ABSTRACT
The prognosis of hepatocellular carcinoma (HCC) is extremely poor, of which hepatitis B virus-related hepatocellular carcinoma (HBV-HCC) accounts for the majority in China. Immune checkpoint inhibitors have become an effective immunotherapy method for the treatment of HCC, but they are mainly used for T cells. NK cells play a vital role as the first line of defense against tumors. Therefore, we explored the characteristic expression pattern of immune checkpoints on NK cells of HBV-HCC patients. We analyzed the correlation between the co-expression of TIGIT and TIM-3 and the clinical progress of patients with HBV-HCC. The co-expression of TIGIT and TIM-3 on NK cells is elevated in patients with HBV-HCC. TIGIT+TIM-3+NK cells showed exhausted phenotypic characteristics and displayed dysfunction manifested as weakened killing function, reduced cytokine production, and proliferation function. TIGIT+TIM-3+NK cells participate in NK cells function exhaustion and are closely related to the disease progression of patients with HBV-HCC, suggesting a new target for future immunotherapy.
Background
Hepatocellular carcinoma (HCC) is a common malignant tumor. According to the latest global cancer statistics in 2020, the mortality rate of HCC ranks third among global cancer.Citation1 In China, more than 80% of patients with HCC are induced by chronic hepatitis B virus (HBV) infection.Citation2–4 Therefore, HBV infection is the most critical cause of HCC, and the high incidence and mortality rates of HBV related hepatocellular carcinoma (HBV-HCC) have always been the focus of research.
Liver is the dominant organ of natural immunity, which contains a large number of natural killer (NK) cells. NK cells mediate immune surveillance and immune clearance functions in cancer.Citation5,Citation6 In various malignant tumors such as lung cancer,Citation7 breast cancer,Citation8 head and neck cancer,Citation9 and liver cancer,Citation10 NK cells are immunosuppressed under the influence of the tumor microenvironment, which is an important cause of tumor progression and metastasis. Significantly elevated levels of inhibitory receptors mediate NK cell immunosuppression and exhaustion in liver cancer and other cancers,Citation11,Citation12 such as T-cell immunoglobulin and immunoreceptor tyrosine–based inhibitory motif (ITIM) domain (TIGIT), Natural killer group 2 member A(NKG2A), T cell immunoglobulin domain and mucin domain protein-3 (TIM-3) and programmed cell death-1 (PD-1).Citation13–15 Compared with other organs, the normal liver is rich in a large number of NK cells. The enhanced activity of NK cells plays a vital role in the immune surveillance of HCC, which may be mediated by the production of perforin, Granzyme, and IFN-γ.Citation6 Meanwhile, the significant increase in the level of inhibitory receptors, the suppression of such NK cells is significantly associated with the progression of HCC.Citation16
The inhibitory receptor TIGIT is considered as an immune checkpoint that mainly mediates T cell exhaustion.Citation17 Moreover, in tumor-bearing mice and colon cancer patients, blocking TIGIT can reverse NK cell exhaustion and promote NK cell’s immune anti-tumor function.Citation13 Recent studies have shown that TIM-3 is a marker of NK cell activation and exhaustion.Citation18,Citation19 The up-regulation of TIM-3 expression inhibits the cytotoxicity of NK cells in CHB patients, suggesting that TIM-3 may mediate the NK cell’s exhaustion function in CHB patients.Citation20 We identify a two-parameter biomarker for the detection of HBV-HCC progression from numerous co-inhibitory molecules. The two-parameter biomarker, the co-expression of TIGIT and TIM-3, was positively correlated with tumor progression and significantly associated with the poor clinical prognosis of HCC patients. TIGIT and TIM-3 synergistically mediate the exhaustion of NK cells in HBV-HCC patients. More importantly, the level of TIGIT+TIM-3+NK cells significantly correlated with the poor clinical prognosis in HBV-HCC patients.
Materials and methods
Patients
Peripheral blood samples were collected from 133 patients with HBV-HCC, 25 patients with HBV-LC, 23 patients with CHB, and 32 healthy donors by Center for Integrative Medicine, Beijing Ditan Hospital, Capital Medical University (Beijing, China). This study is in accordance with the Declaration of Helsinki and has been approved by the ethical committee of Beijing Ditan Hospital, and each individual provides informed consent.
We reviewed the data according to the 2019 China guidelines to confirm HCC diagnosis.Citation21 Moreover, patients with HBV-HCC were defined as positive serum for the hepatitis B surface antigen (HBsAg ≥ 6 months) and conformed to the HCC diagnosis. All patients had no HIV, HCV infection, no hepatitis caused by non-phagocytic hepatitis, and no metastatic liver cancer. The diagnostic criteria for HBV-LC are implemented in accordance with the diagnostic criteria in the “Guidelines for the Diagnosis and Treatment of Liver Cirrhosis” (2019 Edition) formulated by the Liver Disease Branch of the Chinese Medical Association: the diagnosis criteria of chronic hepatitis B have been previously diagnosed, and the liver cirrhosis can be divided into compensatory stage, decompensated stage, recompensation stage and liver cirrhosis reversion stage.Citation22 The clinical characteristics of enrolled all populations have been summarized in .
