ABSTRACT
The effect of anti-programmed cell death 1 (PD-1) antibody in Epstein-Barr virus-associated gastric cancer (EBVaGC) was debatable, and no predictive biomarkers for efficacy have been reported. Public reports on anti-PD-1 antibody monotherapy-treated EBVaGC with available programmed death ligand-1 (PD-L1) expression status were summarized and analyzed. Relevance with clinicopathologic characteristics of PD-L1 expression by immunohistochemistry was analyzed in 159 patients diagnosed with EBVaGC. Relevance with genomic transcriptome and mutation profile of PD-L1 status in EBVaGC was assessed with three datasets, the cancer genome atlas (TCGA), Gene Expression Omnibus (GEO) GSE51575, and GSE62254. Based on the data from 8 reports, patients with positive PD-L1 expression (n = 30) had significantly superior objective response rate (ORR) than patients with negative PD-L1 expression (n = 9) (63.3% vs. 0%, P = .001) in EBVaGC receiving anti-PD-1 antibody monotherapy. PD-L1 positivity was associated with less aggressive clinicopathological characteristics and was an independent predictor for a longer disease-free survival (hazard ratio [HR] and 95% CI: 0.45 [0.22–0.92], P = .03) and overall survival (HR and 95% CI: 0.17 [0.06–0.43], P < .001). Analysis of public EBVaGC transcriptome and mutation datasets revealed enhanced immune-related signal pathways in PD-L1high EBVaGC and distinct mutation patterns in PD-L1low EBVaGC. PD-L1 positivity indicates a subtype of EBVaGC with ‘hot’ immune microenvironment, lower aggressiveness, better prognosis, and higher sensitivity to anti-PD-1 immunotherapy.
Introduction
Gastric cancer (GC) is one of the most aggressive malignancies worldwide, ranking the third on the list of the commonest causes of cancer-related death.Citation1 For decades, chemotherapy has served as the backbone of the treatment for advanced GC.Citation2 In the era of targeted therapy, only a few drugs succeeded in the challenge to conventional treatment.Citation3 However, the choices remain limited and the median overall survival (OS) of advanced GC patients is only approximately 1 year.Citation3 Programmed cell death 1 (PD-1) inhibitors have been approved in refractory GC recently, which benefits only a small subset of patients.Citation4–8 Identifying this subset of patients is one of the most focused issues in immunotherapy of GC.
The Cancer Genome Atlas (TCGA) molecular classification of GC indicated Epstein-Barr virus (EBV)-associated GC (EBVaGC) as a special subtype, which presents with abundant programmed death-ligand 1/programmed death-ligand 2 (PD-L1/PD-L2) expression.Citation9 Since then, EBVaGC has attracted attention and has been intensively investigated as a subset potentially benefiting from immunotherapy. EBV infection induces immune responses, recruits immune cells and modulates immune-related molecular components.Citation10,Citation11 Compared with EBV-negative GC (EBVnGC), EBVaGC shows a high proportion of lymphoepithelioma-like GC,Citation12 and has better patient survival.Citation13 EBVaGC harbors deregulation of immune response genes,Citation14 strong evidence of immune infiltration and high-level activation of immune checkpoint pathways.Citation15 Although theoretically EBVaGC is suitable for immunotherapy, relevant clinical trials of PD-1 inhibitors in EBVaGC are limited and the efficacy was equivocal.Citation5,Citation16 S.T. kim et al. presented a 100% objective response rate (ORR) in 6 EBVaGC patients in a phase II clinical trial.Citation16 However, according to our phase II clinical trial reported previously, only 1 in 4 (25%) EBVaGC patients achieved PR.Citation5 Notably, All six patients reported by S.T. kim et al. and the one patient with PR in our clinical trial had positive PD-L1 expression in tumor tissues, while the remaining 3 patients who didn’t achieve PR in our clinical trial had negative PD-L1 expression.Citation5,Citation16 This finding triggers an interest to investigate the potential impact of PD-L1 expression on efficacy in EBVaGC patients receiving immunotherapy, which has never been reported.
