ABSTRACT
Patients with locally advanced esophageal squamous cell carcinoma (ESCC) show poor survival after concurrent chemoradiotherapy. This study investigated the safety and feasibility of combining concurrent chemoradiotherapy with the anti-PD-1 antibody camrelizumab as first-line treatment for these patients. In this phase 1b study (ClinicalTrials.gov NCT03671265), patients received concurrent chemotherapy (cisplatin [25 mg/m2] plus docetaxel [25 mg/m2] for 4 weeks) and radiotherapy (2.0 Gy/fraction, total 60 Gy) with camrelizumab (200 mg every 2 weeks for 32 weeks). Primary endpoints were safety and tolerability, and health-related quality of life. Secondary endpoints were radiological and pathological response rates, overall survival (OS), and progression-free survival (PFS). Candidate biomarkers in tumor and peripheral blood were monitored at baseline and after 40 Gy radiation. Twenty patients were enrolled. The most common treatment-related grade 3 adverse events included radiation esophagitis (20%) and esophageal fistula (10%). Serious treatment-related adverse events occurred in eight (40%) patients. No treatment-related deaths were reported. Health-related quality of life did not deteriorate. Thirteen (65%) patients had an objective response after 40 Gy radiation. At a median follow-up of 23.7 months (95% CI 21.9–24.5), OS and PFS time ranged from 8.2–28.5 and 4.0–28.5 months, respectively. The 12-month and 24-month OS rate was 85.0% and 69.6%; PFS rate was 80.0% and 65.0%. Tumor PD-L1 expression and CD11c+ dendritic cells and peripheral-blood IL-27, IL-15, Eotaxin-3, and IL-22 were associated with OS. First-line concurrent chemoradiotherapy plus camrelizumab had a manageable safety profile and promising antitumour efficacy for ESCC, and deserves further study.
Introduction
Esophageal cancer was the seventh most common and sixth most deadly form of cancer worldwide in 2018Citation1. The prevalence of esophageal squamous cell carcinoma (ESCC) in China was 477 900 in 2015, placing ESCC among the six most common cancers in China.Citation2 Definitive radiotherapy concurrent with cisplatin plus fluorouracil is the standard care for unresectable, locally advanced esophageal cancer based on the results of the Radiation Therapy Oncology Group (RTOG) 85–01 trial.Citation3 Subsequent studies attempted to improve treatment outcome by optimizing the chemoradiotherapy regimenCitation4 and adding a novel agent, the anti-epidermal growth factor receptor (EGFR) antibody cetuximab, to systemic therapies.Citation5 However, the results were not satisfactory.Citation4,Citation5
Inhibitors of programmed cell-death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) block PD-1/PD-L1 signaling, thus reversing T cell suppression and enhancing antitumour immune response.Citation6 PD-1 blockade yields promising results in advanced/metastatic ESCC. The phase 3 ATTRACTION-3 study showed that nivolumab improved survival compared with chemotherapy.Citation7 Pembrolizumab prolonged overall survival versus chemotherapy in patients with a PD-L1 combined positive score (CPS) ≥10.Citation8 Camrelizumab (SHR-1210) is a selective, humanized, high-affinity IgG4-kappa PD-1 monoclonal antibody that has exhibited encouraging antitumour activity for multiple solid tumors.Citation9–11 The phase 3 ESCORT study undertaken in China showed that camrelizumab significantly improved overall survival compared with chemotherapy.Citation12
Chemoradiotherapy promotes antitumour immune response; meanwhile, expression of immune checkpoints is also upregulated as a negative feedback mechanism.Citation13 The new tumor microenvironment created by chemoradiotherapy may increase the sensitivity to immunotherapy. In the KEYNOTE-590 phase 3 study, first-line pembrolizumab plus chemotherapy versus chemotherapy significantly improved survival in patients with locally advanced esophageal cancer.Citation14 And our recent phase 1b study of first-line camrelizumab concurrently combined with radiotherapy indicated manageable toxicity and antitumour efficacy for locally advanced ESCC.Citation15 Here, we performed a phase 1b study to assess the safety and feasibility of first-line camrelizumab concurrent with chemoradiotherapy in locally advanced ESCC. Prognostic biomarkers in tumor tissues and peripheral blood were also explored.
Methods
Study design and participants
We performed a single-arm, single-center, investigator-initiated, exploratory, phase 1b clinical study (ClinicalTrials.gov NCT03671265) at Tianjin Medical University Cancer Institute & Hospital in Tianjin, China.
