ABSTRACT
It has been an open conundrum why primary sclerosing cholangitis (PSC) is a major risk factor for developing cholangiocarcinoma (CAA), while primary biliary cholangitis (PBC) is not. In mouse models of PSC and PBC, it turned out that the latter condition, an autoimmune disease affecting the bile ducts, reduces transgene-induced cholangiocarcinogenesis, as well as the progression of subcutaneously implanted CCA. This CCA-delaying effect is lost upon depletion of T lymphocytes and involves tumor infiltration by T cell clonotypes that are also found in PBC lesions. Hence, organ-specific autoimmunity may improve immunosurveillance.
Patients with primary sclerosing cholangitis (PSC), which often develops in the context of inflammatory bowel disease, have a 20% lifetime risk to develop cholangiocarcinoma (CAA), which likely results from the chronic inflammation-associated proliferation of cholangiocytes, the epithelial cells of the biliary tract. The prognosis of CAA is grim because it is usually detected at a locally advanced, inoperable stage and barely responds to standard chemotherapy, radiotherapy, and immunotherapy.Citation1,Citation2 In strict contrast to PSC, another type of cholangitis referred to as primary biliary cholangitis (PBC), which has a strong autoimmune component, does not predispose to CAA ().
Figure 1. Epidemiological and immunological links between cholangitis and cholangiocarcinoma. (A) Patients with primary sclerosing cholangitis (PSC) often develop cholangiocarcinoma (CAA), while patients with primary biliary cholangitis (PBC) usually do not develop CAA. (B) In preclinical models, PBC, but not PSC, prevents or delays the development of CCA. This protective effect mediated by PBC is specific to CCA as it does not prevent the outgrowth of other cancers such as hepatocellular carcinoma (HCC). (C) In mice, some CD4+ and CD8+ T cell clonotypes with immune effector functions were infiltrating and expanding in both liver and CCA tissues upon PBC. These T cell clones responsible for concomitant detrimental and beneficial autoimmunity are likely to recognize non-mutated autoantigens shared between nonmalignant and malignant cholangiocytes
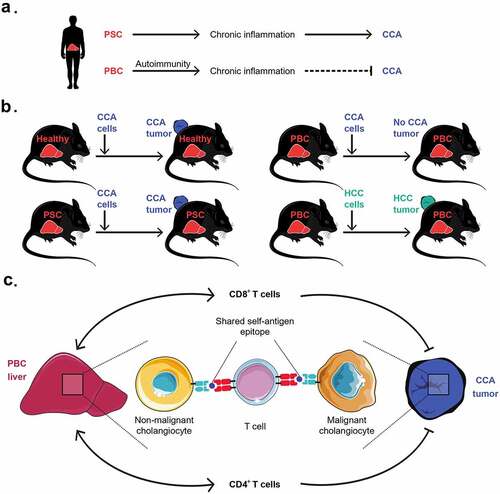
Intrigued by these epidemiological associations, we characterized mouse models of PSC (induced by the cholestasis inducing compound 3,5-dicarbethoxy-1,4-dihydrocollidine) and PBC (induced by intraperitoneal injection of 2-octynoic acid coupled to bovine serum albumin) by bulk RNA sequencing (RNA-seq) of affected livers. This approach led to the conclusion that PSC exhibits a predominantly autoinflammatory pattern marked by the enrichment of myeloid cells, while PBC exhibits an additional autoimmune signature with a strong local enrichment of B and T lymphocytes. This infiltration pattern of PBC was corroborated by cytometry-based immunophenotyping.Citation3
Of note, a CCA cell line that was injected subcutaneously in control mice or mice with PSC developed tumors indistinguishably in healthy control mice and animals with PSC. In sharp contrast, such CCAs either failed to develop palpable tumors or grew more slowly in mice with PBC. This effect was specific because subcutaneous fibrosarcoma, hepatocellular carcinoma and lung adenocarcinoma developed indistinguishably on mice with or without PBC (). Moreover, it extended to orthotopic cholangiocarcinogenesis (induced by hydrodynamic injection of oncogene-expressing vectors) that was prevented upon PBC as opposed to control mice without cholangitis.Citation3
The transplantable CCAs that slowly developed on mice with PBC exhibited a more favorable ratio of tumor-infiltrating CD8+ cytotoxic T lymphocytes (CTLs) over FoxP3+ regulatory CD4+ T cells (Tregs) than CCAs growing in control mice or in the context of PSC. Moreover, in the context of PBC, CCA tumor-draining lymph nodes contained higher levels of granzyme B-positive T cells producing several cytokines (such as IFNγ, IL2, IL4, IL-17 and TNFα) than in CCA-bearing mice without cholangitis or post-PSC. Depletion of T cells (by injections of antibodies specific to CD4 and CD8), elimination of B cells (by anti-CD20) or neutralization of IFNγ or IL4 (but not that of IL17) by means of suitable neutralizing antibodies, accelerated the growth of CCA effects post-PBC, yet did not affect CCA progression in control or PSC mice.Citation3