Table 1. Characteristics of HDs, CHB, HBV-LC, and HBV-HCC patients
Sample collection and PBMC isolation
In this study, 5 ml of peripheral blood from all the population was collected, placed in EDTA anticoagulant blood collection tube. The procedure for isolating peripheral blood mononuclear cells (PBMC) is shown here.Citation23
Multi-parametric flow cytometric analysis
NK cell-surface markers were used the following reagents for multi-parametric flow cytometric: anti-humanCD3-BV786 (clone SK7; 563800), anti-CD19-APC-H7 (clone SJ25C1; 560177), anti-CD14-BV650 (clone M5E2; 563419), anti-CD56-BV510 (clone HCD56; 318340), anti-CD16-BV711 (clone 3G8; 563127), anti-TIGIT-PE-CY7 (clone MBSA43; 25–9500-42), anti-TIM-3-FITC (clone F38-2E2; 11–3109-42), anti-PD-1-PE (clone EH12.2H7; 329906), anti-CD39-APC (clone A1; 328210), anti-LAG-3-AF700 (clone T47-530; 565775), anti-BTLA-PE-CY7 (clone MIH26; 344515), anti-CD160-FITC (clone BY55; 562351), anti-2B4-BV421 (clone 2–69; 565750), and the corresponding isotype controls.
Detection of NK cytokine secretion function: PBMC were isolated from peripheral blood of patients with HBV-HCC, and then stimulated with stimulant system, including IL-12 (100 ng/ml), IL-15 (20 ng/ml), IL-18 (100 ng/ml), 1 μl of BD GolgiPlug™ (1 ml, cat 555029) and anti-CD107a-BUV395 (clone H4A3; 565113), incubated in 37°C and 5% CO2 incubator for 4 hours. Cells were washed in PBS, the extracellular stained with CD3, CD14, CD19, CD56, CD16, TIGIT and TIM-3 antibodies, respectively, add 100 μl of reagent A for cell fixation, and then add 50 μl of reagent B solution for cell permeabilization (BD IntraSure Kit), further the intracellular stained with anti-IFN-γ-AF700 (clone 4S.B3; 56–7319-42) and anti-TNF-α-BV421 (clone MAb11; 502932) antibodies.
NK cell transcription factor expression analysis: PBMC were isolated from peripheral blood of patients with HBV-HCC. Cells were washed in PBS, the extracellular stained with CD3, CD14, CD19, CD56, CD16, TIGIT, and TIM-3 antibodies, respectively, adding TF Fix/Perm Working Solution for cell fixation and permeabilization and then add TF Perm/Wash Working Solution to serve as an antibody diluent and cell wash buffer (BD pharmingen™ Kit). Intracellular standing with anti-T-bet-BV421 (clone O4-46; 563318), anti-Eomes-PE (clone WD1928; 12–4877-41) and anti-Ki67-BV605 (clone Ki-67; 350521) antibiotics were used. Use LSR Fortessa flow cytometer to obtain experimental data, and then use FlowJo software (Version 10) for analysis.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 and SPSS 22.0 statistical software. Fisher’s exact test was used for classification, and t test or nonparametric test was used to evaluate continuous variables. For more than two independent samples, one-way ANOVA was used by Tukey’s multiple comparison test. Mann–Whitney U-test was used to analyze the non-normally distributed data. The Kaplan–Meier curve was used for survival analysis, and then the log-rank test was used to compare survival time of two groups. P values < .05 were considered statistically significant.
Results
TIGIT+TIM-3+NK cells expression was elevated in patients with HBV-HCC
To evaluate the expression levels of TIGIT and TIM-3, we used flow cytometry analysis to detect the respective expression levels in healthy donors, CHB, HBV-LC, and HBV-HCC patients. The basic characteristics of the four groups were presented in . Firstly, we found that the proportion of total NK cell among lymphocytes in HBV-HCC patients was significantly decreased than that of healthy donors and CHB patients (P < .01), but there was no significant difference between patients with HBV-HCC and patients with HBV-LC (, b). Subsequently, we compared the changes in the proportions of TIGIT and TIM-3 in the total NK cells and subgroups of patients with HBV-HCC compared with healthy donors, patients with CHB, and HBV-LC. The expression level of TIGIT+ NK cells was elevated in HBV-HCC patients, compared with healthy donors, CHB, and HBV-LC patients in total NK cells (P < .01) (). The expression level of TIM-3+ NK cells was also elevated in HBV-HCC patients, compared with healthy donors and CHB patients (P < .001). However, there was no statistically significant difference in the expression of TIM-3 in total NK cells between HBV-HCC patients and HBV-LC patients (). The fluorescence minus one (FMO) control of TIGIT+ or TIM-3+ was shown in supplementary Figure 1(Supplementary Figures, Figure S1). Interestingly, we found that TIGIT and TIM-3 had a certain proportion of co-expression; the co-expression level of TIGIT and TIM-3 was also significantly increased in HBV-HCC patients (P < .01) (, b). At the same time, the expression levels of TIGIT and TIM-3 of total NK cells in HBV-HCC patients were significantly positively correlated (r= 0.5218, P < .0001) ().