Eligible patients were aged 18–75 years and had locally advanced ESCC confirmed by pathological and imaging diagnosis (T3-4 N0M0 or T1-4N+M0, stage I–IVa according to the 8th [2017] edition of the American Joint Committee on Cancer staging system). No patients had received prior antitumour treatment and were amenable to surgery. Other key inclusion criteria were evaluable lesions per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; an Eastern Cooperative Oncology Group performance status score of 0 or 1; life expectancy of at least 6 months; normal bone marrow reserve and blood cell counts; normal renal function; and normal liver function. Key exclusion criteria were any active autoimmune diseases or a history of autoimmune diseases; ongoing systemic immunosuppressive therapy; abnormal heart disease; pulmonary fibrosis, interstitial pneumonitis, pneumoconiosis, radiation pneumonitis, drug-associated pneumonitis, and severely impaired lung function; congenital or acquired immunodeficiency; and clinically significant concurrent cancer. The protocol (Supplement 1) was approved by the institutional review board at Tianjin Medical University Cancer Institute & Hospital (E2018142). All patients were discussed by the multidisciplinary team (MDT) in our hospital before included. All patients provided written informed consent before study participation. This study followed the Consolidated Standards of Reporting Trials (CONSORT, Figure S1 in supplement 2) reporting guideline.
Procedures
Camrelizumab was delivered concurrently with radiotherapy for 6 weeks, and with chemotherapy for 4 weeks. Camrelizumab was given intravenously over 30 minutes at a dose of 200 mg on day 1 of every 2-week period from the beginning of radiotherapy up to 32 weeks, based on previous data;Citation16,Citation17 cisplatin (25 mg/m2) plus docetaxel (25 mg/m2) were given intravenously on days 1, 8, 15, and 22 of camrelizumab treatment; apatinib (250 mg/d) was administrated orally once a day for five consecutive days per week from the week 11 to the end of camrelizumab treatment; and radiotherapy was delivered as volumetric arc intensity-modulated radiotherapy with a simultaneous integrated boost, until disease progression, death, unacceptable toxicity, withdrawal of consent, or investigator decision. The radiotherapy was given according to Chinese treatment guidelines for esophageal carcinoma,Citation4,Citation18 and was prescribed to cover 95% of the planning gross tumor volume (PGTV), given at 2.0 Gy per fraction, five fractions per week, to a total of 60 Gy over 6 weeks. The dose prescribed to cover 95% of the planning target volume (PTV) was 1.8 Gy per fraction, five fractions per week, for a total of 54 Gy over 6 weeks. Target volumes were as described previously (Methods in Supplement 2).Citation19
Patients received cervical, thoracic, and upper-abdomen CT scans and upper gastrointestinal radiography at baseline, every 8 weeks during treatment, and every 12 weeks after treatment until disease progression. All patients underwent endoscopic ultrasonography, the standard clinical practice for potential tumor biopsy, at baseline and after 40 Gy radiationCitation20 (ie, at the end of 4 weeks of radiotherapy) in order to confirm pathological response rates and perform exploration tests. Disease progression in this study was defined as local regional recurrence or distant metastasis. Laboratory tests included hematology, biochemistry, urinalysis, liver/kidney function, and immunological and hormone tests.
Health-related quality of life was assessed using European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire-core 30 (QLQ -C30) and EORTC QLQ-OES18 scales, at baseline and every 2 weeks till the last dose (Methods in Supplement 2).
Baseline tumor specimens were sequenced by FoundationOne CDx (F1CDx) and U.S. Food & Drug Administration–approved 324-gene panel assay conducted by DIAN (Hangzhou Lab, China) with licensed technologies, to detect base substitutions, short insertions and deletions, focal gene amplification and homozygous deletions, and select gene fusions and biomarkers (tumor mutation burden [TMB] and microsatellite status).Citation21
The tumor-immune microenvironment at baseline and during treatment (after 40 Gy radiation) was identified by multicolor immunofluorescence to quantify CD4+ and CD8+ T cells, CD11c+ dendritic cells, CD68+ macrophages, the immunosuppressive receptor PD-1 and its ligand PD-L1, and tumor cells labeled with pan-keratin. PD-L1 expression, evaluated by tumor proportion score (PD-L1–positive tumor cells/total tumor cells × 100%).
T, B and natural killer (NK) cells in peripheral blood were assessed by flow cytometry at baseline and during treatment (after 40 Gy radiation). Thirty-four circulating cytokines involved in chemotaxis, inflammation, immune regulation, and Th-17 cytokines, such as IP-10/CXCL10, interferon-γ, interleukin (IL)-27, IL-15, Eotaxin-3, and IL-22, were measured using an electrochemiluminescence assay (Meso Scale Discovery, Rockville, MD, USA) according to the manufacturer’s instructions. The details of methods were described in Supplement 2. Samples that were available are shown in Table S1 in Supplement 2.
Study End Points
The primary endpoints were safety and tolerability, and health-related quality of life (as measured by EORTC QLQ-C30 and EORTC QLQ-OES18). Adverse events and serious adverse events were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Secondary endpoints were radiological and pathological responses, scored according to RECIST 1.1 by individual clinicians. Outcome measures included objective response rate, overall survival (OS), and progression-free survival (PFS). A major pathological response was defined as <10% viable residual tumor cellsCitation22 on a deep biopsy collected from the tumor primary site where residual tumor cells were most likely located guided by the endoscopic ultrasound image after 40 Gy radiation.