The aforementioned results strongly suggest that PBC induces an autoimmune response that cross-reacts against CCA, hence specifically enhancing immunosurveillance against this specific cancer type. To obtain formal proof in favor of this conjecture, we used single-cell RNA-seq and single-cell TCR sequencing to compare T lymphocytes purified from PBC livers, peripheral blood and CCA tumors. We found that, in the same mouse with PBC, a substantial proportion of CCA-infiltrating and intrahepatic CD4+ and CD8+ T cells shared identical TCRs (), strongly suggesting that T cells that recognize PBC-relevant autoantigens also recognize CCA.Citation3
Circumstantial evidence suggests that immune responses against antigens expressed by normal, non-transformed cells can contribute to anticancer immunosurveillance. For example, the CTL/Treg ratio detectable in normal, nonmalignant breast tissue, predicts the risk to develop breast cancer in spite of the surgical removal of ductal in situ carcinomas.Citation4 Moreover, immune responses against normal melanocytes causing vitiligo, and against normal thyroid epithelial cells causing thyroiditis, have a positive prognostic impact on patients with melanoma and thyroid carcinoma, respectively.Citation5 Moreover, in preclinical experiments, the development of mammary carcinoma and colorectal cancer can be delayed by prior vaccination with anthracycline-treated or irradiated normal breast epithelial cells and oxaliplatin-treated normal ileal epithelial cells, respectively.Citation6,Citation7
In the context of this epidemiological and experimental evidence, it appears highly plausible that organ-specific autoimmune responses specific to cholangiocytes, mammary gland cells, melanocytes, and thyrocytes can improve immunosurveillance against CCA, breast cancer, melanoma, and thyroid cancer, respectively. Hence, from the point of view of the cancer immunologist and immunotherapist, such autoimmune responses may be dubbed as “beneficial”. It is noteworthy that such a “beneficial autoimmunity” involves the recognition of non-mutated autoantigens, at odds with the popular view that the clinically most important tumor antigens arise from mutations.Citation5–9
The precise nature of autoantigens shared between the target cells of organ-specific autoimmunity, and malignant cells originating from such target cells remains to be determined. Theoretically, such autoantigens may either be constitutively expressed or might be restricted to stressed (but not unstressed) parenchymal cells. In the former case, clinical signs of autoimmunity would be ineluctable (at least in the presence of Tregs dysfunction), while the latter scenario would be compatible with a physiological state. Indeed, there is mounting evidence that immunosurveillance is not only involved in the destruction of (pre-)malignant cells but may also assure the elimination of stressed and senescent cells that otherwise would accumulate as a result of local damage and aging.Citation5,Citation10 It is tempting to speculate that “beneficial autoimmunity” is in charge of this phenomenon, calling for the identification of such health-relevant autoantigens.
Disclosure of Statement
GK is a co-founder of Samsara Therapeutics, everImmune, and Therafast Bio. All other authors declare that they have no potential conflicts of interest.
Additional information
Funding
References
- Boileve A, Hilmi M, Smolenschi C, Ducreux M, Hollebecque A, Malka D. Immunotherapy in advanced biliary tract cancers. Cancers (Basel). 2021;13:1569. doi:10.3390/cancers13071569.
- Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–3.
- Paillet J, Plantureux C, Lévesque S, Le Naour J, Stoll G, Sauvat A, Caudana P, Tosello Boari J, Bloy N, Lachkar S, et al. Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma. J Exp Med. 2021. In press. doi:10.1084/jem.20200853.
- Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi:10.1007/s10549-011-1647-3.
- Zitvogel L, Perreault C, Finn OJ, Kroemer G. Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol. 2021. doi:10.1038/s41571-021-00508-x.
- Buque A, Bloy N, Perez-Lanzon M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 2020;11(1):3819. doi:10.1038/s41467-020-17644-0.
- Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon L, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–931. doi:10.1038/s41591-020-0882-8.
- Tuohy VK. Retired self-proteins as vaccine targets for primary immunoprevention of adult-onset cancers. Expert Rev Vaccines. 2014;13:1447–1462. doi:10.1586/14760584.2014.953063.
- Kooreman NG, Kim Y, de Almeida PE, Termglinchan V, Diecke S, Shao NY, Wei -T-T, Yi H, Dey D, Nelakanti R, et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell. 2018;22:501–13 e7. doi:10.1016/j.stem.2018.01.016.
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9(1):5435. doi:10.1038/s41467-018-07825-3.
