ABSTRACT
Combination immunotherapy with sequential administration may enhance metastatic melanoma (MM) patients with long-term disease control. High Dose Aldesleukin/Recombinant Interleukin-2 (HD rIL-2) and ipilimumab (IPI) offer complementary mechanisms against MM. This phase IV study assessed the sequenced use of HD rIL-2 and IPI in MM patients. Eligible Stage IV MM patients were randomized to treatment with either two courses of HD rIL-2(600,000 IU/kg) followed by four doses of IPI 3 mg/kg or vice-versa. The primary objective was to compare one-year overall survival (OS) with historical control (46%, Hodi et al., NEJM 2010). Secondary objectives were 1-year progression-free survival (PFS), objective response rate (ORR), and adverse events (AEs) profile. Evaluable Population (EP) included patients who received at least 50% of planned treatment with each drug. Thirteen and 16 patients were randomized to receive HD rIL-2 first, and IPI first, respectively. One-year OS rate was 75% for intention to treat population. Eighteen patients were included in EP, 8 in HD rIL-2, 10 in IPI first arm. In EP, 1-year OS, PFS and ORR rates were 87%, 68%, and 50%, respectively. The frequency of AEs was similar in both arms with 13 patients experiencing Grade 3 or higher AEs, 3 resulting in the end of study participation. There was one HD rIL-2-related death, from cerebral hemorrhage due to thrombocytopenia. In this study with small sample size, HD rIL-2 and IPI were safe to administer sequentially in MM patients and showed more than additive effects. 1-year OS was superior to that of IPI alone from historical studies.
INTRODUCTION
Melanoma causes most skin cancer-related deaths. The estimated number of new melanoma cases in the U.S. in 2020 is 100,350, with 6,850 estimated deaths, and the number of new cases has been increasing for the past few decades.Citation1 Before the development of new therapies, five-year overall survival (OS) of metastatic melanoma (MM) was as low as 2%Citation2 two decades ago, increasing to 16%Citation3 a decade ago and now up to 52% with new therapiesCitation4 Recombinant Interleukin-2 was first described in 1976 as a T cell growth factorCitation5,Citation6 and quickly found its way to clinical trials in the high-dose form.Citation7 High-dose interleukin-2 (HD rIL-2) was the first successful immunotherapy in melanoma and was approved by the U.S. Food and Drug Administration (FDA) for melanoma in 1998.Citation8 Analysis of seven HD rIL-2 trials, enrolling 270 patients, showed an objective response rate (ORR) of 16%, of which 6% were of complete response (CR), especially in fit patients without previous immunotherapy exposure.Citation2 Critically, these responses were found to be durable without relapses after 30 months in responders. The median duration of the CR was more than 59 months.Citation9 Through these trials, HD rIL-2, for the first time, raised the expectations of curing select MM patients despite the drawbacks of the severe adverse events (AEs).
Development of the immune checkpoint inhibitors of cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) was a groundbreaking event for MM patients. Ipilimumab (IPI), monoclonal antibody blocking CTLA-4, augments T-cell activation, and proliferation.Citation10,Citation11 Hodi et al. conducted a phase III study comparing IPI alone versus IPI plus gp100 versus gp100 alone in patients with MM.Citation12 The control, gp100, was a Human Lymphocyte Antigen-restricted peptide vaccine, which was not anticipated to have significant activity as monotherapy. The median OS with gp100 alone was 6.4 months, whereas, for IPI alone and IPI with gp100, the median OS was 10 months. The hazard ratio for death was significantly reduced by IPI alone (p = .003) and IPI plus gp100 (p < .001) when compared to gp100 alone. The one-year OS rate for the IPI alone cohort was 46%.