Exploratory End Points
Exploratory analyses included the associations between the efficacy of this combination therapy and PD-L1 expression, immune microenvironment, TMB and microsatellite status in tumor tissues, and lymphocytes and cytokines in peripheral blood.
Statistical analysis
Data were analyzed from August 11, 2018 to January 31, 2021. OS and PFS were assessed by the Kaplan–Meier method. Differences in survival were compared with log-rank tests for all baseline and on-treatment biomarkers. Statistical significance between groups was compared using non-parametric two-sided Mann-Whitney U tests for two independent samples. The SPSS (v.21.0; STATA, College Station, TX, USA) or SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) were used for all analyses. All reported p values were two-sided, and the significance level was set at 0.05 (Methods in Supplement 2).
Results
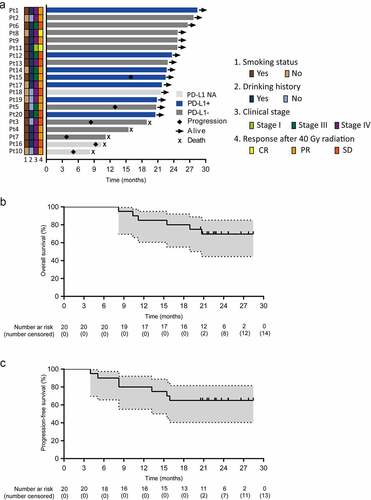
Between August 11, 2018 and May 17, 2019, 20 treatment-naïve patients with locally advanced ESCC were enrolled. One patient was at stage I (T1bN1M0), and 19 (95%) patients were at stage III–IVa. Although one patient was at stage I according to AJCC 8th stage and stage IIb according to AJCC 6th stage, he had been approved by MDT and was included. Because his tumor was located in the upper thoracic segment with a 5.2 cm length, and total laryngectomy was required if receiving surgery. All the patients received camrelizumab plus chemoradiotherapy (). By January 31, 2021, the median follow-up duration was 23.7 months (95% confidence interval [CI] 21.9–25.4) and six (30%) patients had died.
Table 1. Baseline patient characteristics
All patients received ≥52 Gy radiation. Of these, 14 (70%) completed the 60 Gy radiotherapy regimen; two (10%) refused radiotherapy after 54 and 58 Gy radiation, respectively; three (15%) were intolerant after 56 Gy radiation; and one (5%) was intolerant after 52 Gy radiation. Seventeen (85%) patients completed full cycles of camrelizumab. Three (15%) patients discontinued camrelizumab, two (10%) for progressive disease after 20 and 24 weeks, respectively, and one (5%) for esophageal fistula after 28 weeks. Ten (50%) patients completed all cisplatin plus docetaxel cycles. Ten (50%) patients completed 3 weeks of cisplatin plus docetaxel. Eleven (55%) patients completed full cycles of apatinib. Eight (40%) patients discontinued apatinib, three (15%) for progressive disease and five (25%) for intolerance. One (5%) patient did not receive apatinib treatment because of hypertension and cardiac stent implantation (Table S2 in Supplement 2).
All 20 patients were included in the safety analysis. All patients experienced some form of treatment-related adverse events (). Grade 3 adverse events occurred in nine (45%) patients, and no grade 4 or 5 adverse events were observed. Grade 3 radiation esophagitis occurred in four (20%) patients. Other grade 3 adverse events included esophageal fistula, pain, and decreased white blood cell count, each in two (10%) patients, and fatigue and cough, each in one (5%) patient. No patients died during treatment.
Table 2. Treatment-related adverse events
Serious treatment-related adverse events occurred in eight (40%) patients, including radiation esophagitis in four (20%) patients, and esophageal fistula, radiation pneumonitis, and pulmonary infection each in two (10%) patients (Table S3 in Supplement 2). Both the two patients received gastrostomy after esophageal fistula occurred. One patient developed esophageal fistula 6.1 months later after completion of radiotherapy, and died of a pulmonary infection 9.4 months after the esophageal fistula occurred (Figure S2 in Supplement 2). The other patient experienced esophageal fistula 4.3 months later after radiotherapy started (Figure S3 in Supplement 2). The OS time of these two patients was 15.5 and 21.8 months, individually. And the latter patient was still alive 17.5 months after the esophageal fistula at the follow-up cutoff time.
Immune-related adverse events occurred in 11 (55%) patients. The most common immune-related adverse events were reactive capillary endothelial proliferation in 10 (50%) patients, and hypothyroidism in three (15%) patients, all in grade 1. Other immune-related adverse events, such as enteritis, diarrhoea/colitis, hepatitis, nephritis, hypophysitis, and diabetes, were not observed.
Late adverse events (more than 6 months after radiotherapy) occurred in five (25%) patients. Of these, one (5%) experienced grade 2 esophageal stenosis, two (10%) experienced grade 1 pulmonary fibrosis, one (5%) experienced grade 1 pericardial effusion, and one (5%) experienced grade 1 pulmonary fibrosis accompanied by grade 1 pericardial effusion.