PD-L1 is a hallmark of inhibition of adaptive immune response.Citation17 It has been widely proved to be a predictive biomarker for immunotherapy in various malignancies.Citation18 Compared with EBVnGC, PD-L1 is highly expressed in EBVaGC.Citation9,Citation19 About 50% of EBVaGC were PD-L1 positive in tumor tissues by immunohistochemistry.Citation19,Citation20 However, its association with clinicolpathological characteristic and prognosis were inconsistency by limited previous studies with small sample numbers.Citation20–22
Here, literature were reviewed to investigate the impact of PD-L1 expression on efficacy of immunotherapy in EBVaGC. Data of responses to immunotherapy according to PD-L1 expression in EBVaGC was collected and summarized. Moreover, the clinicolpathological and prognostic association of PD-L1 expression in EBVaGC was analyzed in a large consecutive EBVaGC cohort from Sun Yat-sen University Cancer Center. Furthermore, publicly available tumor sequencing data was used to compare molecular characteristics between PD-L1low and PD-L1high EBVaGC.
Materials and methods
Ethics statement
For patients involved in PD-L1 immunohistochemistry analysis in the present study, all of them provided written informed consent for their information and archived tumor tissues to be stored and used for scientific research in hospital database of Sun Yat-sen University Cancer Center. This study was approved by the independent ethics committee of the cancer center.
Literature search and data collection
We conducted a literature search for reports of the efficacy of PD-1/PD-L1 inhibitors monotherapy in patients with EBVaGC in the Pubmed database. Studies which contained original information of clinical results of PD-1/PD-L1 inhibitor treatment in EBVaGC were screened, including original researches and case reports. While those without enough information on PD-L1 expression by immunohistochemistry and tumor response data for analyses were excluded.
Patients
For PD-L1 immunohistochemistry analysis in the present study, a total of 4,436 consecutive cases diagnosed with GC from January 2015 to December 2019 were screened, only 190 (4.3%) cases were EBV-encoded RNAs (EBERs) positive by routine fluorescence in situ hybridization (FISH) detection. Excluding those without available archived tumor tissues in our cancer center or complete clinical and survival data, 159 EBVaGC cases were finally included for PD-L1 immunohistochemistry analysis.
PD-L1 immunohistochemistry and scoring
The protein levels of PD-L1 were assessed in formalin-fixed, paraffin-embedded primary GC tissues. Immunohistochemistry staining of tissue sections was performed as described previously.Citation23 An antihuman PD-L1 monoclonal antibody (E1L3N, Cell Signaling Technology) was used per the manufacturer’s instructions. PD-L1 expression was evaluated using the scoring method of the combined positive score (CPS), which is the number of positive cells (tumor cells, lymphocytes, and macrophages)/the total number of viable tumor cells, and multiplied by 100. Positive PD-L1 expression was defined as a CPS ≥ 1, and negative PD-L1 expression was defined as a CPS <1. The clinicopathological features was compared between patients with positive and negative PD-L1 expression.
Public sequencing database
Datasets of TCGA mRNA expression matrix and mutation profiling were downloaded from the UCSC Xena browser (https://xenabrowser.net/datapages/). Datasets of GSE51575 and GSE62254 mRNA expression profiling were downloaded from the Gene Expression Omnibus (GEO, www.ncbi.nlm.nih.gov/geo/, GSE51575, GSE62254) database. There were respectively 23 (TCGA), 12 (GSE51575), and 24 (GSE62254) EBVaGC specimens. Gene mutation and expression profiles were compared between PD-L1low and PD-L1high subgroup with cutoff values of PD-L1 mRNA expression level set at the median.
Statistics
Statistical analysis were performed using SPSS for Windows V.13.0 (SPSS Inc., Chicago, IL, USA) and R version 3.4.0. The associations of PD-L1 level by immunohistochemistry staining with clinicopathological characteristics were evaluated using the chi-square test. The prognostic relevance of PD-L1 level was assessed using univariate and multivariate COX regression analysis. Survival curves were plotted by Kaplan-Meier method and log-rank test. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) biological process enrichment analysis was calculated by using the R package “clusterProfile” based on gene expression matrix. Mutation landscape and comparison between PD-L1high and PD-L1low tumors were performed using the package “maftools”. All statistical hypothesis performed in this paper were two-tailed, and a P value≤0.05 was considered significant.
