ABSTRACT
Human Vγ2Vδ2 (also termed Vγ9Vδ2) T cells play important roles in microbial and tumor immunity by monitoring foreign- and self-prenyl pyrophosphate metabolites in isoprenoid biosynthesis. Accumulation of isoprenoid metabolites after bisphosphonate treatment allows Vγ2Vδ2 T cells to recognize and kill tumors independently of their MHC expression or burden of non-synonymous mutations. Clinical trials with more than 400 patients show that adoptive immunotherapy with Vγ2Vδ2 T cells has few side effects but has resulted in only a few partial and complete remissions. Here, we have tested Vγ2Vδ2 T cells for expression of inhibitory receptors and determined whether adding PD-1 checkpoint blockade to adoptively transferred Vγ2Vδ2 T cells enhances immunity to human PC-3 prostate tumors in an NSG mouse model. We find that Vγ2Vδ2 T cells express PD-1, CTLA-4, LAG-3, and TIM-3 inhibitory receptors during the 14-day ex vivo expansion period, and PD-1, LAG-3, and TIM-3 upon subsequent stimulation by pamidronate-treated tumor cells. Expression of PD-L1 on PC-3 prostate cancer cells was increased by co-culture with activated Vγ2Vδ2 T cells. Importantly, anti-PD-1 mAb treatment enhanced Vγ2Vδ2 T cell immunity to PC-3 tumors in immunodeficient NSG mice, reducing tumor volume nearly to zero after 5 weeks. These results demonstrate that PD-1 checkpoint blockade can enhance the effectiveness of adoptive immunotherapy with human γδ T cells in treating prostate tumors in a preclinical model.
Introduction
Recent advances in cancer immunotherapy have revolutionized treatment. Checkpoint blockade can treat a variety of different cancers by releasing neoantigen-specific T cells directed against the cancer that are held dormant by inhibitory receptors. Treatment with chimeric-antigen receptor (CAR) T cells specific for B cell surface receptors can induce durable remissions in lymphoma, acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma. Despite these clinical advances, efficacy is still limited. Present CAR T cells can only treat B cell malignancies and the majority of patients treated with checkpoint blockade do not achieve durable remissions. Thus, new approaches are needed.
The use of γδ T cells for cancer treatment is one such approach. γδ T cells represent a third lineage of adaptive immune cells along with αβ T cells and B cells that arose in the earliest vertebrates.Citation1 γδ T cells bridge adaptive and innate immunity with their ability to rearrange their T cell antigen receptors (TCR) and develop immunological memory but they use their TCRs more for pattern-recognition.Citation2 Their TCRs mediate unconventional recognition of non-MHC proteins, such as butyrophilins (BTN), and non-peptide compounds leading to unique niches in immunity and in roles beyond the conventional immune system.
Human γδ T cells play important roles in tumor and microbial immunity that are now being more clearly defined. The major circulating V-gene subset express diverse Vγ2Vδ2 (also termed Vγ9Vδ2) TCRs. Vγ2Vδ2 T cells respond to both eukaryotic apicomplexan parasites, such as those causing malaria and toxoplasmosis, as well as many prokaryotic bacteria, such as those in the Enterobacteriaceae and Mycobacteriaceae families, with rapid expansions to high numbers (up to 50% of circulating T cells), production of interferon (IFN)-γ and tumor necrosis factor (TNF)-α, along with killing of infected cells and reduction in the number of circulating bacteria.Citation3
Stimulation of Vγ2Vδ2 T cells by this wide variety of microbial pathogens is through their TCRs. Unlike conventional CD8 and CD4 αβ T cells that respond to peptides presented by MHC class I and class II molecules, Vγ2Vδ2 TCRs are triggered by shared intracellular isoprenoid metabolites and other phosphorylated metabolites also commonly termed as phosphoantigens. Foreign phosphorylated metabolites, such (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate in the 2-C-methyl-D-erythritol 4-phosphate isoprenoid synthesis pathway, or overproduced endogenous isoprenoid metabolites, such as isopentenyl pyrophosphate (IPP) in the mevalonate pathway,Citation4 are sensed by the intracellular B30.2 domain of BTN3A1.Citation5,Citation6 Binding is signaled to its extracellular domain through an unknown mechanism and, in conjunction with BTN2A1,Citation7,Citation8 leads to recognition by the Vγ2Vδ2 TCR and T cell activation. This sensor system allows Vγ2Vδ2 T cells to surveil the cytoplasm of cells and eliminate those with aberrant isoprenoid metabolism because of mutagenesis or infection.