All 20 patients were included in the response analysis. Objective response rate evaluated after 40 Gy radiation showed two (10%) complete responses, 11 (55%) partial responses, and seven (35%) cases of stable disease (Table S4 in Supplement 2). Of 17 patients evaluable for pathological response by endoscopic biopsy after 40 Gy radiation, 14 (70%) were without residual tumors, two (10%) with <10% residual tumors, and two (10%) with ≥ 10% residual tumors (Table S4 in Supplement 2). Treatment effects were maintained in 14 (70%) patients at the cutoff date. Median duration of response was 21.13 months (95% CI 18.77–23.48). Tumor recurrence occurred in six (30%) patients, including one (5%) local regional failure, three (15%) cases of distant metastasis, and two (10%) cases of concurrent regional failure and distant metastasis (Table S5 in Supplement 2). The median time to recurrence was 8.25 months (95% CI 4.68–11.82).
The OS and PFS time ranged from 8.2–28.5 and 4.9–28.5 months, respectively (). Neither median OS nor median PFS were available at the cutoff date. The 12-month and 24-month OS rates were 85.0% and 69.6%, respectively (). The 12-month and 24-month PFS rates were 80.0% and 65.0%, respectively ().
Figure 1. Duration of response and survival
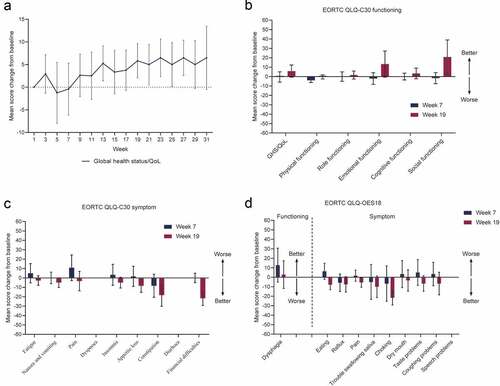
EORTC QLQ-C30 completion and compliance were close to or greater than 80% from baseline to week 27 (Table S6 in Supplement 2). EORTC QLQ-OES18 completion and compliance were almost identical to those observed for the EORTC QLQ-C30 (Table S6 in Supplement 2). Relative to baseline, mean global health status/quality-of-life score increased at week 3, then decreased to the lowest value at week 5. Subsequently, it increased and was maintained above 5 after week 13 except for a slight drop between weeks 15 and 17 ( and Supplement 3). EORTC QLQ-C30 functioning and most symptom domain scores were observed to be lowest between weeks 5 and 7 (Supplement 3 and and c). At week 19, most functioning symptom domains appeared better than at baseline. In particular, improvement was observed in the emotional functioning, social functioning, constipation symptom, and financial difficulties symptom domains at week 19 (improvement in functioning was defined as a 10-point or greater increase from baseline; symptom improvement was defined as a 10-point or greater decrease from baseline; and c). Improved EORTC QLQ-OES18 functioning and symptom domains were also observed during treatment. Dysphagia, choking, and trouble swallowing saliva were improved at weeks 7, 9, and 13, respectively (Supplement 3 and ). Other EORTC QLQ-OES18 symptoms remained relatively stable from treatment start up to week 33 (Supplement 3 and ).
Figure 2. Changes from baseline in EORTC QLQ-C30 and QLQ-OES18
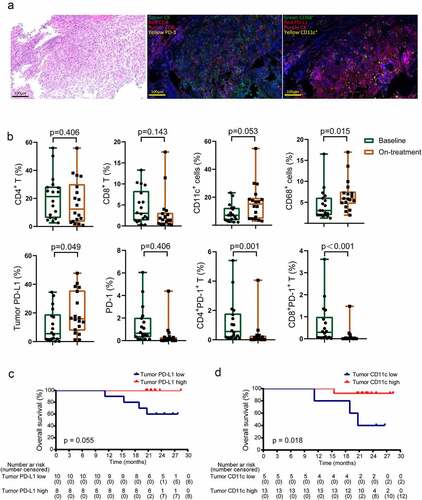
We applied multi-color immunofluorescence to dynamically monitor the tumor immune microenvironment ( and b). Of the 18 patients who were assessed for baseline PD-L1 expression, 8 (44%) had PD-L1-positive tumors using a cutoff value of 7.613%. High baseline tumor PD-L1 expression tended to be associated with longer OS (). Radiation could convert the tumor into an in situ vaccine, promoting cross-presentation of tumor-derived antigens by dendritic cells to T cells.Citation23 And macrophages programmed by radiotherapy might exhibit double activity to anti-tumor effect.Citation24 Both dendritic cells and macrophages partially contributed to the expression of PD-L1 except tumor cells. As a result, we identified dendritic cells and macrophages in the tumor microenvironment before and during treatment. We found high baseline levels of tumor CD11+ dendritic cells were related to improved OS (). None of immune-cell subsets during treatment was associated with patient survival.