Both HD rIL-2 and IPI are effective immunotherapies for the treatment of MM, but their mechanisms of action differ. IL-2 is a cytokine stimulant for T cells, NK cells, and B cells, whereas IPI is an antibody that blocks the binding site of CTLA-4, a receptor that downregulates T cells when it is attached to its ligand. Theoretically, the two agents could demonstrate synergy in the clinic. The rationale for synergy is based on the idea that IL-2 turns on the immune system while IPI prevents the immune system from turning off. Previously, a phase I/II study of the two drugs in combination was conducted at the National Cancer Institute (NCI) and reported in 2005.Citation13 In this study, 36 patients were treated with an initial dose of IPI followed by two cycles of both drugs at three-week intervals and concluded by a final dose of IPI. The combination was tolerated with AEs consistent with the use of both drugs when given alone. An ORR of 22% was observed with a 9% CR rate. Subsequently, two additional cycles were given to patients whose tumors did not progress and who did not experience dose-limiting toxicities and were reevaluated for the response after every two cycles. When the cohort of patients was followed up 5–6 years after treatment, 17% had an unmaintained CR.Citation14
There is concern for overlapping toxicity when these agents are given in combination, and thus a sequential strategy could be attempted. It is unclear whether the effects of these drugs would be additive or synergistic when used in sequence. This randomized, two-arm, multicenter phase IV study was designed to answer the question about the additive or synergistic effects of the drugs when closely sequenced in the initial therapy of MM. In addition, it provided insight into whether the sequence of HD rIL-2 and IPI influences the ability to treat with both drugs, the toxicity associated with the drugs, and if the order of administration is essential in inducing and maintaining responses.
MATERIALS AND METHODS
Patients
Patients were eligible if they had confirmed MM with at least one measurable lesion per immune-related response criteria (irRC),Citation15 were 18 years or older, treatment naïve, or had previously received only one systemic therapy apart from adjuvant therapy. At least four weeks since the last adjuvant therapy or other cancer treatment must have passed for the patients to receive any treatments in the study. Patients with untreated brain metastases were ineligible; however, patients with brain metastases that had been treated, which no longer required corticosteroid therapy and were without progression by MRI at least six weeks after definitive therapy, were allowed entry. Before starting the study procedures, all patients provided written informed consent.
Study design and treatments
This was an open-label, randomized, two-arm, multicenter phase IV study that assessed the sequence of HD rIL-2 and IPI in patients with MM. Eligible subjects were randomly assigned (1:1 randomization scheme) to one of two treatment arms. The primary objective of the study was to compare one-year OS of the intention to treat population (ITT) in each treatment arm separately with historical control in the pivotal Phase III ipilimumab study by Hodi and colleagues.Citation12
HD rIL-2 was given as intravenous (IV) bolus, at the dose of 600,000 IU/kg, every 8 hours, up to 14 doses per cycle, and one course was two cycles of HD rIL-2. HD rIL-2 is given as one course (2 cycles) in patients who progress and 2–3 courses of the drug in patients who respond or have stable disease after course 1. IPI was administered as an IV infusion at the dose of 3 mg/kg every 3 weeks, up to 4 doses per course regardless of disease status during treatment. Dose reductions of the two agents (e.g., for toxicity) were not allowed, in favor of either skipping one or more doses or permanently discontinuing one or both agents, consistent with labeling.
A course of HD rIL-2 was defined as 2 cycles (with up to 14 doses per cycle) of HD rIL-2. A course of IPI was 4 cycles (one dose per cycle) of IPI. In treatment arm 1, subjects received one or two courses (two to four cycles) of HD rIL-2 followed by one course (four doses) of IPI. In treatment arm 2, subjects received one course (four doses) of IPI followed by two courses (four cycles) of HD rIL-2 (see Figure, Supplementary , which demonstrates the study schema).
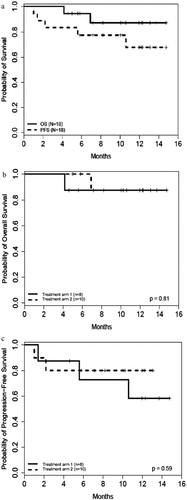
Figure 1. Kaplan-Meier Survival Curves for Overall and Progression-Free Survival in Evaluable Population
A 3–6-week interval between the administration of the two drugs was planned. Investigators adjusted this interval as necessary to resolve treatment-related toxicities, especially discontinuation of corticosteroids. Subjects requiring corticosteroid treatment for IPI toxicities were to have steroid treatment discontinued at least 2 weeks before treatment with HD rIL-2. HD rIL-2 toxicities were likewise required to have resolved or stabilized before starting treatment with IPI.
Patients were scheduled for four post-baseline disease response assessments: between 5 and 11 weeks, 13–19 weeks, 24–30 weeks, and one year after initiating therapy in either treatment arm. The timing of the response assessments could be adjusted to facilitate clinical procedures and treatment decisions at the investigator’s discretion. Patients with stable or responding disease continued per protocol. Patients with progressive disease (PD) without substantial clinical deterioration after receiving at least 50% of the first drug doses (defined as 1 cycle of HD rIL-2, or 2 doses of IPI), either continued per protocol or transferred to the second drug at the investigator’s discretion. Patients with a rapid PD requiring systemic non-immunologic therapy were removed from the study. Patient treatment tolerability and safety events were monitored and managed.