Both BTN3A1 and BTN2A1 are expressed by all malignant human cells tested,Citation9 allowing Vγ2Vδ2 T cells to recognize and kill tumor cells with altered isoprenoid metabolism, either through pharmacological manipulation by aminobisphosphonate drugs, such as pamidronate or zoledronic acid,Citation10 or through undefined defects, such as those present in some B cell tumors such as Daudi,Citation11 RPMI 8226,Citation12 and XG-7.Citation13–15 Vγ2Vδ2 TCR stimulation by these tumor cells is clearly mediated by BTN3A114 and BTN2A115. A number of different alterations in endogenous isoprenoid metabolism such as overexpression of HMG-CoA reductase,Citation16 inhibition of FDPS or isopentenyl diphosphate isomerase through siRNA/shRNA treatment,Citation10,Citation17 or addition of the rate limiting metabolite, mevalonateCitation10 can convert tumor cells into activators for Vγ2Vδ2 T cells. However, it is not clear what specific genomic mutations or epigenetic modifications are present in activating tumors. But treatment with bisphosphonates, prenyl pyrophosphates, or activating anti-BTN3 or anti-BTN2A mAbs can render all lineages of tumor cells stimulatory for Vγ2Vδ2 T cells.
The presence of γδ T cells infiltrating solid tumors has been found to be the most favorable prognostic cell association when ~18,000 tumor across 39 malignancies were studied.Citation18 Indeed, in prostate cancers, both Vγ2Vδ2 and Vδ1 T cells infiltrate these tumors with a trend for lower numbers of infiltrating γδ T cells with higher-grade tumors.Citation19 The ability of Vγ2Vδ2 T cells to recognize all lineages of malignant cells without off-target autoreactivity and their ability to mediate tumor immunity suggests an explanation for the favorable prognostic association. Tumor recognition by Vγ2Vδ2 T cells requires neither MHC protein expression nor non-synonymous genomic mutations in the tumor. Vγ2Vδ2 T cells are not alloreactive and therefore can be used as “off-the-shelf” treatments by cryopreserving expanded Vγ2Vδ2 T cells from normal donors foregoing lengthy isolation and expansion from patient’s blood or tumor biopsies. Large numbers of cytotoxic Vγ2Vδ2 T cells can be grown ex vivo by stimulating with aminobisphosphonate or prenyl pyrophosphates. Thus far, the results of 15 clinical trials have been reported for 444 patients treated with the adoptive transfer of Vγ2Vδ2 T cells including those with non-small cell lung cancer (NSCLC), renal cell cancer, melanoma, colorectal cancer, breast cancer, cholangiocarcinoma, gastric cancer, multiple myeloma, and pancreatic cancer.Citation20–34 There have been relatively few grade 3/4 adverse events unless patients are concurrently treated with zoledronic acid and IL-2.Citation22,Citation23 Adoptive transfer of Vγ2Vδ2 T cells has induced a durable remission in a patient with metastatic kidney cancer,Citation35 induced remission in patient with breast cancer,Citation22 and increased overall survival of patients with advanced liver and lung cancer.Citation32 However, the most common beneficial clinical response was stable disease underscoring the need to improve the efficacy of this treatment.
One potential reason for the poor efficacy of Vγ2Vδ2 T cell treatments is the tumor microenvironment (TME). Tumors employ a variety of defenses against attack by tumor-reactive T cells.Citation36 One major defense is the expression of ligands on tumor cells or on other cells in the TME for inhibitory receptors expressed by T cells, such as programmed cell death ligand 1/2 (PD-L1/PD-L2) for PD-1 and B7-1/B7-2 for cytotoxic T lymphocyte-associated protein 4 (CTLA-4). Besides PD-1 and CTLA-4, T cells can express T cell immunoglobulin- and mucin-domain-containing molecule 3 (TIM-3), lymphocyte-activation gene-3 (LAG-3), T cell immunorecepter with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), and V-domain immunoglobulin suppressor of T cell activation (VISTA).Citation37 Some of these receptors can be concurrently expressed on both CD4 and CD8 tumor infiltrating lymphocytes through a co-inhibitory gene module.Citation38
Engagement of these inhibitory receptors blocks T cell effector functions. Direct evidence for PD-1/PD-L1 checkpoint inhibition has been found in melanoma patients.Citation39 Patients who responded to PD-1 blockade had higher densities of CD8 αβ T cells at their tumor margins prior to treatment. Releasing T cells from PD-1 inhibition further increased CD8 αβ T cell densities, T cell proliferation, and granzyme B levels. CD8 αβ T cells reacting to tumor antigens secrete IFN-γ. As predicted, phosphoSTAT1 (the immediate downstream effector upon IFN-γ binding to its receptor) and PD-L1 at the invasive margins increased after PD-1 blockade.Citation39 The close proximity of PD-1+ CD8 T cells and PD-L1+ cells (tumor cells, lymphocytes, and macrophages) prior to and during treatment correlated with response to PD-1 checkpoint blockade.Citation39
Besides expression on “exhausted” T cells,Citation40 these inhibitory receptors are also expressed in CD8 αβ T cells after activation.Citation41 PD-1 blockade, either by anti-PD-1 mAbs or by PD-1 disruption, has been shown to enhance the activity of CAR T cells against solid tumors in preclinical models and in patients providing evidence that checkpoint blockade can also enhance adoptive T cell therapies.Citation42 Solid tumors, such as prostate cancer, have low mutation rates and have shown minimal responses to PD-1 checkpoint blockade (although they can express high levels of PD-L1Citation43) and limited responses to CAR T cells.Citation44
Here, we have investigated the efficacy of combining adoptive immunotherapy with Vγ2Vδ2 T cells with PD-1 checkpoint blockade for immunity against human prostate cancer. We find that Vγ2Vδ2 T cells upregulate multiple inhibitory receptors, including PD-1, CTLA-4, TIM-3, and LAG-3 during ex vivo expansion. PC-3 prostate cancer cells express high level of PD-L1 and PD-L2 ligands. We find that combination treatment of PC-3 prostate cancers in NSG mice by adoptively transferring Vγ2Vδ2 T cells along with anti-PD-1 blockade greatly enhanced tumor immunity.