Figure 3. Tumor biomarkers associated with survival
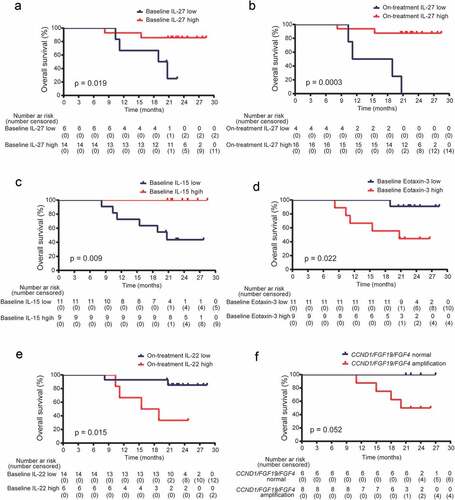
Because systematic immune response could reflect the local anti-tumor immune activity during immunotherapy.Citation25,Citation26 And radiotherapy could translate tumors from “cold” to “hot”, where the inflammatory response was robustly induced.Citation27 We evaluated the levels of immune-related cytokines in baseline and on-treatment peripheral blood from all the 20 patients. The peripheral cytokines detected here included chemotaxis, inflammation, immune regulation, and Th-17 cytokines, which have been reported to be closely associated with tumor inflammatory microenvironment.Citation28–30 We found both baseline and on-treatment IL-27 levels, and high baseline IL-15 levels in peripheral blood were associated with better OS (). While a high level of baseline Eotaxin-3, and a high level of on-treatment IL-22 correlated with worse OS (Figure D and E). The other cytokines were not related with survival. We also performed a FoundationOne CDx assay in 14 baseline tumors. The result showed all baseline tumors were microsatellite stable. The mutation count per tumor ranged from 1 to 29, with a median of 6 mutations/Mb. There was no association between TMB and survival. While, patients with amplification of any of the three genes, CCND1, FGF19, or FGF4, had borderline significantly different OS compared with those with normal expression (). We did not find the association of peripheral T, B, and NK cells with survival.
Figure 4. Gene amplification and peripheral cytokines associated with survival
Discussion
To our knowledge, this phase 1b trial is the first to study the safety and feasibility of camrelizumab combined with concurrent chemotherapy as first-line treatment in patients with local advanced ESCC. Our findings showed that the toxicity was well tolerated and the risk of deterioration in quality of life did not increase. The 24-month OS rate was 69.6%, which was higher than with trials with concurrent chemoradiotherapy without immunotherapy.Citation4,Citation5,Citation31
Chen et al.’s phase 3 study of concurrent chemoradiotherapy with a cisplatin-fluorouracil regimen in 219 patients with ESCC (all Chinese) showed the 24-month OS rate was 61.5%.Citation4 A 44.0% 24-month OS rate was reported in the phase 3 NRG Oncology RTOG 0436 trial (38.1% ESCC vs 61.9% esophageal adenocarcinoma).Citation5 The local regional failure was 34.2%Citation4 and 48.8%Citation5 in these two studies. In the present report, median OS and PFS were not available at the data cutoff (23.7 months median follow-up time). However, our findings showed the 24-month OS rate was 69.6% and local regional failure was 15.0%. Although we acknowledge the limitations of cross-trial comparisons, the addition of anti-PD-1 to concurrent chemoradiotherapy may probably improve survival and decrease recurrence compared with concurrent chemoradiotherapy.
The toxicity profile was similar to previous reports of either modality given alone in patients with advanced ESCC.Citation4,Citation5,Citation12,Citation17,Citation31 All adverse events were no more than grade 3 and manageable. The incidence of radiation pneumonitis did not increase in our study compared with concurrent chemoradiotherapy (10% vs 9.6–20.3% in grade 2; 0% vs 1.8–7.4% in grade 3).Citation4,Citation5,Citation31 The two patients who developed esophageal fistula both had T4 stage tumors, and one tumor was an ulcerative type (Figure S2 and 3 in Supplement 2). The incidence of esophageal fistula was 10% (2/20) in our study, which accounted for 20% in all 10 patients with T4 tumors. T4 stage and ulcerative ESCC were risk factors associated with esophageal fistula.Citation32,Citation33 High esophageal fistula rates of 22% (31/140),Citation34 24% (28/116)Citation35 and 30.1% (41/136)Citation36 were reported in patients with ESCC and receiving definitive chemoradiotherapy, which accounted for 76%,Citation34 100%Citation35 and 100%Citation36 in all patients with T4 or T4b tumors, individually. Relative low rate of 10.38% (22/212) was also reported in ESCC patients who received definitive chemoradiotherapy, which accounted for 15.09% in T4 patients.Citation37 Combined chemoradiotherapy and anti-PD-1 antibody did not increased the occurrence of esophageal fistula (10% vs 5–24%).Citation32 We found more frequent radiation esophagitis compared with concurrent chemoradiotherapy (40% vs 16.9–32.7% in grade 2; 20% vs 3.2–5.6% in grade 3),Citation4,Citation5,Citation31 which might be due to the relatively higher radiation dose per fraction (2 Gy vs 1.8 Gy),Citation4,Citation5,Citation31 total radiation dose (60 Gy vs 54 Gy), Citation5,Citation31 and incorporation of the anti-PD-1 antibody in the present trial.