The primary endpoint of the study was the one-year OS rate. Secondary endpoints included progression-free survival (PFS), ORR, number of planned treatments received, safety, and tolerability for each treatment arm sequence.
ITT included all randomized subjects. The Safety Population (SP) was identified as all randomized subjects who received at least one dose of either study drug. The Evaluable Population (EP) consisted of all randomized subjects who received at least 50% of the planned cycles of therapy of both study drugs (i.e., ≥2 doses IPI and ≥1 cycle HD rIL-2). Subjects who exhibited objective PD or died before the end of one course of treatment were also considered evaluable.
Assessments
Tumor response was assessed by computerized tomographic scans at screening (up to 6 weeks before intervention), 5–11 weeks, 13–19 weeks, 24–30 weeks, and one year after the initiation of the treatment. The irRC was determined based on tumor burden calculated by the World Health Organization (WHO)_method of summation of the multiplied perpendicular dimensions of all lesions are summed to obtain the tumor burden.Citation15
Clinical and laboratory assessments for safety and toxicity evaluations were carried out at baseline and consecutive clinical appointments in 5–11 weeks, 13–19 weeks, 24–30 weeks, and one year after the first treatment. The severity of the adverse events was graded using the NCI’s Common Toxicity Criteria (NCI-CTCAE), version 4.03.Citation16
Statistical analysis
OS was computed from the start of the first treatment date to date of death from any cause, and patients alive at their last evaluation date were censored. OS was estimated using the Kaplan-Meier method, and the difference between treatment arms was evaluated using the log-rank test. One-year OS rates, along with 95% confidence intervals (CI), were estimated for the entire subject population and each treatment arm separately.
The historical control was a randomized phase III trial comparing IPI alone versus IPI plus gp100 versus gp100 alone in patients with MM.Citation12 In that study, the one-year OS for patients randomized to IPI alone (ITT) was 46%. The primary objective of the present study was to compare the one-year OS of the ITT within each treatment arm separately with historical control. Using a one-year OS rate of 46% for the historical control, and assuming a one-year OS rate of 60% for each treatment arm, an accrual rate of 2 years, and a follow-up time of 3 years, a sample size of 50 patients for each treatment arm was initially estimated to provide 88% power to detect the 14% difference in OS rates using a one-sided alpha (α) level of 0.05. The power computation was determined using the statistical tools on the SWOG Statistical Center website.Citation17
Provided the early termination of enrollment without reaching the target accrual, the observed one-year OS rates of EP in entire cohort was compared with the historical control rate of 46%,Citation12 using a one-sample binomial test (one-sided).
PFS was computed from the start of the first treatment date to the date of objective PD or death from any cause, and patients alive who did not experience objective PD were censored at their last evaluation date. PFS was estimated using the Kaplan-Meier method, and the difference between treatment arms was evaluated using the log-rank test. One-year PFS rates, along with 95% CI, were estimated for the entire subject population and each treatment arm separately.
Planned treatment received was evaluated in both treatment arms. The number of IPI and HD rIL-2 doses and cycles were counted for each treatment arm. The difference in the number of cycles between treatment arms was assessed using Fisher’s exact test. The difference in the number of doses between treatment arms was assessed using Student’s t-test.
CR, partial response (PR), stable disease (SD), and PD were reported using irRC.Citation18 CR rate, ORR (CR + PR), and disease control rate (DCR = CR + PR + SD) were summarized with frequencies and percentages by treatment group and both arms combined in the EP. The difference in tumor response between treatment arms was assessed using Fisher’s exact test.
The best response was defined as the best objective response achieved at any assessment during the study. Duration of best response was computed from the date of the best response to death or the last evaluation date. The difference in duration of best response between the two treatment arms was assessed by the Wilcoxon rank sum test (exact p-value).
RESULTS
Patients and treatment
Initially, enrolling 100 patients, 50 in each treatment arm, was expected to have a study power of 88% to detect a difference in OS. However, the study was terminated after randomizing 29 patients from September 2013 through August 2015 due to slow enrollment. Three patient populations were identified for further analysis, including ITT, EP, and SP, as described in the methods section. Thirteen patients were randomized to treatment arm 1 and 16 patients to treatment arm 2 in the ITT group, where all randomized subjects were included. One patient in treatment arm 1 withdrew consent before starting any treatment. SP consisted of all randomized subjects who received at least one dose of either study drug, including 12 patients in treatment arm 1 and 16 patients in treatment arm 2. EP was defined as all patients who completed at least 50% of the expected therapy cycles of both research drugs (≥2 doses IPI and ≥1 cycle HD rIL-2). In EP, treatment arm 1 and 2 consisted of 8 and 10 patients, respectively.