Results
Efficacy of PD-1/PD-L1 inhibitors in EBVaGC according to PD-L1 expression
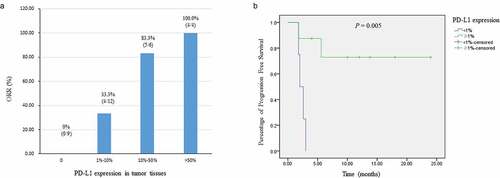
Altogether, eight reports from 2018 to 2020 (3 prospective studies, 4 retrospective studies, and 1 case report) were qualified for use in investigating the impact of PD-L1 expression on the efficacy of PD-1 antibody immunotherapy in EBVaGC (Supplementary Table 1S). These studies contributed to a total of 39 EBVaGC patients treated with PD-1/PD-L1 inhibitors monotherapy and with available data on PD-L1 expression status in tumor tissues and tumor response (). All of the 8 studies assessed PD-L1 expression combining staining on both tumor cells and immune cells by immunohistochemistry. From the studies, nine patients were PD-L1 negative (PD-L1 < 1%), and none of them achieved PR (ORR 0%). Whereas the ORR was 63.3% in 30 patients with positive PD-L1 expression (PD-L1≧1%), and the difference was significant (P = .001). In patients with PD-L1 ≧10% and PD-L1 ≧50%, the ORRs were, respectively, 83.3% and 100.0%. Furthermore, the ORRs increased with the rising level of PD-L1 expression (, ). Among the 39 patients, there were 12 patients with available survival information, including progression-free survival (PFS) and OS (Supplementary Table 2S). Compared with patients with negative PD-L1 expression (n = 4, median and 95% confidence interval [CI]: 2.0 [1.2–2.8 m], mean: 2.4 m), those with positive PD-L1 expression (n = 8, median and 95% CI not reached, mean: 18.5 m) had significantly superior PFS (P = .005, ). While OS data was not sufficiently mature due to several patients were early-censored.
Table 1. The effective rate of PD-1/PD-L1 inhibitors stratified by PD-L1 expression in EBVaGC
Association of PD-L1 expression with clinicopathological characteristics in EBVaGC
Altogether, 159 consecutive patients with EBVaGC were included, who were diagnosed as positive in EBERs staining by FISH (supplementary Figure 1S). The baseline characteristics were shown in Supplementary Table 3. The median age was 57 years. Most patients were males (n = 141, 88.7%), with tumors located in proximal stomach (n = 75, 47.2%), poorly differentiated (n = 90, 56.6%) and diagnosed at stage III (n = 78, 49.1%). Median size of primary tumor was 4.5 cm. Positive PD-L1 expression was detected in 87 (54.7%) patients. Representative slides of HE staining and PD-L1 immunohistochemistry staining are shown in Supplementary Figure 2S.
The proportion of positive PD-L1 expression was higher in female patients compared with male patients (77.8% vs. 51.8%, P = .04). Patients with positive PD-L1 expression had significantly smaller size of tumor (P = .05), less advanced N category (P = .001), less frequent distant metastasis (P = .01), less advanced TNM classification (P = .004), less frequent nervous invasion (P = .01), less frequent venous invasion (P = .02), and lower HER2 immunohistochemistry score (P = .01). The proportion of positive PD-L1 expression was higher in patients with stage T1+ T2 tumors than in those with stage T3+ T4 tumors, although the difference was not significant (65.1% vs. 51.9%, P = .14). While PD-L1 status was not associated with age, tumor location, tumor differentiation and Lauren classification ().
Table 2. The clinicopathological relevance of PD-L1 expression in EBVaGC
Interestingly, PD-L1 expression was significantly associated with lymphoepithelioma-like GC in patients with EBVaGC (). In lymhoepithelioma-like GC, 69.2% of patients were PD-L1 positive, while only 32.1% of patients were PD-L1 positive in non-lymhoepithelioma-like GC. Thus that lymhoepithelioma-like GC may be an important subtype of EBVaGC which deserves further research in the area of immunotherapy. We further analyzed the clinicopathological and prognostic association of lymhoepithelioma-like GC in our 159 patients diagnosed with EBVaGC. Lymhoepithelioma-like GC was significantly correlated with smaller tumor size (P < .001), less advanced T category (P = .003), less advanced N category (P < .001), less frequent distant metastasis (P < .001), less advanced TNM classification (P < .001), less frequent nervous invasion (P = .01), and less frequent venous invasion (P = .001) (Supplementary Table 4S). A significantly superior disease-free survival (DFS) and OS were also found in lymhoepithelioma-like GC subtype compared with non-lymhoepithelioma-like GC subtype in EBVaGC patients (Supplementary Figure 3S).