Materials and methods
Reagents
Monoclonal antibodies (mAb) used for flow cytometric analyses in this study are detailed in Supplemental Table I in the Supplementary material and methods. Pamidronate (3 mg/ml) was from Hospira, Inc. (Lake Forest, IL). Zoledronic acid was provided by Dr. Eric Oldfield. For in vivo studies, anti-human PD-1 (CD279) (clone J116) and control mouse IgG1 (clone MOPC-21) antibodies were from Bio X Cell (West Lebanon, NH).
Ex vivo expansion of Vγ2Vδ2 T cells by pulse zoledronic acid stimulation
PBMC were isolated from random donor leukopaks (AllCells, LLC, Alameda, CA) by density centrifugation over Ficoll-Hypaque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and cryopreserved for later use. For expansions, complete media (C-Media) was used that was prepared as previously described with prescreened 8% fetal calf serum, 2% human serum, and 1000 IU/ml IL-2 (Proleukin, Chiron, Emeryville, CA).Citation45 For T cell functional assays and for culturing tumor cells, human serum and IL-2 were omitted and 12 ml of FCS was substituted.
For expansion of Vγ2Vδ2 T cells, thawed PMBC were suspended at 1 × 106 cells/ml in C-media lacking IL-2 with 100 μM zoledronic acid for 4 h at 37°C and 5% CO2, washed three times with PBS, and resuspended in C-Media without zoledronic acid. Expansions were performed in 75 cm2 flasks standing upright. On day 3, 50% of the media was removed and replaced with media containing 2000 IU/ml IL-2. PBMC were incubated for 14 d at 37°C and 5% CO2. Cell growth was monitored by microscopic examination and media color. Every 2–4 d, 50% of the media was changed with fresh C-Media (containing IL-2 but without zoledronic acid) and the cells were split 1:2 depending on cell density. On day 14, the cells were harvested, washed twice with PBS, and counted.
Purification and cryopreservation of expanded Vγ2Vδ2 T cells and flow cytometry
After ex vivo expansion for 14 d, Vγ2Vδ2 T cells were positively purified using anti-allophycocyanin (APC)-coated magnetic beads (MACS, Miltenyi Biotec, San Diego, CA) as detailed.Citation45 Briefly, expanded cells were washed once with PBS and reacted with APC-conjugated anti-human Vδ2 mAb (clone B6). Cells were then washed twice with purification buffer and anti-APC magnetic beads added. After incubation on ice for 15 min, the cells and beads were washed twice, resuspended in 500 μl of purification buffer, and then loaded onto an LS column for positive selection using a Miltenyi VarioMACS separator. Retained Vδ2 T cells were washed on the column three times with 3 ml of buffer, removed from the magnetic field, and then eluted, washed, and either immediately used or cryopreserved. Purified Vγ2Vδ2 T cells were cryopreserved by resuspending cells in 4°C FCS and adding dropwise an equal volume of FCS with 20% DMSO (Hybri-Max, Sigma-Aldrich, St. Louis, MO). Cells were frozen at −1°C/min in Mr. Frosty freezing containers (Nalgene) in a − 80°C freezer and then transferred to liquid N2 on the next day. Levels of γδ and Vγ2Vδ2 T cells and their differentiation state were assessed by flow cytometric analysis using an LSR II flow cytometer (BD Biosciences) using BD CompBead particles to set compensation. Analysis was performed using FlowJo software (FlowJo, LLC) and plotted using bi-exponential axes.