The baseline characteristics of the patients in the ITT are described in . In ITT, there were 17 (58.6%) patients that had mutation testing. BRAF was the most frequent mutation identified, as it was reported by 9 (31.0%) patients. Additionally, two patients (6.9%), one from each treatment arm, tested positive for an NRAS mutation. Twenty-two out of 29 (75.9%) patients in ITT, 11 patients from each treatment arm received prior treatment before enrolling in the clinical trial. None of the patients received prior dacarbazine, temozolomide, platinum, vinca alkaloids, paclitaxel, or nitrosourea. No patients had prior treatment with antibodies against PD-1 or PD-L1. Details of the treatments are given in . The most common metastatic sites were lymph nodes (44.8%), lung (37.9%), liver (17.2%), and bone (13.8%), similar in both treatment arms ().
Table 1. Baseline Characteristics of the Patients
There were 22 patients in ITT who received at least one dose of HD rIL-2; 12 in treatment arm 1 and 10 in treatment arm 2. On the other hand, at least one dose of IPI was given to 26 patients, 10 and 16 patients in treatment arm 1 and 2, respectively. Comparative analysis was conducted for certain aspects of the treatment with HD rIL-2 and IPI (i.e., dosing, duration, compliance). Dosing information details of the treatment with HD rIL-2 and IPI by treatments arm 1, 2, and all EP were presented in Supplementary (see Supplementary , which shows the dosing of HD rIL2 and IPI for each treatment arms in EP). There was no statistically significant difference between the treatment arms in mean total doses (p-value = 0.76) of HD rIL-2 or IPI (p-value = 0.19). In addition, no statistically significant differences were observed between the treatment arms in the highest cycle completed for IPI (p-value = 0.76) and HD rIL-2 (p-value = 1.0) or the level of HD rIL-2 compliance (p-value = 0.27). The most common reason for stopping treatment with IPI and HD rIL-2 was the completion of the planned dosing.
Seven of the patients (24.1%) enrolled in the study completed all study requirements. The most common reason patients discontinued early from the study was PD, which was seen in 10 out of the 29 (34.5%) patients, followed by other reasons (4 patients, 13.8%), AEs (3 patients, 10.3%) and death (3 patients, 10.3%). One patient withdrew consent in each treatment arm (). Other reasons included discontinuation from the study due to noncompliance for one patient in treatment arm 2 and study closure by the sponsor for three patients, one in treatment arm 1, and two in treatment arm 2. Two deaths occurred in treatment arm 2 due to PD, but not due to treatment-related adverse events. One treatment-related death occurred in treatment arm 1. The patient experienced Grade 4 hypoxia, acute respiratory failure, and Grade 5 cerebral hemorrhage from thrombocytopenia after receiving cycle 1 of HD rIL-2 and deceased from the complications of these adverse events during hospitalization. Details of the reasons for study discontinuation are presented in .
Table 2. Reason for End of Study
Efficacy
OS, PFS and, best response data are presented in , and Kaplan-Meier survival curves are shown in . Median follow up of all EP was 10.1 months; 11.3 and 9.1 months for treatment arm 1 and 2, respectively.
Table 3. Summary of Outcomes of EP
Median OS was not reached in EP at one-year. The estimated one-year OS rate for the 18 patients in EP was 87% (95% CI: 57–97%). One-year OS rate in both treatment arms in EP was 88% (95% CI: 39–98%) (p = .81).
The estimated one-year OS rate for all 29 patients in the ITT was 75% (95% CI: 51–88%), with the median OS not reached. The estimated one-year OS rates were 73% (95% CI: 38–91%) and 75% (95% CI: 40–92%) for treatment arm 1 and 2, respectively. Statistically significant differences were observed for both and individual treatment arms of ITT compared to the historical control of 46%Citation12 (p-value = 0.001, 0.03, and 0.01 for all ITT, treatment arm 1 and 2, respectively).
Median PFS was not reached for patients in the EP. The one-year estimated PFS rate was 68% (95% CI: 37–86%). The one-year PFS rate in treatment arm 1 in EP was 58% (95% CI: 18–84%), while 80% (95% CI: 41–95%), in treatment arm 2 (p-value = 0.59).