Prognostic relevance of PD-L1 expression in EBVaGC
During a median follow-up time of 23 months, 32 patients experienced disease recurrence and/or metastasis among the 132 patients at stages I–III, and 36 patients died of GC among the total 159 patients. The univariate and multivariate analyses were conducted to assess the impact of PD-L1 expression and other factors on DFS and OS in patients with EBVaGC.
Patients with positive PD-L1 expression had an estimated mean DFS (95% CI) of 48.9 (44.9–52.9) m (median DFS and 95% CI: not reached), whereas patients with negative PD-L1 expression had an estimated mean DFS (95% CI) of 38.3 (31.5–42.0) m (median DFS and 95% CI: not reached). The difference of DFS between PD-L1 negative and positive EBVaGC was significant (P = .004, ). Other factors significantly associated with DFS included tumor size (P = .01), TMN classification (P = .001), nervous invasion (P = .02), and venous invasion (P < .001). After being adjusted by these confounding factors, positive PD-L1 expression remained to be independently associated with superior DFS (hazard ratio [HR] and 95% CI: 0.45 [0.22–0.92)], P = .03, ).
Table 3. Univariate and multivariate Cox proportional hazards analysis for progression-free survival and overall survival in EBVaGC
Figure 2. The impact of PD-L1 expression in tumor tissues on disease-free survival and overall survival in EBVaGC. Survival curves of disease-free survival stratified by PD-L1 expression (a); Survival curves of overall survival stratified by PD-L1 expression (b)
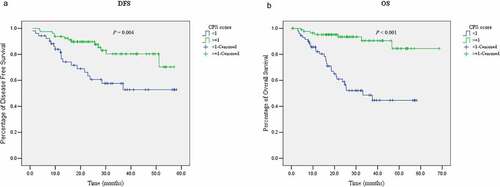
Similarly, patients with positive PD-L1 expression had an estimated mean OS (95% CI) of 62.5 (58.1–66.9) m (median OS and 95% CI: not reached), whereas patients with negative PD-L1 expression had an estimated mean OS (95% CI) of 35.7 (29.9–41.5) m (median OS and 95% CI: 33.2 [18.0–48.3] m). The survival differences were significant (P < .001, ). Other factors significantly associated with OS were the same with those for DFS, including tumor size (P < .001), TMN classification (P = .001), nervous invasion (P = .003), and venous invasion (P < .001). After adjusting for these confounding factors, PD-L1 expression remained as an independent prognostic factor for OS in EBVaGC (HR and 95% CI: 0.17 [0.06–0.43], P < .001, ).
Association of PD-L1 high expression with enhanced activity of immune signal pathways in EBVaGC
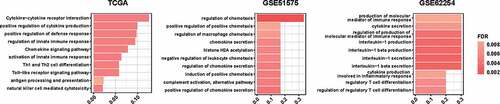
The gene expression profile was compared between PD-L1high and PD-L1low tumors according to the mRNA expression level of PD-L1 in EBVaGC using TCGA, GSE51575, and GSE62254 datasets. The cutoff value of PD-L1 mRNA expression was set at the median considering the around 50% PD-L1 positive rate by immunohistochemistry in EBVaGC by our present study and previous reports.Citation19,Citation20 Distinct profiles between PD-L1high and PD-L1low tumors were shown. Immune-related signal pathways were frequently up-regulated in PD-L1high tumors in all the three datasets (), such as pathways related with cytokine interaction and production, and regulation of chemotaxis. Whereas pathways up-regulated in PD-L1low tumors were involved in other signal pathways, such as metabolism and epithelial cell proliferation (Supplementary Figure 4S).