Reactive capillary endothelial proliferation was the most common immune-related adverse event reported in camrelizumab monotherapy (75–79%).Citation12,Citation17 In our recent phase 1b study of camrelizumab combined with radiotherapy in locally advanced ESCC, it was observed in 89% (17/19) patients (79% [15/19] grade 1, 10% [2/19] grade 2).Citation15 Apatinib independently developed in China is an oral small-molecule tyrosine kinase inhibitor that selectively binds to and inhibits vascular endothelial growth factor receptor 2, with a decrease in VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular density. Apatinib has been approved for use in gastric adenocarcinoma or gastroesophageal junction in China.Citation38 The latest phase 2 study of camrelizumab plus apatinib and chemotherapy in advanced ESCC reported the incidence of reactive capillary hemangiomas at 60% (18/30) and high objective response rate at 80%.Citation39 In the present study, we added apatinib during the maintenance treatment of camrelizumab originally to decrease the occurrence of reactive capillary endothelial proliferation. The result showed reactive capillary endothelial proliferation occurred in a much lower frequency (50%, [10/20]), and was all in grade 1 without special treatment. These results suggest that combining camrelizumab with apatinib could inhibit reactive cutaneous capillary endothelial proliferation. We also found noninfectious pneumonia and dermatitis occurred in three (15%) patients each. However, it was hard to determine whether these resulted from activated immune response or radiotherapy.
Camrelizumab reduces the risk of deterioration in quality of life compared with chemotherapy in the second-line setting.Citation12 In our studies, we found that the risk of deterioration in quality of life does not increase when adding camrelizumab to chemoradiotherapy compared with chemoradiotherapy alone.Citation40,Citation41 Similar to the previous reports in esophageal cancerCitation12,Citation42 and non-small cell lung cancer,Citation43–45 large variations were observed in each domain. These variations were partially associated with limited sample size, and most probably resulted from large individual differences in the quality of life measures. However, we could find the obvious tendency that quality of life deteriorated most after chemoradiotherapy, and then improved during camrelizumab-continued treatment, indicating chemoradiotherapy may have played a more important role in deterioration in quality of life than camrelizumab. Because of the single-arm design of our study, we could not eliminate the contribution of gradual recovery after chemoradiotherapy. We will further focus on the health-related quality of life in ESCC patients receiving the combination therapy of radiotherapy and immunotherapy in the future studies.
The increased PD-L1 expression and CD11c+-cell infiltration, and decreased PD-1 expression, in tumors during treatment suggested that anti-PD-1 combined with chemoradiotherapy could harmonize the tumor-immune microenvironment. It was probably this combination treatment not only reactivated antitumour immune response, but also promoted T-cell priming by inducing tumor antigen presentation. Patients with high PD-L1 expression tended to have longer survival, although this did not become a statistically significant difference. In the KEYNOTE-181 study, patients with high PD-L1 expression (PD-L1 CPS ≥10) had better survival after pembrolizumab versus chemotherapy.Citation8 In the ESCORT study, high tumor PD-L1 expression (PD-L1 ≥ 1%) was associated with improved survival in camrelizumab subgroup compared with chemotherapy subgroup.Citation12 In the ATRACTION-3 study, patients benefit more from nivolumab than chemotherapy irrespective of PD-L1 expression.Citation7 The PD-L1 threshold here was 7.613% by using fluorescent staining. The different cutoff value of positive PD-L1 expression in these studies indicates that different anti-PD-1 antibodies (22C3, 6E8 or E1L3N), testing methods and treatment regimens might influence the correlation of PD-L1 status and treatment outcome. The fluorescent staining used in testing PD-L1 expression has a higher sensitivity compared with conventional immunochemistry assay.
We found both high level of IL-27 and high level of IL-15 in peripheral blood were associated with better survival. The cytokine IL-15 has a key role in promoting survival, proliferation and activation of natural killer and T cells.Citation46,Citation47 IL-27 had double ways in antitumor efficacy.Citation48 Both IL-15 and IL-27 contribute to maintain homeostasis of memory T cells.Citation49,Citation50 As far, their association with survival in checkpoint blockade with or without conventional anti-tumor therapies was little known. Eotaxin-3 was the most highly induced gene in eosinophilic esophagitis patients compared with its expression level in healthy individuals.Citation51 Eotaxin-3 had two receptors CCR3 and CX3CR1. Eotaxin-3 expressed by hepatocellular carcinoma cells could recruit CX3CR1+ MDSCs to the tumor tissue.Citation52 IL-22 is exclusively produced by immune cells. Increased numbers of intratumoral and peripheral IL-22 secreting cells have been reported in lung, gastric, colorectal, pancreatic and hepatocellular carcinomas and enhanced tumor progression.Citation53 Neither Eotaxin-3 nor IL-22 was reported in esophageal cancer. Our findings in peripheral blood illustrate the important role of systemic immune response in antitumour treatment and provide convenient biomarkers for patient selection. Lastly, for patients with CCND1/FGF19/FGF4 amplification, combining targeted therapy might improve treatment efficiency, and deserves further study.