In EP, CR rate was 17%, PR rate was 33%, ORR was 50%, and DCR was 83% in both treatment arms combined. In treatment arm 1, 1 CR (13%) and 3 PRs (38%) were observed, while 2 patients had CR (20%) and 3 patients had PR (30%) in treatment arm 2 (). There was no statistically significant difference in tumor response between the two treatment arms (p = 1.00) ().
The median duration of the best response (CR+PR) for all EP was 6.8 months with a range of 4.2 to 11.0 months of response. The median duration of best response was 6.9 months for treatment arm 1, and 6.8 months for treatment arm 2 (p = .56) (). The median duration of clinical benefit, defined as CR/PR/SD, is 6.8 months (range: 1.5– 11.0 months) for all patients (n = 15); 7.0 months (range: 2.1– 10.7 months) for patients in treatment arm 1 (n = 7); 6.5 months (range: 1.5– 11.0 months) for patients in treatment arm 2 (n = 8) ( and ).
Figure 2. Swimmer’s Plot for Efficacy in Evaluable Population
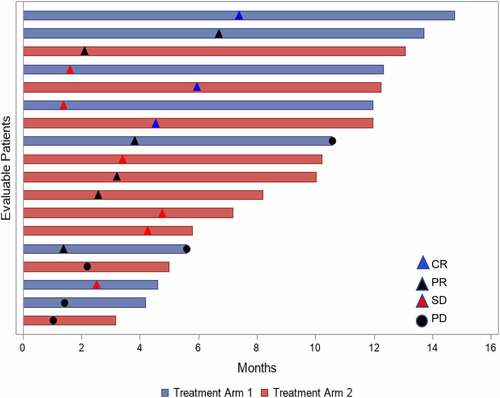
In treatment arm 1, of the 8 patients who had response assessment after each treatment, 6 patients had the same response after both treatments. One patient had SD after HD rIL-2 followed by PR after IPI, whereas another patient had PR after HD rIL-2, then PD after IPI. In treatment arm 2, 7 patients had response assessment after each treatment and 3 patients had the same response with both treatments. Notably, 4 patients had potentiation of the response with 2 patients having PR with IPI then CR after HD rIL-2, one patient with PD on IPI followed by SD after HD rIL-2; and one patient SD with IPI then PR with HD rIL-2.
Adverse events
Common AEs that happened in two or more patients, as well as CTCAE version 4.03 Grade 3 and 4 or more AEs that were reported for the SP (n = 28), are described in . AEs were listed in descending order of system organ class then preferred term. Overall, there were 19 patients (68%) that reported at least one AE; nine patients (75%) in treatment arm 1 and 10 (63%) patients in treatment arm 2. Thirty AEs were reported as serious by 10 patients; five patients in each treatment arm. The most common AEs were acute kidney injury (10 patients, 36%), diarrhea (5 patients, 18%), back pain (4 patients, 14%), peripheral edema (4 patients, 14%), hypotension (4 patients, 14%), and thrombocytopenia (4 patients, 14%) ().
Table 4. Adverse Events
Seven patients (25%) from the SP with 23 AEs were reported as immune-mediated; three patients in treatment arm 1 (25%) and four patients (25%) in treatment arm 2. The most frequently reported irAEs were gastrointestinal disorders with severity ranging from Grade 1 to Grade 5. Two patients had Grade 4 irAEs. One of the patients was in treatment arm 1 receiving HD rIL-2, and had Grade 4 thrombocytopenia, hypoxia, respiratory failure, supraventricular and ventricular tachycardia, as well as a Grade 5 intracranial hemorrhage resulting in death from AEs. All of these irAEs were related to HD rIL-2 as they happened following cycle 1 of HD rIL-2 treatment before receiving IPI. The other patient with a Grade 4 AE was from treatment arm 2 and had colitis and intestinal perforation that was related to IPI, causing the conclusion of IPI treatment. This patient withdrew consent and never received HD rIL-2 while on study. In treatment arm 2, two patients had repeating irAEs. One patient had a repeating renal failure (Grade 3), while the other patient had a repeating hyperbilirubinemia (Grade 1 and Grade 3), which were definitely related to HD rIL-2 and caused the conclusion of HD rIL-2 cycle.
Steroid use was required in one patient on treatment arm 1 and two patients on treatment arm 2. The (ir)AEs requiring use of steroids were diarrhea, possible Addison’s disease and bilateral leg weakness. The duration of the steroid use ranged between 21 and 45 days.