Difference of genetic mutation profile in PD-L1high and PD-L1low EBVaGC
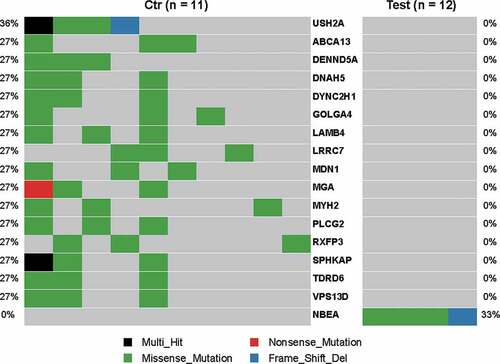
The gene mutation profile between PD-L1high and PD-L1low tumors in EBVaGC was compared using TCGA data. A dozen of genes were highly mutated in PD-L1low tumors (mutation rate 27%, mainly missense mutations) but not mutated in PD-L1high tumors (). Those included some of genes which were reported to be associated with malignancies, such as USH2A, DENND5A, DNAH5, DYNC2H1, and MYH2. While NBEA gene mutation was frequently found in PD-L1high tumors (mutation rate: 33%).
Discussion
EBVaGC is an important subtype of GC, which is supposed to be potentially sensitive to PD-1/PD-L1 inhibitors due to the relatively ‘hot’ immune microenvironment.Citation9,Citation14 However, according to clinical reports,Citation5,Citation15,Citation16,Citation24–29 the efficacy of PD-1 inhibitors in EBVaGC may not be universally satisfactory. The underlying biomarkers for immunotherapy in EBVaGC haven’t been reported. This study demonstrated PD-L1 expression to be a crucial biomarker in EBVaGC. According to our study, positive PD-L1 expression was associated with less aggressive clinical and pathological features, predicted superior prognosis, and better efficacy of immunotherapy in EBVaGC.
EBVaGC constitutes only less than 10% of GC, and an even smaller proportion in stage Ⅳ GC.Citation13,Citation30 Thus, it’s relatively difficult to recruit enough EBVaGC patients in clinical trials. To date, all clinical reports on immunotherapy in EBVaGC are of small sample numbers, none of which exceeded 10 EBVaGC patients, and the efficacy has been debatable, with ORRs ranging from 25% to 100%.Citation5,Citation15,Citation16,Citation24–29 Collectively, an ORR of 48.7% was concluded combining available clinical reports, as shown in our study. However, this result was evidently biased, as 30 out of 39 (76.9%) patients were PD-L1 positive, which was far beyond the rates of approximately 50% in unselected EBVaGC population by our immunohistochemistry staining result and literature reports.Citation19,Citation20 Furthermore, stage Ⅳ patients would have a lower PD-L1 positive rate as suggested by our present study. Considering PD-L1 positivity was significantly associated with a higher ORR in EBVaGC receiving anti-PD-1 immunotherapy found by our present study, the efficacy of anti-PD-1 immunotherapy in EBVaGC have been generally overestimated in literature reports. As a conclusion, the authentic efficacy of PD-1/PD-L1 inhibitors in EBVaGC are still not clear. Clinical trials recruiting consecutive EBVaGC patients are needed to reveal the answer.
By collecting and analyzing public clinical data, our present study is the first to provide an important perspective on biomarkers for anti-PD-1 immunotherapy in EBVaGC. This is the first study to indicate the potential role of PD-L1 expression in predicting benefit from immunotherapy in EBVaGC. The ORR was 0% in PD-L1 negative EBVaGC, while 63.3% in PD-L1 positive EBVaGC. The ORR was even higher in those with PD-L1 10–50% positive (ORR: 83.3%) and ≧50% positive (ORR: 100%). A significantly prolonged PFS was also observed in PD-L1 positive EBVaGC compared with PD-L1 negative EBVaGC. However, these results were based on retrospective analyses of various public clinical reports, the value of PD-L1 in immunotherapy in EBVaGC need to be verified in prospective clinical trials.