Figure1. Co-expression of TIGIT and TIM-3 is upregulated on NK cells in HBV-HCC patients. a-b The Proportion of total NK cells and NK cell subsets(CD56brightNK cells,CD56dimNK cells, and CD56−NK cells) from HBV-HCC (n = 133), compared with HDs (n = 32), CHB (n = 23),and HBV-LC patients (n = 25) by flow cytometry analysis. c-d The expression of TIGIT (c) and TIM-3 (d) on total NK cells and NK cell subsets(CD56brightNK cells,CD56dimNK cells, and CD56−NK cells) from HBV-HCC, HDs, CHB and HBV-LC patients. P values were calculated by using the Kruskal–Wallis nonparametric H test.* P < .05, **P < .01, ***P < .001, ****P < .0001
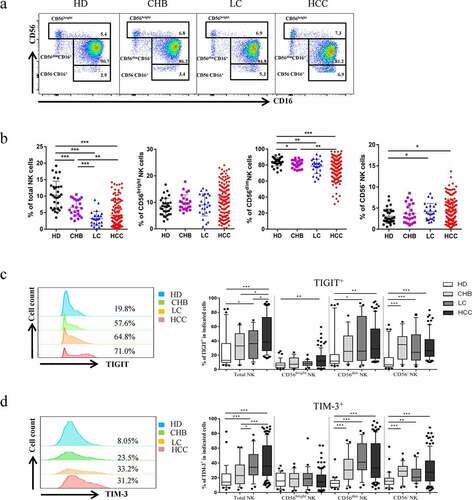
Figure2. The co-expression of TIGIT and TIM-3 is elevated on NK cells of progression patients with HBV-HCC. a-b Percentages of TIGIT+TIM-3+NK cells on total NK cells and NK cell subsets(CD56brightNK cells,CD56dimNK cells, and CD56−NK cells) from HBV-HCC, HDs, CHB and HBV-LC patients by flow cytometry analysis. c Correlation analysis of TIGIT and TIM-3 on NK cells from patients with HBV-HCC. d-e Flow-cytometry analyses (d) of TIGIT and TIM-3 were performed on PBMCs collected from HBV-HCC patients. Representative plots (e) display the expression of TIGIT+NK cells, TIM-3+NK cells and total TIGIT+TIM-3+NK cells from patients with progression (n = 61) and no progression (n = 72). P values were calculated by using the Kruskal–Wallis nonparametric H test (a-c). P values were obtained by the unpaired t test (d-e). * P < .05, **P < .01, ***P < .001, ****P < .0001
High expression of TIGIT+TIM-3+NK cells was associated with the progression of HBV‑HCC patients
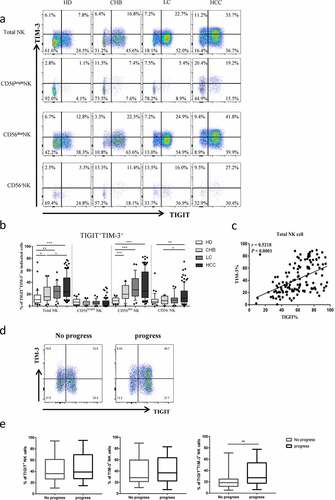
To evaluate the relationship between TIGIT+TIM-3+ NK cells and the clinical outcome of HBV-HCC patients, we enrolled 133 patients with HBV-HCC. We divided them into progress group and no progress group according to whether they had progressed. The expression levels of TIGIT+, TIM-3+and TIGIT+TIM-3+ NK cells were compared between the two groups. We found that only the expression of TIGIT+TIM-3+ NK cells in HBV-HCC patients in the progress group was significantly higher than that of patients in the no progress group (P < .001) (, e). Further, we analyzed the CD56dimCD16+ subgroup and CD56−CD16+ subgroup, respectively, and found that the proportion of co-expression of TIGIT+TIM-3+ in the progress group was higher than that in the non-progress group, but no significant statistical difference was found between the two groups (Supplementary Figures, Figure S2). According to the proportion of TIGIT+TIM-3+NK cells, the median of TIGIT+TIM-3+NK cells expression level (20.5%) was used as cutoff value. We divided HBV-HCC patients (n = 133) into two groups: TIGIT+TIM-3+high (>20.5%) and TIGIT+TIM-3+low (≤20.5%). A total of 65 patients were defined as the TIGIT+TIM-3+ high group, and 68 patients were defined as the TIGIT+TIM-3+low group. We analyzed clinical characteristics and found no significant difference between the two groups in terms of age, gender, hypertension, diabetes, coronary disease, BCLC stage, Child-pugh, HBV-DNA, HBeAg, tumor size, tumor multiplicity, HGB, PLT, ALT, ALB,γ-GGT, AFP, ascites, hepatic encephalopathy, vascular metastasis and distant metastasis (). Portal hypertension was higher in the TIGIT+TIM-3+high group, but there was no statistical difference between the two groups. Moreover, there were no statistical differences among different treatment methods. We further observed the correlation of tumor progression in the two groups of patients. We found that HBV-HCC patients in the TIGIT+TIM-3+high group had a higher rate of tumor progression (38/27(58.5%) vs. 23/45(33.8%), P = .004, ).