Three patients had an AE that led to their early discontinuation from the study. The AEs which resulted in removal from the study were diarrhea and autoimmune encephalopathy caused by IPI in the treatment arm 1. In treatment arm 2, acute renal failure and respiratory failure secondary to HD rIL-2, and intestinal perforation and perihepatic abscess due to IPI were the other AEs causing removal from the study.
DISCUSSION
This phase IV HD rIL-2 and IPI trial was performed to determine the efficacy and tolerability of sequencing these drugs in metastatic melanoma. The study showed an improved one-year OS rate of 75% for ITT over the pre-specified historical control OS rate of 46%. The median OS was not reached for the patients in the EP. In EP, the one-year OS rate was 87%, PFS rate was 68%, ORR was 50%, with a DCR of 83%. Note that EP is a smaller population than ITT and might reflect a bias favoring those who remained healthy enough to receive both treatments. The side effect profile was consistent with each treatment agent’s established AEs, with the most common Grade 3–4 side effects being pneumonia, respiratory failure, and back pain, seen in 2 patients (7%) each. There were 3 total deaths in this study (10.3%), including one patient (3.4%) who died of side effects of HD rIL-2 treatment.
Metastatic melanoma treatment entered into a new era after the approval of HD rIL-2 (1998) and IPI (2011). These drugs have distinctly different mechanisms of action. IL-2 acts via the cytokine stimulation of T-cells. IPI inhibits the immunosuppressive CTLA-4 checkpoint receptor. Both drugs are known to activate the T-cell response, which is crucial in tumor cell killing.Citation10,Citation19
After the approval of both agents, the possibility of enhanced activity through combination treatment was proposed. The combination of interest, concomitant use of HD rIL-2 and IPI in a Phase I/II study showed an ORR of 25%Citation13,Citation14 and another Phase II study with a higher dose of IPI at 10 mg/kg showed ORR of 11%.Citation20 In a retrospective study that analyzed 52 patients treated with HD rIL-2 after progression on IPI showed ORR benefit of 21%, compared to ORR 12% of 276 patients treated with HD rIL-2 without prior immune checkpoint blockade.Citation21 Our study sought to explore this further by asking whether the sequential use of HD rIL-2 and IPI was additive or synergistic. This study with a limited sampling size showed an ORR of 50%, which is more than the addition of individual ORR of each agent, which is 16% for HD-IL-2Citation2 and 11% for IPICitation12 or concomitant use of the agents.Citation20
The primary endpoint of the study, one-year OS rate of 75%, in ITT was statistically higher than the historical control of 46% in IPI only arm of the study.Citation12 While there was no difference between the treatment groups, both arms had better OS than the historical control. Although our study was relatively small, it demonstrated a similar 1-year OS when compared to outcomes obtained using the BRAF- and MEK-targeting agents dabrafenib and trametinib,Citation22 which had 1-year OS of 72%.
Several toxicity issues may interfere with the sequential use of these drugs, especially if HD rIL-2 is used as the follow-up drug to IPI. Immune-related AEs (irAEs) related to IPI are frequently treated with glucocorticoids, which are contraindicated during HD rIL-2 administration. Following ineffective treatment with IPI, patients may have experienced PD, and thus their performance status might have declined, potentially contraindicating the use of HD IL-2. Therefore, it was of interest to explore the feasibility of sequential use of those drugs.
There was little difference between the treatment arms in terms of compliance with both study drugs. In both treatment arms, the numbers of patients for each reason for early discontinuation from the study were similar; 10 patients (34.5%) had PD, 3 patients (10.3%) died, and 3 patients (10.3%) had serious AEs. Thrombocytopenia, hypoxia, respiratory failure, intracranial hemorrhage that occurred in one patient due to HD rIL-2 in treatment arm 1 were the AEs that caused the early discontinuation from the study and death. In treatment arm 2, the other AEs causing early discontinuation from the study were acute renal failure and respiratory failure secondary to HD rIL-2, and intestinal perforation, and perihepatic abscess due to IPI.