PD-1 is strictly regulated and strongly induced when T cells are activated,Citation31 when engaged with its ligand PD-L1, it mediates immunosuppression in tumor microenvironment.Citation32 Thus, PD-L1 is considered a marker of the existence of adaptive immune response. Its expression is positively associated with infiltration of immune cells in GC,Citation33 as well as in EBVaGC.Citation22 Consistently, we found PD-L1 positivity to be associated with benign tumor features, less advanced tumor stage, and better prognosis in EBVaGC. Our results are in line with reports by Min et al.Citation21 and Raghav et al.Citation22 It may be speculated that EBVaGC with higher PD-L1 expression is more likely to be immune-inflamed, thus is more sensitive to immunotherapy.
To further support our assumption, we conducted a gene expression profile analysis in EBVaGC to compare the differences between PD-L1high and PD-L1low tumors. It was found that immune-related pathways were activated in PD-L1high EBVaGC in three different datasets, suggesting that EBVaGC with higher PD-L1 expression was inclined to be immune-inflamed.
EBVaGC is well-known to be a subtype of GC characterized by the activation of immune-related genes,Citation9,Citation14 it’s important to understand why PD-L1low EBVaGC break the routine and induce resistance to immunotherapy. By comparing the mutational profile between PD-L1high and PD-L1low EBVaGC, it was found that PD-L1low tumors possessed more frequent tumor mutations in several genes. Among them, PLCG2 mutation was related with marked humoral immunodeficiency,Citation34 VPS13D genetic variant was reported to increase the production of IL-6,Citation35 an immunosuppressive cytokine.Citation36 Although some of the other involved genes have been reported to be associated with malignancies, such as USH2ACitation37 and DNAH5,Citation38 whether those genes have any role in modulating cancer immune microenvironment remains unclear. Our mutational profile analysis was based on a small TCGA patient sample of EBVaGC, intensive studies with large patient samples are warranted for better characterization of mutational profile between PD-L1high and PD-L1low EBVaGC. And such works may shed new light on overcoming resistance to immunotherapy in EBVaGC.
Lymhoepithelioma-like GC, also indicated as gastric carcinoma with lymphoid stroma, is a unique subtype of GC, and over 80% of them is infected with EBV. This subtype of GC commonly harbors intense lymphocyte infiltration.Citation39 EBV associated lymphoepithelial-like GC was considered a typical hot tumor.Citation40 Compared with conventional histological type, lymhoepithelioma-like GC was reported to have significantly prolonged DFS and OS in EBVaGC.Citation41 Our findings are consistent with previous reports; here we reported that lymhoepithelioma-like GC to be associated with less aggressive tumor features and better prognosis. Additionally, our study was the first to report an association of lymhoepithelioma-like GC with higher PD-L1 positive rate in EBVaGC. Although the correlation of lymhoepithelioma-like GC with efficacy of anti-PD-1 immunotherapy in EBVaGC has not been investigated, lymhoepithelioma-like EBVaGC also has the potential to be a highly sensitive subtype. Future clinical trials on immunotherapy in EBVaGC should include both PD-L1 expression and lymhoepithelioma-like subtype as stratification factors.
Several limitations need to be highlighted. First of all, the analyses for the impact of PD-L1 status on the efficacy of immunotherapy were based on public data, which might result in certain biases. However, due to the small proportion of EBVaGC among the general GC population, our analysis is a good supplementary to the scarce clinical trial data about immunotherapy in EBVaGC. Secondly, the number of samples for transcriptome and mutation analyses was relatively small, and further validation was warranted for solid conclusions to guide in-depth research. Nevertheless, combining public data and tumor samples in our center, our present study proved an important impact of PD-L1 expression on immunotherapy in EBVaGC with corresponding clinical and molecular basis.
In summary, our study revealed the association between PD-L1 expression and effect of the PD-1 antibody in EBVaGC. Positive PD-L1 expression indicated a subtype of EBVaGC with less aggressive clinicopathological features, and superior survival compared with negative PD-L1 expression. Genetically, a higher level of PD-L1 expression was associated with enhanced immune-related signal pathways and different mutation pattern compared with a lower level of PD-L1 expression. Our findings provide significant clues for future clinical and experimental researches on immunotherapy in EBVaGC.
Declaration of interests
The authors declare that they have no competing interests.
Contributors
WXL, YAL and LYH for study concept and design, WXL, LQW, LFR and LXF for analysis and interpretation of data, YSS and LJN for technical and material support, WXL, LQW, LFR, YAL and LYH for drafting of the manuscript. WXL, YAL and LYH for obtained funding and study supervision. All authors read and approved the final manuscript.