Table 2. Demographic and clinical characteristics of different level of TIGIT+TIM-3+NK cells in patients with HBV-HCC
High expression of TIGIT+TIM-3+NK cells was more sensitive to the poor prognosis of patients with early-stage HBV-HCC
To further determine the effects of tumor progression on TIGIT+TIM-3+ co-expression, we used the Kaplan–Meier survival curve to compare the progression-free survival (PFS) time of the two groups (TIGIT+TIM-3+high group and TIGIT+TIM-3+low group) in the clinical subgroup. In the total HBV-HCC population, we found that the TIGIT+TIM-3+high group had lower PFS rates than the TIGIT+TIM-3+low group (; HR = 2.05, 95% CI 1.24–3.04, P = .005). Next, we further compared the effects of the TIGIT+TIM-3+high group and TIGIT+TIM-3+low group on tumor progression in different tumor stages and liver function grades. For patients in BCLC stages 0-B and patients in Child-Pugh A, the PFS rates of patients with TIGIT+TIM-3+high NK cells populations were significantly lower than those with TIGIT+TIM-3+low NK cells populations (BCLC stage 0-B, P= .010; Child-Pugh A, P = .031, , d), but not in the subgroups of patients in BCLC stage C-D and Child-Pugh B + C (BCLC stage C-D, P= .443; Child-Pugh B + C, P = .087, , e). Furthermore, we also divided the tumor burden, transaminase level, and hepatitis B virus level into subgroups to compare the effects of TIGIT+TIM-3+high group and TIGIT+TIM-3+low group on PFS. The PFS rate of the TIGIT+TIM-3+high group was obviously lower than the TIGIT+TIM-3+low group in patients with tumor size ≤5 cm (; HR = 2.38, 95% CI 1.28–4.45, P = .006) and tumors solitary (; HR = 2.11, 95% CI 1.01–4.39, P = .045) and the level of AFP ≤ 400 ng/mL (; HR = 2.34, 95% CI 1.30–4.22, P = .004), but not in the subgroups of patients with tumors size >5 cm (P = .342; ) and tumor multiple (P = .068; ) and the level of AFP > 400 ng/mL (P = .549; ). The PFS rate of the TIGIT+TIM-3+high group was obviously lower than the TIGIT+TIM-3+low group in patients with the level ALT ≤ 50 U/L (; HR = 2.21, 95% CI 1.26–3.89, P = .005), but not in the subgroup of patients with the level ALT > 50 U/L (P = .446; h). In the process of chronic viral infection, hepatitis B virus and HBeAg were also important reasons that affect the tumor progress of patients. Therefore, observe the effect of the virus level and HBeAg level of the TIGIT+TIM-3+high group and TIGIT+TIM-3+low group of co-inhibitory molecules on the PFS of HBV-HCC patients. The PFS of TIGIT+TIM-3+high NK cells patients was significantly lower than that of TIGIT+TIM-3+low NK cells patients in HBV-DNA level <100 IU/mL (; HR = 2.63, 95% CI 1.45–4.77, P = .001) and HBeAg negative group (; HR = 2.17, 95% CI 1.23–3.84, P = .007), but not in the subgroups of patients with HBV-DNA level ≥100 IU/mL (P = .726) and HBeAg positive group (P = .418).
Figure3. Kaplan–Meier curve analysis showing the efficacy of TIGIT+TIM-3+NK cells levels as a predictor of progression-free survival (PFS) in HBV-HCC patients across different tumor stage and liver function classification. a Progression-free survival in HBV-HCC patients, b-d Subgroup analysis of patients with BCLC stage 0-B (b), BCLC stage C-D (c), Child-Pugh A (d), and Child-Pugh B + C (e). P values and HRs were obtained using the log-rank test
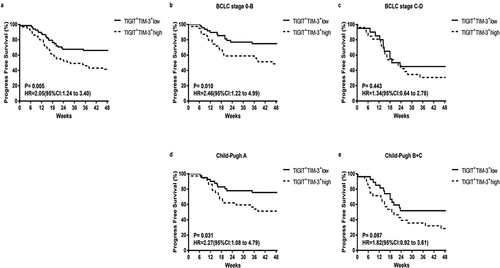
