.
- Jose SS, Bendickova K, Kepak T, Krenova Z, Fric J. 2017. Chronic Inflammation in Immune Aging: role of Pattern Recognition Receptor Crosstalk with the Telomere Complex?. Front Immunol. 8:1078. doi:10.3389/fimmu.2017.01078.