Data sharing statement
Data are available on reasonable request. All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Ling on reasonable request.
Supplemental Material
Download ()Acknowledgments
The study was supported by the following funding sources: National Key R&D Program of China (2018YFC1313300); Guangdong Basic and Applied Basic Research Foundation (2019A1515110171 and 2020A1515110034); National Natural Science Foundation of China (81801895 and 82002476).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–10. doi:10.3322/caac.21492.
- Cervantes A, Roda D, Tarazona N, Rosello S, Perez-Fidalgo JA. Current questions for the treatment of advanced gastric cancer. Cancer Treat Rev. 2013;39:60–67. doi:10.1016/j.ctrv.2012.09.007.
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3.
- Folprecht G. Tumor mutational burden as a new biomarker for PD-1 antibody treatment in gastric cancer. Cancer Commun (Lond). 2019;39:74. doi:10.1186/s40880-019-0417-1.
- Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479–1486. doi:10.1093/annonc/mdz197.
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chuang HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi:10.1016/S0140-6736(17)31827-5.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal S, Shah MA, Metges J, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013.
- Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi:10.1016/S1470-2045(16)00175-3.
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature.Cancer Genoma Atlas Research Network. 2014; 513: 202–209. doi:10.1038/nature13480.
- Ouaguia L, Mrizak D, Renaud S, Morales O, Delhem N. Control of the inflammatory response mechanisms mediated by natural and induced regulatory T-cells in HCV-, HTLV-1-, and EBV-associated cancers. Mediators Inflamm. 2014;2014:564296. doi:10.1155/2014/564296.
- Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67–76.
- Huang SC, Ng KF, Yeh TS, Cheng CT, Lin JS, Liu YJ, Chuang H-C, Chen T-C. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145:3218–3230. doi:10.1002/ijc.32215.
- Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJY, Herrera-Goepfert R, Meneses-Gonzalez F, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63(2):236–243. doi:10.1136/gutjnl-2013-304531.
- Kim SY, Park C, Kim HJ, Park J, Hwang J, Kim JI, Choi MG, Kim S, Kim K-M, Kang M-S, et al. Deregulation of immune response genes in patients with Epstein-Barr virus-associated gastric cancer and outcomes. Gastroenterology. 2015;148(1):137–147. doi:10.1053/j.gastro.2014.09.020.
- Panda A, Mehnert JM, Hirshfield KM, Riedlinger G, Damare S, Saunders T, Kane M, Sokol L, Stein MN, Poplin E, et al. Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst. 2018;110(3):316–320. doi:10.1093/jnci/djx213.
- Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi:10.1038/s41591-018-0101-z.
- Avendano-Ortiz J, Maroun-Eid C, Martin-Quiros A, Toledano V, Cubillos-Zapata C, Gomez-Campelo P, Varela-Serrano A, Casas-Martin J, Llanos-González E, Alvarez E, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1alpha. J Infect Dis. 2018;217(3):393–404. doi:10.1093/infdis/jix279.
- Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi:10.1136/bmj.k3529.
- Saito R, Abe H, Kunita A, Yamashita H, Seto Y, Fukayama M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017;30:427–439. doi:10.1038/modpathol.2016.202.
- Seo AN, Kang BW, Kwon OK, Park KB, Lee SS, Chung HY, Yu W, Bae HI, Jeon SW, Kang H, et al. Intratumoural PD-L1 expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Br J Cancer. 2017;117(12):1753–1760. doi:10.1038/bjc.2017.369.
- Dong M, Wang HY, Zhao XX, Chen JN, Zhang YW, Huang Y, Xue L, Li H-G, Du H, Wu X-Y, et al. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2016;53:25–34. doi:10.1016/j.humpath.2016.02.007.
- Sundar R, Qamra A, Tan A, Zhang S, Ng C, Teh BT, Lee J, Kim K-M, Tan P. Transcriptional analysis of immune genes in Epstein-Barr virus-associated gastric cancer and association with clinical outcomes. Gastric Cancer. 2018;21(6):1064–1070. doi:10.1007/s10120-018-0851-9.