Figure4. Kaplan–Meier curve analysis showing the efficacy of TIGIT+TIM-3+NK cells levels as a predictor of progression-free survival in HBV-HCC across different tumor load, liver function level and HBV virus load. a-b Patients with different tumor size, tumor size ≤ 5 cm (a), tumor size > 5 cm (b); c-d patients with different tumor number, tumor solitary (c), tumor multiple (d); e-f patients with different AFP level, AFP ≤ 400 ng/ml (e), AFP > 400 ng/ml (f); g-h patients with different ALT level, ALT≤ 50 U/L (g), ALT > 50 U/L (h); i patients with different HBV-DNA level; j patients with or without HBeAg. P values and HRs were obtained using the log-rank test
TIGIT+TIM-3+NK cells from HBV‑HCC patients showed functional exhaustion
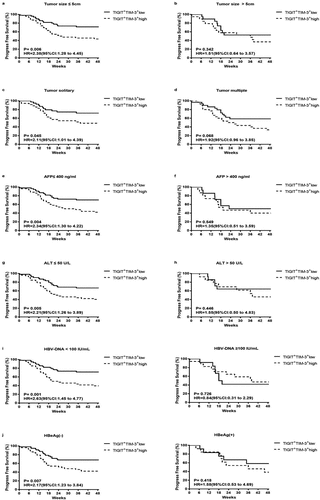
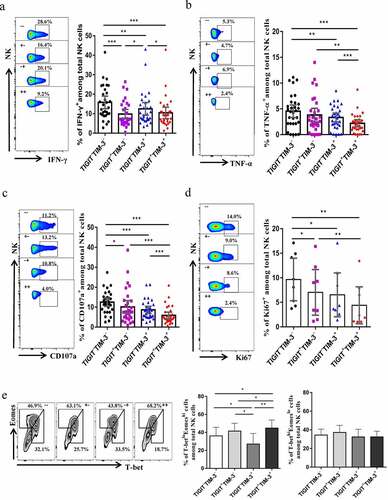
To further study whether the TIGIT+TIM-3+ NK cells in HBV-HCC patients were functionally exhausted, we tested the cytokines release and cell killing function. TIGIT+TIM-3+NK cells in HBV-HCC patients exhibited a reduced capacity to produce IFN-γ compared with TIGIT−TIM-3− and TIGIT−TIM-3+NK cells (P < .05, ). Meanwhile,TIGIT+TIM-3+NK cells in HBV-HCC patients exhibited a reduced capacity to produce TNF-α and CD107a compared with TIGIT−TIM-3−, TIGIT+TIM-3−and TIGIT−TIM-3+NK cells (P < .05, , c). We next tested the proliferation of NK cells in HBV-HCC patients and found that the expression level of Ki67 of TIGIT+TIM-3+NK cells in HBV-HCC patients was significantly reduced compared with TIGIT−TIM-3− and TIGIT+TIM-3−NK cells, indicating that their proliferation ability was weakened (P < .05, ). In addition, we detected NK cell transcription factors T-bet and Eomes in HBV-HCC patients, and found that percentage of T-betloEomeshi on TIGIT+TIM-3+NK cells in HBV-HCC patients increased compared with TIGIT−TIM-3−,TIGIT+TIM-3−and TIGIT−TIM-3+NK cells, while T-bethiEomeslo expression levels did not change significantly (P < .05, ).
Figure5. TIGIT+TIM-3+NK cells in HBV-HCC patients are functionally exhausted. a-c Intracellular staining for IFN-γ (a), TNF-α (b) and CD107a (c) in TIGIT+TIM-3+NK cells from HBV-HCC patients (n = 30) upon in vitro stimulation for IL-12, IL-15, and IL-18 (100, 20, and 100 ng/ml, respectively).d Percentage of expression of Ki67 (d) on TIGIT−TIM-3−, TIGIT+TIM-3−, TIGIT−TIM-3+ and TIGIT+TIM-3+ NK cells from patients with HBV-HCC (n = 8).e Representative flow cytometry data and histogram showing the percentage of T-betloEomeshi and T-bethiEomeslo cells (e) in different subpopulations of TIGIT+TIM-3+ NK cells from patients with HBV-HCC (n = 8). P values were obtained by the Kruskal–Wallis ANOVA test. *P < .05, **P < .01, ***P < .001
TIGIT+TIM-3+NK cells from HBV‑HCC patients exhibited an exhausted phenotype
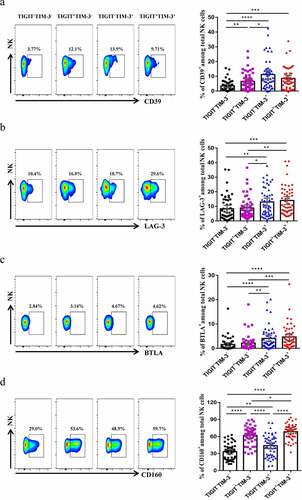
To characterize the phenotype of NK cells that co-expressed TIGIT+TIM-3+ in HBV-HCC patients, we detected the expression of several inhibitory receptors on TIGIT+TIM-3+ NK cells. We found that the expression level of CD39 was significantly higher in the TIGIT+TIM-3+ NK cells than the TIGIT−TIM-3− NK cells in HBV-HCC patients (P < .0001, ). The expression level of LAG-3 and BTLA was significantly higher in the TIGIT+TIM-3+ NK cells than TIGIT−TIM-3− and TIGIT+TIM-3− NK cells in HBV-HCC patients (P < .001, , c). Besides, the expression level of CD160 on TIGIT+TIM-3+ NK cells increased significantly, compared with TIGIT−TIM-3−, TIGIT+TIM-3−and TIGIT−TIM-3+NK cells (P < .05, ). TIGIT+TIM-3+ NK cells highly expressed co-inhibitory molecules, such as CD160, so they exhibited an exhausted phenotype.