Hypotension and supraventricular tachycardia related to HD rIL-2 were less common than in other studies, while respiratory events and renal failure were seen at similar frequency.Citation2 The most common irAE related to IPI were gastrointestinal disorders, including diarrhea and colitis, similar to historical control.Citation12,Citation23–25 Unlike other studies, rash was less common in this study.Citation12
Although this study demonstrated an increase in OS relative to historical controls, it has some major limitations. First, the study ended early after only 29 patients had been enrolled due to a poor enrollment rate. Hence, the sample size was less than originally expected. Although the sample size of the study is considerably less than what was anticipated, if a power computation for a future study using the estimates from this study compared with the same historical controls were performed, treatment arm 1 would achieve 75% power to detect the 42% difference in one-year OS rates with 8 patients, while treatment arm 2 would achieve 89% power with 10 patients. In addition, this study does not report analyses of long-term survival and tumor response that would be useful for measuring long-term OS for the durability of response in respondents and comparing whether there is an increase in ORR as in the previous analysis.Citation14
Until the advent of checkpoint inhibitors, HD rIL-2 was the only immunotherapy in melanoma documented to induce long-term (>10 years) remissions reliably in metastatic melanoma, albeit only in a fraction of treated patients. That being said, the drug could prove much more useful if its therapeutic index could be improved, either by lowering toxicity or increasing the proportion of treated patients experiencing clinical benefit. Unlike HD rIL-2, presently approved checkpoint inhibitors targeting the PD-1 pathway, which have come to dominate melanoma treatment, do not have long-term data documenting disease control, and even cures, in metastatic melanoma.
Our study suggests that HD rIL-2, relegated to a secondary or tertiary role in melanoma management, warrants further investigation as a complement to checkpoint inhibitor therapy. Checkpoint inhibitor combination therapy (IPI with PD-1 inhibitor) is in wide use for the treatment of melanoma and a wide variety of other malignancies. Adding HD rIL-2 to this combination treatment regimen or to PD-1 inhibitor therapy may be able to enhance activity. This may allow a higher rate of complete treatment response than either therapy alone or shorter durations of therapy. In either case, this could yield further improvement in melanoma therapy.
In addition, the hypothesis assessed here, whether sequencing of immunotherapy treatment has an impact on the outcome, remains a worthwhile hypothesis to explore. Sequencing versus the combination of IPI after PD-1 progression is being investigated in S1616 (NCT03033576).Citation26 The concept of sequencing PD-1 with IPI or with HD rIL-2 before PD-1 failure has not been studied. Given the complementary mechanisms of action, this may represent a reasonable avenue for future investigation.
In summary, this phase IV study suggests a potentially more than additive effect between IPI and HD-IL2 when administered sequentially in metastatic melanoma patients. The toxicity profile was acceptable, with expected AEs of each treatment agent. These results provide an impetus for a renaissance of investigation in the role of IL-2 in melanoma treatment.
Abbreviations:
AEs: Adverse events
CI: Confidence Interval
CR: Complete response
CTLA-4: Cytotoxic T-lymphocyte–associated antigen 4
DCR: Disease control rate
EP: Evaluable population
FDA: the U.S. Food and Drug Administration
HD rIL-2: High Dose Aldesleukin/Recombinant Interleukin-2
IPI: Ipilimumab
irAEs: Immune-related adverse events
irRC: Immune-related response criteria
ITT: Intention to treat population
IV: Intravenous
MM: Metastatic melanoma
NCI: the National Cancer Institute
NCI-CTCAE: the NCI’s Common Toxicity Criteria
ORR: Objective response rate
OS: Overall survival
PD: Progressive disease
PFS: Progression-free survival
PR: Partial response
SD: Stable disease
SP: Safety population
WHO: the World Health Organization
Ethics Statement
The study was approved by the local ethical review committee of each participating institution and was conducted according to the Declaration of Helsinki. Informed consent was obtained from each patient before the clinical trial enrollment (Clinicaltrials.gov identifier: NCT01856023)
Novelty and Impact:
This phase IV study aimed to improve ORR and 1-year OS in metastatic melanoma by sequencing two approved immunotherapy agents, aldesleukin and ipilimumab. We summarized the 29 enrolled patients and their outcomes with a noticeable ORR of 50% and 1-year OS of 87% in the evaluable population. This study highlights the importance of considering the mechanism of action when designing and executing studies. It raises the possibility of sequencing or combining PD-1 inhibitors with cytokine therapy such as aldesleukin as a reasonable treatment strategy.
Supplemental Material
Download ()Disclosure statement
MH, DRM, RH, BT, LFl, LFe have no conflicts of interest to disclose.
WHS reports grants and personal fees from Bristol-Myers Squibb, during the conduct of the study; grants and personal fees from Merck, grants and personal fees from Novartis, personal fees from Regeneron, grants from Genentech, outside the submitted work.