This study has some limitations. Firstly, small numbers of patients were included. Secondly, the high fractional radiation dose (2 Gy) and total radiation dose (60 Gy) might increase the incidence of adverse events. Thirdly, the potential biomarkers which were associated with survival need to be confirmed.
In conclusion, this is the first study to show that camrelizumab combined with concurrent chemoradiotherapy as first-line therapy has a promising efficacy and a tolerable safety profile in patients with locally advanced ESCC. Our results further revealed several potential immune biomarkers in tumor tissues and peripheral blood. Based on these findings, a phase 3, randomized, double-blind, placebo-controlled study of camrelizumab versus placebo in combination with concurrent chemoradiotherapy in locally advanced ESCC (NCT04426955) is ongoing. In this phase 3 study, we adjusted the radiation dose according to the National Comprehensive Cancer Network Guideline 2020 (1.8 Gy/fraction, total 50.4 Gy), with the expectation to further increase therapeutic efficiency and safety.
Acknowledgments
Author contributions: WZ and CY contributed equally to this trial. WZ, CY, PW, and QP conceived and designed the study. WZ, CY, TZ, XC, JD, JZ, DH, GZ, FC, and DZ collected the data. WZ and CY analyzed and interpretated the data. JW, HJ, PT, LZ, ZY and QW reviewed literature and validated the data. All authors participated in writing, reviewing, and approving the manuscript for submission.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–11.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132.
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr., Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–1627.
- Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, Tang H, Fan M, Li L, Lin Q, et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: a Randomized, Multicenter, Phase III Clinical Trial. J Clin Oncol. 2019;37(20):1695–1703.
- Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar H, Horiba N, et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: the NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017;3(11):1520–1528.
- Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak M, Dionne D, Xia J, Rozenblatt-Rosen O, et al. Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1(-)CD8(+) Tumor-Infiltrating T Cells. Immunity. 2019;50(1):181–194. e186.
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517.
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol. 2020;38(35):4138–4148
- Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580.
- Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, Li X, Liu J, Ku W, Zhang Y, et al. Addition of Low-Dose Decitabine to Anti-PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma. J Clin Oncol. 2019;37(17):1479–1489.
- Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350.
- Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842.
- Kelly RJ, Zaidi AH, Smith MA, Omstead AN, Kosovec JE, Matsui D, Martin SA, DiCarlo C, Werts ED, Silverman JF, et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann Surg. 2018;268(6):992–999.
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, T, Kojima T, Metges JP, Li Z, Kim SB, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759-771.
- Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, Zhao J, Er P, Zhang T, Chen X, et al. Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma. Oncologist. 2021;26(7):e1110-e1124.
- Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: an Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25(2):515–523.
- Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24(6):1296–1304.
- Esophageal Carcinoma Cooperative Group of Radiation Oncology Society of Chinese Medical Association. Treatment guideline of radiotherapy for Chinese esophageal carcinoma (draft). Chin J Cancer. 2010;29(10):855–859.
- Wang D, Zhang W, Qian D, Guan Y, Chen X, Zhang H, Wang J, Pang Q. Efficacy and safety of weekly nab-paclitaxel plus cisplatin with concurrent intensity-modulated radiotherapy in patients with inoperable, locally advanced esophageal cancer: a pilot trial. Onco Targets Ther. 2018;11:6333–6338.
- Xie Y, Wang Q, Cao B, Lv J, Wang Y, Wu L, Dong M, Li T. Textural features based enhanced contrast CT images predicts prognosis to concurrent chemoradiotherapy in stage III esophageal squamous cell cancer. Cancer Biomark. 2020;27(3):325–333.
- Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031.
- Qian D, Wang Y, Zhao G, Cao F, Er P, Chen X, Cheng J, Zhang W, Li X, Zhang B, et al. Tumor Remission and Tumor-Infiltrating Lymphocytes During Chemoradiation Therapy: predictive and Prognostic Markers in Locally Advanced Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2019;105(2):319–328.
- Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–7422.
- Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini JL. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017;40:200–211.
- Axelrod ML, Nixon MJ, Gonzalez-Ericsson PI, Bergman RE, Pilkinton MA, McDonnell WJ, Sanchez V, Opalenik SR, Loi S, Zhou J, et al. Changes in Peripheral and Local Tumor Immunity after Neoadjuvant Chemotherapy Reshape Clinical Outcomes in Patients with Breast Cancer. Clin Cancer Res. 2020;26(21):5668–5681.
- Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918.
- McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–217.
- Nalbant A. IL-17, IL-21, and IL-22 Cytokines of T Helper 17 Cells in Cancer. J Interferon Cytokine Res. 2019;39:56–60.
- Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019;39:6–21.
- Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572.
- De Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, de Araujo Lima Franca B, Del Giglio A, Lazaretti NS, Alvares MN, Pedrini JL, et al. A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer. 2018;88:21–30.
- Zhu C, Wang S, You Y, Nie K, Ji Y. Risk Factors for Esophageal Fistula in Esophageal Cancer Patients Treated with Radiotherapy: a Systematic Review and Meta-Analysis. Oncol Res Treat. 2020;43:34–41.
- Hu B, Jia F, Zhou H, Zhou T, Zhao Q, Chen Y, Li B, Huang W. Risk Factors Associated with Esophageal Fistula after Radiotherapy for Esophageal Squamous Cell Carcinoma. J Cancer. 2020;11(12):3693–3700.
- Tsushima T, Mizusawa J, Sudo K, Honma Y, Kato K, Igaki H, Tsubosa Y, Shinoda M, Nakamura K, Fukuda H, et al. Risk Factors for Esophageal Fistula Associated With Chemoradiotherapy for Locally Advanced Unresectable Esophageal Cancer: a Supplementary Analysis of JCOG0303. Medicine (Baltimore). 2016;95(20):e3699.
- Kawakami T, Tsushima T, Omae K, Ogawa H, Shirasu H, Kito Y, Yoshida Y, Hamauchi S, Todaka A, Machida N, et al. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer. 2018;18(1):573.
- Chen B, Deng M, Yang C, Dragomir MP, Zhao L, Bai K, Xi M, Hu Y, Zhu Y, Li Q. High incidence of esophageal fistula on patients with clinical T4b esophageal squamous cell carcinoma who received chemoradiotherapy: a retrospective analysis. Radiother Oncol. 2021;158:191–199.
- Zhang Y, Li Z, Zhang W, Chen W, Song Y. Risk factors for esophageal fistula in patients with locally advanced esophageal carcinoma receiving chemoradiotherapy. Onco Targets Ther. 2018;11:2311–2317.
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34(13):1448–1454.
- Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, Wang X, Bai L, Huang J. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond). 2020;40(12):711–720.
- Qiu Y, You J, Wang K, Cao Y, Hu Y, Zhang H, Fu R, Sun Y, Chen H, Yuan L, et al. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial. Nutrition. 2020;69:110558.
- Yamashita H, Omori M, Okuma K, Kobayashi R, Igaki H, Nakagawa K. Longitudinal assessments of quality of life and late toxicities before and after definitive chemoradiation for esophageal cancer. Jpn J Clin Oncol. 2014;44:78–84.
- Noordman BJ, Verdam MGE, Lagarde SM, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: results From the Randomized CROSS Trial. J Clin Oncol. 2018;36(3):268–275.
- Garassino MC, Gadgeel S, Esteban E, Felip E, Speranza G, Domine M, Hochmair MJ, Powell S, Cheng SY, Bischoff HG, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(3):387–397.
- Barlesi F, Garon EB, Kim DW, Felip E, Han JY, Kim JH, Ahn MJ, Fidler MJ, Gubens MA, de Castro G, Jr., et al. Health-Related Quality of Life in KEYNOTE-010: a Phase II/III Study of Pembrolizumab Versus Docetaxel in Patients With Previously Treated Advanced, Programmed Death Ligand 1-Expressing NSCLC. J Thorac Oncol. 2019;14(5):793–801.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609.
- Lin JX, Leonard WJ. The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harb Perspect Biol. 2018;10:9.
- Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82.
- Beizavi Z, Zohouri M, Asadipour M, Ghaderi A. IL-27, a pleiotropic cytokine for fine-tuning the immune response in cancer. Int Rev Immunol. 2021;40:319–329.
- Tkachev V, Kaminski J, Potter EL, Furlan SN, Yu A, Hunt DJ, McGuckin C, Zheng H, Colonna L, Gerdemann U, et al. Spatiotemporal single-cell profiling reveals that invasive and tissue-resident memory donor CD8(+) T cells drive gastrointestinal acute graft-versus-host disease. Sci Transl Med. 2021;13:576.
- Huang Z, Zak J, Pratumchai I, Shaabani N, Vartabedian VF, Nguyen N, Wu T, Xiao C, Teijaro JR. IL-27 promotes the expansion of self-renewing CD8(+) T cells in persistent viral infection. J Exp Med. 2019;216(8):1791–1808.
- Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–547.
- Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL, Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology. 2016;64(3):797–813.
- Markota A, Endres S, Kobold S. Targeting interleukin-22 for cancer therapy. Hum Vaccin Immunother. 2018;14:2012–2015.