Figure6. TIGIT+TIM-3+NK cells display an exhausted phenotype in HBV-HCC patients. The percentages of the NK cells inhibitory receptors CD39 (a), LAG-3 (b), BTLA (c) and CD160 (d) expressed on four NK cells subsets (TIGIT−TIM-3−, TIGIT+TIM-3−, TIGIT−TIM-3+ and TIGIT+TIM-3+, respectively) from HBV-HCC patients (n = 51). P values were obtained by the Kruskal–Wallis ANOVA test. * P < .05, **P < .01, ***P < .001, ****P < .0001
Discussion
NK cell exhaustion is usually caused by tumor progression and chronic infection, which weakens the anti-tumor and anti-infection ability of NK cells.Citation24 The blocking of immune checkpoint is an important part of reversing exhaustion. The reversal of T cell exhaustion by anti-PD-1 monoclonal antibody and anti-CTLA-4 monoclonal antibody has shown clinical benefits to some patients, but its clinical effective response rate is about 35%.Citation24–26 Studies have shown that co-inhibitory molecules on T cells such as PD-1, TIGIT, TIM-3, LAG-3, CTLA-4 also exist on NK cells.Citation27,Citation28 In a variety of tumors, the co-inhibitory molecules TIGIT and TIM-3 are closely related to T cell exhausted.Citation29–32 These studies indicate that it is necessary to study the specific exhausted phenotype and function of NK cells in HBV-HCC patients to reveal new anti-tumor targets of NK cells. In this study, we found that the co-expression of TIGIT and TIM-3 mediates NK cells exhaustion in HBV-HCC patients, which is characterized by the decrease of cytokine (IFN-γ, TNF-α), decreased cytotoxicity (CD107a), proliferation (Ki67), and high expression of T-betloEomeshiNK cells in HBV-HCC patients. Importantly, we found that high levels of TIGIT+TIM-3+NK cells are closely associated with poor prognosis of HBV-HCC. To our knowledge, this is the first evidence that TIGIT+TIM-3+NK cells are related to the progression of HBV-HCC. Besides, our results provide a potential therapeutic target for the treatment of HCC to prevent tumor progression.
TIGIT is an inhibitory receptor expressed on both T cells and NK cells. It inhibits the activation of T cells and NK cells by mediating inhibitory signals.Citation33,Citation34 Studies have shown that the expression level of TIGIT is significantly negatively correlated with the IFN-γ secretion capacity of NK cells in tumor patients and autoimmune disease patients.Citation35 TIM-3 is an inhibitory receptor expressed on various immune cells, mainly involved in immune regulation.Citation36,Citation37 In recent years, TIM-3 has been regarded as an important immune checkpoint involved in tumor immunosuppression, which has attracted considerable attention and is expected to become a new immunotherapy target. Studies have reported that the expression of TIM-3 on NK cells in patients with metastatic melanoma is increased, and the function of NK cells is impaired. Simultaneously, the expression level of TIM-3 is related to tumor stage and poor prognosis.Citation38 The expression level of TIM-3 in patients with lung adenocarcinoma is higher in patients with high tumor burden and advanced tumor. Patients with high TIM-3 expression are associated with a shorter overall survival time, and further blocking TIM-3 signaling can improve patients’ NK cell cytotoxicity and IFN-γ production function.Citation39 In this study, we found that the co-expression of TIGIT and TIM-3 was increased in HBV-HCC patients, and they coordinated to mediate the exhaustion of NK cells. Highly expressed TIGIT+TIM-3+NK cells had a significant correlation with the progression of HCC patients.
It is generally believed that tumor factors and chronic infection factors are important reasons for the exhaustion of NK cells.Citation24 Therefore, we further divided the related clinical indicators into subgroups to observe the relationship between increased expression of TIGIT and TIM-3 and tumor progression in different subgroups. In this study, we found that patients with high expression of TIGIT+TIM-3+NK cells were more likely to develop tumor progression in the early stage of the tumor, better liver function, lighter tumor load, lower hepatitis B virus level, and HBeAg negative patients than those with low expression of TIGIT+TIM-3+NK cells. This may be because there is a more sensitive correlation between the increase of inhibitory receptors and the adverse outcomes in the early stage of disease development and in the mild condition. In the future, we need to expand the sample size and continue to further verify in prospective studies.
In the process of anti-tumor and anti-virus infection, transcription factors T-bet and Eomesodermin (Eomes) not only regulate the differentiation and maturation of NK cells, but also play an anti-tumor role by regulating the function of NK cells. Eomes is mainly involved in the function of mature NK cells. Some studies have found that the decrease of Eomes levels is related to the damage of cytotoxicity of NK cells.Citation40,Citation41 Animal studies have found that the down-regulation of Eomes chromosomes can inhibit the anti-tumor activity of adoptive metastasis mouse.Citation42 In peripheral blood NK cells of patients with renal cell carcinoma, it was found that higher Eomes mRNA expression is an independent good prognostic factor for OS and PFS.Citation43 These reports speculate that the expression level of Eomes was positively correlated with the cytotoxicity of NK cells. However, there are also reports that the expression of Eomes is down-regulated due to the stronger cytotoxicity of mature NK cells, which indicated that Eomes was negative regulator of NK cytotoxicity.Citation44,Citation45–46 This study is consistent with our findings that EomeshiNK cells express more inhibitory receptors and lower cytotoxicity. In our study, the expression of Eomes was up-regulated on TIGIT+TIM-3+NK cells in peripheral blood of patients with HBV-HCC, and there was a characteristic change of subsets. However, T-bet can affect the differentiation and maturation of end-stage NK cells and stabilize immature NK cells. The decrease of T-bet is related to the damage of NK cytotoxicity, and affects the anti-tumor effect of NK cells.Citation40–42 Whether the up-regulation of T-bet is the cause of its increased cytotoxicity remains controversial.Citation44 In our study, we found that there was no significant difference in the expression proportion of T-bet in TIGIT+TIM-3+NK cells of HBV-HCC patients.