LDC’s institution (University of Arizona) received payments from Prometheus Laboratories to support the conduct of this study. LDC receives research funding, paid to the institution (University of Washington), from Eli Lilly, AADi, BluePrint Medicine, Iterion, Gradalis, Philogen, Advenchen Laboratories, and CBA Pharma. LDC has received honoraria or has served on advisory boards for Daaichi Sankyo, BluePrint Medicines, and Regeneron.
GAD reports position at the advisory board for Clinigen and institutional research support from Bristol-Myers Squibb and Clinigen.
SH reports position at advisory board for Cardinal Health, Bristol-Myers Squibb, Immunomedics, Pfizer, and Speakers bureau at Bristol-Myers Squibb.
MM reports other interests from Exicure, Blueprints Medicine, Immunocore, Amgen, Trieza, Array Biopharma, Biontech, and Novartis, outside the submitted work.
GD reports fees from Novartis, Merck & Co, and Bristol-Myers Squibb.
NG is employed by study sponsor Prometheus Labs, Clinigen, Inc.
SPP reports institutional clinical trial support from Bristol Myers Squibb, InxMed, Novartis, Provectus, and Reata; advisory board participation for Cardinal Health and Castle Biosciences; data safety monitoring board activities for Immunocore, and personal fees from Merck outside the submitted work.
Data Availability Statement
Data that support the findings of this study is available from the corresponding author, [SPP], upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–10. doi:10.3322/caac.21590.
- Atkins MB, Lotze MT, Dutcher JP. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi:10.1200/JCO.1999.17.7.2105.
- DeSantis CE, Lin CC, Mariotto AB. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi:10.3322/caac.21235.
- Larkin J, Chiarion-Sileni V, Gonzalez R. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi:10.1056/NEJMoa1910836.
- Mier JW, Gallo RC. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980;77:6134–6138. doi:10.1073/pnas.77.10.6134.
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193(4257):1007–1008. doi:10.1126/science.181845.
- Rosenberg SA, Lotze MT, Muul LM. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi:10.1056/NEJM198512053132327.
- Herzberg B, Fisher DE. Metastatic melanoma and immunotherapy. Clin Immunol. 2016;172:105–110. doi:10.1016/j.clim.2016.07.006.
- Atkins MB, Kunkel L, Sznol M. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6:S11–4.
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383(9919):816–827. doi:10.1016/S0140-6736(13)60802-8.
- Fong L, EJ S. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi:10.1200/JCO.2008.17.8954.
- FS H, Sj O, DF M. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi:10.1056/NEJMoa1003466.
- Maker AV, Phan GQ, Attia P. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi:10.1245/ASO.2005.03.536.
- Prieto PA, Yang JC, Sherry RM. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi:10.1158/1078-0432.CCR-11-1823.
- Jd W, Hoos A, O’Day S. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi:10.1158/1078-0432.CCR-09-1624.
- U.S. Department of Health and Human Services NIoH, National Cancer Institute: Common terminology criteria for adverse events: (CTCAE) v4.03, CTCAE; 2010.
- SWOG: SWOG Statistical Center website statistical tools.
- Seymour L, Bogaerts J, Perrone A. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi:10.1016/S1470-2045(17)30074-8.
- Batus M, Waheed S, Ruby C. Optimal management of metastatic melanoma: current strategies and future directions. Am J Clin Dermatol. 2013;14:179–194. doi:10.1007/s40257-013-0025-9.
- Silk AW, Kaufman HL, Curti B. High-dose ipilimumab and high-dose interleukin-2 for patients with advanced melanoma. Front Oncol. 2019;9:1483. doi:10.3389/fonc.2019.01483.
- Buchbinder EI, Gunturi A, Perritt J. A retrospective analysis of high-dose interleukin-2 (HD IL-2) following Ipilimumab in metastatic melanoma. J Immunother Cancer. 2016;4:52. doi:10.1186/s40425-016-0155-8.
- Robert C, Karaszewska B, Schachter J. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi:10.1056/NEJMoa1412690.
- Sj O, Maio M, Chiarion-Sileni V. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi:10.1093/annonc/mdq013.
- Weber J, Thompson JA, Hamid O. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi:10.1158/1078-0432.CCR-09-1024.
- Wolchok JD, Neyns B, Linette G. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi:10.1016/S1470-2045(09)70334-1.
- ClinicalTrials.gov. Testing Treatment With Ipilimumab and Nivolumab Compared to Treatment With Ipilimumab Alone in Advanced Melanoma. 2020. ls.gov/ct2/show/NCT03033576?term=NCT03033576&draw=2&rank=1