- Wei XL, Luo X, Sheng H, Wang Y, Chen DL, Li JN, Wang F-H, Xu R-H. PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer. J Transl Med. 2020;18(1):475. doi:10.1186/s12967-020-02636-x.
- Qiu MZ, He CY, Yang DJ, Zhou DL, Zhao BW, Wang XJ, Yang L-Q, Lu S-X, Wang F-H, Xu R-H, et al. Observational cohort study of clinical outcome in Epstein-Barr virus associated gastric cancer patients. Ther Adv Med Oncol. 2020;12:431414822. doi:10.1177/1758835920937434.
- Xie T, Liu Y, Zhang Z, Zhang X, Gong J, Qi C, Li J, Shen L, Peng Z. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43(4):139–144. doi:10.1097/CJI.0000000000000316.
- Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7(1):24. doi:10.1186/s40425-019-0514-3.
- Kim J, Kim B, Kang SY, Heo YJ, Park SH, Kim ST, Kang WK, Lee J, Kim K-M. Tumor mutational burden determined by panel sequencing predicts survival after immunotherapy in patients with advanced gastric cancer. Front Oncol. 2020;10:314. doi:10.3389/fonc.2020.00314.
- Kubota Y, Kawazoe A, Sasaki A, Mishima S, Sawada K, Nakamura Y, Kotani D, Kuboki Y, Taniguchi H, Kojima T, et al. The impact of molecular subtype on efficacy of chemotherapy and checkpoint inhibition in advanced gastric cancer. Clin Cancer Res. 2020;26(14):3784–3790. doi:10.1158/1078-0432.CCR-20-0075.
- Kwon M, Hong JY, Kim ST, Kim KM, Lee J. Association of serine/threonine kinase 11 mutations and response to programmed cell death 1 inhibitors in metastatic gastric cancer. Pathol Res Pract. 2020;216:152947. doi:10.1016/j.prp.2020.152947.
- Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi:10.1053/j.gastro.2009.05.001.
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi:10.1093/intimm/8.5.765.
- Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6. doi:10.1126/sciadv.abd2712.
- Xing X, Guo J, Ding G, Li B, Dong B, Feng Q, Li S, Zhang J, Ying X, Cheng X, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7:e1356144. doi:10.1080/2162402X.2017.1356144.
- Martín-Nalda A, Fortuny C, Rey L, Bunney TD, Alsina L, Esteve-Solé A, Bull D, Anton MC, Basagana M, Casals F, et al. Severe autoinflammatory manifestations and antibody deficiency due to novel hypermorphic PLCG2 mutations. J Clin Immunol. 2020;40:987–1000. doi:10.1007/s10875-020-00794-7.
- Nakada TA, Boyd JH, Russell JA, Aguirre-Hernández R, Wilkinson MD, Thair SA, Nakada, E, McConechy MK, Fjell CD, Walley KR. VPS13D gene variant is associated with altered IL-6 production and mortality in septic shock. J Innate Immun. 2015;7:545–553. doi:10.1159/000381265.
- Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson 3rd WE, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4⁺ T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi:10.1007/s00262-011-1029-z.
- Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, Rubinstein JC, Choi M, Kiss N, Nelson-Williams C, et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24:2318–2329. doi:10.1093/hmg/ddu749.
- Xiao WH, Qu XL, Li XM, Sun YL, Zhao HX, Wang S, Zhou X. Identification of commonly dysregulated genes in colorectal cancer by integrating analysis of RNA-Seq data and qRT-PCR validation. Cancer Gene Ther. 2015;22:278–284. doi:10.1038/cgt.2015.20.
- Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, et al. Clinical importance of Epstein⁻Barr virus-associated gastric cancer. Cancers (Basel). 2018;10:167. doi:10.3390/cancers10060167.
- Fang WL, Chen MH, Huang KH, Lin CH, Chao Y, Lo SS, Li AFY, Wu C-W, Shyr Y-M. The clinicopathological features and genetic alterations in Epstein-Barr virus-associated gastric cancer patients after curative surgery. Cancers (Basel). 2020;12:1517. doi:10.3390/cancers12061517.
- Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, Chung HY, Yu W, Kang H, Kim JG. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol. 2016;27:494–501. doi:10.1093/annonc/mdv610.