The subjects in this study were all peripheral blood samples, not tumor tissues. Because tumor tissue is difficult to obtain in a short period of time, we want to obtain new immune targets for HCC patients through simple, rapid and noninvasive peripheral blood, so as to provide new ideas for future immunotherapy. At the same time, many studies have shown that the inhibitory molecules of NK cells are increased in tumor tissues and peripheral blood. Qing Z et al. found that the high expression of TIGIT on tumor infiltrating NK cells was related to the progression of colon cancer patients and NK cell exhaustion.13 Tan S et al. found that the expression of TIM-3 was up-regulated in tumor infiltrating NK cells and peripheral blood NK cells of patients with hepatocellular carcinoma, which inhibited the cytotoxicity of NK cells.Citation47 Moreover, in our previous study, we found that the expression levels of PD-1 and TIGIT on tumor infiltrating T cells in HCC patients were consistent with those in peripheral blood T cells.Citation48
Conclusions
In conclusion, the present study has provided new insights into the phenotype and function of TIGIT+TIM-3+NK cells and tumor progression in patients with HBV-HCC. We have demonstrated that TIGIT+TIM-3+NK cells population is elevated by the progression in HBV-HCC patients, characterized by NK cells exhaustion. More importantly, TIGIT+TIM-3+NK cells subset may represent a novel indicator to predict the poor prognosis of HBV-HCC patients.
Abbreviations
AFP Alpha-fetoprotein
ALB Albumin
ALT Alanine aminotransferase
BCLC Barcelona Clinic Liver Cancer
BTLA B and T lymphocyte attenuator
CHB Chronic hepatitis B virus infection
CTLA-4 Cytotoxic T-lymphocyte antigen 4
Eomes Eomesodermin
FITC Fluorescein Isothiocyanate
GGT Glutamyl Transferase
HBeAg Hepatitis B e Antigen
HBV Hepatitis B Virus
HCC Hepatocellular Carcinoma
HCV Hepatitis C Virus
HGB Hemoglobin
HIV Human immunodeficiency virus
IFN-γ Interferon-gamma
IL Interleukin
LAG-3 lymphocyte activation gene-3
LC Liver cirrhosis
NK cells Natural Killer cells
NKG2A Natural killer cell receptor group 2A
OS Overall Survival
PBMC Peripheral Blood Mononuclear Cell
PD-1 Programmed cell death-1
PE Phycoerythrin
PFS Progression-Free Survival
PLT Platelet
TIGIT T cell immunoreceptor with Ig and ITIM domain
TIM-3 T cell immunoglobulin domain and mucin domain protein-3
TNF-α Tumor necrosis factor-alpha
Declarations
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZY and JD designed the study; LY and JD performed experiments and wrote the manuscript; XL, XW, FY and PW provided patients data; YJ was responsible for the interpretation of data and revision. ZY approved for final revision and approval.
Supplemental Material
Download ()Acknowledgments
We would like to thank Huiwen Yan and Dongdong Zhou for their assistance with the follow-up of survival information in HBV-HCC patients.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–13. doi:10.3322/caac.21660.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi:10.1053/j.gastro.2011.12.061.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338.
- Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21(8):835–847. doi:10.1038/s41590-020-0728-z.
- Chen Y, Tian Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol Immunol. 2021;18(1):57–72. doi:10.1038/s41423-020-00561-z.
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean MC, Alifano M, Régnard JF, et al. coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. doi:10.1158/0008-5472.CAN-10-4179.
- Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609–3622. doi:10.1172/JCI45816.
- Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RLCTLA. 4⁺ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75(11):2200–2210. doi:10.1158/0008-5472..
- Zhang QF, Yin WW, Xia Y, Yi YY, He QF, Wang X, Ren H, Zhang DZ. Liver-infiltrating CD11b-CD27- NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol Immunol. 2017;14(10):819–829. doi:10.1038/cmi.2016.28.
- Malmberg KJ, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi:10.1016/j.smim.2017.08.002.
- Muntasell A, Ochoa MC, Cordeiro L, Berraondo P. López-Díaz de Cerio A, Cabo M, López-Botet M, Melero I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr Opin Immunol. 2017;45:73–81. doi:10.1016/j.coi.2017.01.003.
- Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi:10.1038/s41590-018-0132-0.
- Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, Wang J, Song J, Zheng M, Sun H, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. 2016;6(1):e1264562. doi:10.1080/2162402X.2016.1264562.
- Seo H, Jeon I, Kim BS, Park M, Bae EA, Song B, Koh CH, Shin KS, Kim IK, Choi K, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun. 2017;8(1):15776. doi:10.1038/ncomms15776.
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–437. doi:10.1016/j.clim.2008.08.012.
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi:10.1038/ni.1674.
- Dao TN, Utturkar S, Atallah Lanman N, Matosevic STIM-3. Expression Is Downregulated on Human NK Cells in Response to Cancer Targets in Synergy with Activation. Cancers (Basel). 2020;12(9):2417. doi:10.3390/cancers12092417.
- So EC, Khaladj-Ghom A, Ji Y, Amin J, Song Y, Burch E, Zhou H, Sun H, Chen S, Bentzen S, et al. NK cell expression of Tim-3: first impressions matter. Immunobiology. 2019;224(3):362–370. doi:10.1016/j.imbio.2019.03.001.
- Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52(3):322–329. doi:10.1016/j.jhep.2009.12.005.
- Department of Medical Administration, National Health and Health Commission of the People’s Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28( 2):112–128. Chinese. doi: 10.3760/cma.j..1007-3418.2020.02.004.
- Chinese Society of Hepatology,Chinese Medical Association. [Chinese guidelines on the management of liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2019;27( 11):846–865. Chinese. doi: 10.3760/cma.j..1007-3418.2019.11.008.
- Song Y, Wang B, Song R, Hao Y, Wang D, Li Y, Jiang Y, Xu L, Ma Y, Zheng H, et al. T-cell Immunoglobulin and ITIM Domain Contributes to CD8+ T-cell Immunosenescence. Aging Cell. 2018;17(2):e12716. doi:10.1111/acel.12716.
- Bi J, Tian Z. NK Cell Exhaustion. Front Immunol. 2017;8:760. doi:10.3389/fimmu.2017.00760.
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi:10.1016/S0140-6736(14)60958-2.
- JS W, SP D, Minor D, FS H, Gutzmer R, Neyns B, Hoeller C, NI K, Jr MWH, CD L, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi:10.1016/S1470-2045(15)70076-8.
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862.
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001.
- Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, Wu H, Bu LL, Kulkarni AB, Zhang WF, et al. Blockade of TIGIT/CD155 Signaling Reverses T-cell Exhaustion and Enhances Antitumor Capability in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res. 2019;7(10):1700–1713. doi:10.1158/2326-6066.CIR-18-0725.
- Ostroumov D, Duong S, Wingerath J, Woller N, Manns MP, Timrott K, Kleine M, Ramackers W, Roessler S, Nahnsen S, et al. Transcriptome Profiling Identifies TIGIT as a Marker of T-Cell Exhaustion in Liver Cancer. Hepatology. 2021;73(4):1399–1418. doi:10.1002/hep.31466.
- Avery L, Filderman J, Szymczak-Workman AL, Kane LP. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc Natl Acad Sci U S A. 2018;115(10):2455–2460. doi:10.1073/pnas.1712107115.
- Zhang Y, Cai P, Li L, Shi L, Chang P, Liang T, Yang Q, Liu Y, Wang L, Hu L. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int Immunopharmacol. 2017;43:210–218. doi:10.1016/j.intimp.2016.12.024.
- Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2):e000957. doi:10.1136/jitc-2020-000957.
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi:10.1038/ni.1674.
- Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45(10):2886–2897. doi:10.1002/eji.201545480.
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi:10.1038/ni1271.
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. Corrigendum: CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2016;536(7616):359. doi:10.1038/nature17421.
- da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2(5):410–422. doi:10.1158/2326-6066.CIR-13-0171.
- Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. 2015;29(2):635–641. doi:10.1016/j.intimp.2015.09.017.
- Shehata HM, Hoebe K, Chougnet CA. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell. 2015;14(2):191–199. doi:10.1111/acel.12303.
- Simonetta F, Pradier A, Bosshard C, Masouridi-Levrat S, Chalandon Y, Roosnek E. NK Cell Functional Impairment after Allogeneic Hematopoietic Stem Cell Transplantation Is Associated with Reduced Levels of T-bet and Eomesodermin. J Immunol. 2015;195(10):4712–4720. doi:10.4049/jimmunol.1501522.
- Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, Florek M, Gibbs KD, Tate K, Ritchie DS, et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 2012;119(24):5758–5768. doi:10.1182/blood-2012-03-415364.
- Dielmann A, Letsch A, Nonnenmacher A, Miller K, Keilholz U, Busse A. Favorable prognostic influence of T-box transcription factor Eomesodermin in metastatic renal cell cancer patients. Cancer Immunol Immunother. 2016;65(2):181–192. doi:10.1007/s00262-015-1786-1.
- Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Björklund AT, Flodström-Tullberg M, Michaëlsson J, Rottenberg ME, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. doi:10.1182/blood-2010-04-281675.
- Collins A, Rothman N, Liu K, Reiner SL. Eomesodermin and T-bet mark developmentally distinct human natural killer cells. JCI Insight. 2017;2(5):e90063. doi:10.1172/jci.insight.90063.
- Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi:10.1038/s41590-018-0132-0.
- Tan S, Xu Y, Wang Z, Wang T, Du X, Song X, Guo X, Peng J, Zhang J, Liang Y, et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020;80(5):1130–1142. doi:10.1158/0008-5472.CAN-19-2332.
- Liu X, Li M, Wang X, Dang Z, Jiang Y, Wang X, Kong Y, Yang Z. PD-1+ TIGIT+ CD8+ T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(12):2041–2054. doi:10.1007/s00262-019-02426-5.