For in vivo experiments, cryopreserved PBMC from the leukopak of one healthy donor (LP.25) were used to diminish variability. The donor’s PBMC had 48.8% CD3+ T cells with Vγ2Vδ2 T cells constituting 6.6% of total CD3+ T cells. Vδ1 T cells and total γδ T cells constituted 1.5% and 9.1% of T cells, respectively. The majority of the Vγ2Vδ2 T cells (81.3%) were early or central memory T cells expressing CD28 and CD27. Other subsets constituted 4.0% CD28+CD27−, 7.3% CD28−CD27+, and 7.4% CD28−CD27− (TEMRA). After stimulation with pulse zoledronic acid, the Vγ2Vδ2 T cells expanded ~774- to 858-fold during the 14-day culture. For example, at the end of expansion but before purification Vγ2Vδ2 T cells constituted 96.5%, 94.5%, and 95.1% of total T cells for three lots. After purification, Vγ2Vδ2 T cells constituted 98.8%, 98.7%, and 98.6% of total T cells with 1.2%, 1.3%, and 1.4% presumed CD3 αβ T cell contamination. After expansion, most Vγ2Vδ2 T cells have lost expression of CD27 and CD27 (89.1%). The donor used in this paper, LP.25, has a slightly higher proportion of such cells (89.1%) compared with other donors. Other memory subsets constituted 5.9% CD28+CD27+, 4.8% CD28+CD27−, and 0.2% CD28−CD27+. LP.25 values are shown as red dots among values for 12 other donors along with the gating strategy used to delineate memory subsets (Supplemental ). Cell yields of cryopreserved Vγ2Vδ2 T cells after overnight incubation in C-media with IL-2 were between 60% and 70% of the cells counted immediately after thawing. Cell purity was evaluated by flow cytometry and the Vγ2Vδ2 T cells used were at least 98% Vδ2 cells.
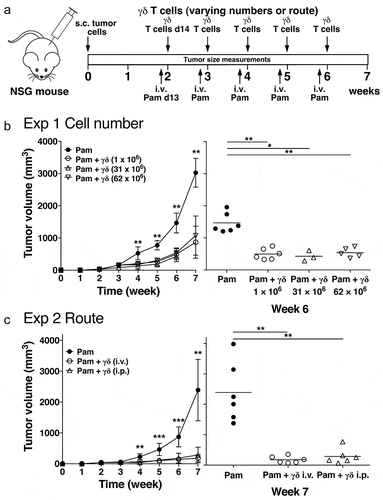
Figure 1. Increasing Vγ2Vδ2 T cell numbers or changing the route of transfer does not improve immunity against human PC-3 prostate tumors in immunodeficient NSG mice. (a) Schema of treatment protocol used to evaluate the anti-tumor efficacy of Vγ2Vδ2 T cells. Human PC-3 prostate cancer cells were injected s.c. into immunodeficient NSG mice on day 0. On day 13, pamidronate (50 μg/kg) was given i.v. On day 14, cryopreserved purified Vγ2Vδ2 T cells expanded using pulse zoledronate stimulation were inoculated i.v. or i.p. Treatments were repeated weekly until week 6. Longitudinal and transverse diameters of the tumors were measured weekly. (b) (left panel) Increasing the number of cryopreserved Vγ2Vδ2 T cells does not improve anti-tumor immunity. Mean PC-3 tumor volume ± SD is shown for 3–8 mice per group treated with either pamidronate alone (closed circles), or pamidronate with 1 × 106 (open circles), 31 × 106 (open triangles), or 62 × 106 (open inverted triangles) purified Vγ2Vδ2 T cells. (right panel) Tumor volumes at week 6 for individual mice. Bars represent mean values. (c) (left panel) Changing the route of adoptively transferred Vγ2Vδ2 T cells does not improve anti-tumor immunity. Mean PC-3 tumor volume ± SD is shown for 5–6 mice per group treated with either pamidronate alone (closed circles), or pamidronate with 1 × 106 purified Vγ2Vδ2 T cells given i.v. (open circles) or i.p. (open triangles). (right panel) Tumor volumes at week 7 for individual mice. Bars represent mean values. *p < .05, **p < .01, ***p < .001 compared with the tumor volume in mice treated with pamidronate alone using the Mann-Whitney U test
Intracellular cytotoxic protein and cytokine expression after stimulation of expanded Vγ2Vδ2 T cells
To assess the functional activity of purified Vγ2Vδ2 T cells after cryopreservation, the intracellular levels of granzyme B and interferon-γ in previously cryopreserved Vγ2Vδ2 T cells were compared to those of fresh Vγ2Vδ2 T cells. Previously, cryopreserved or fresh Vγ2Vδ2 T cells were stimulated with ionomycin (2 μg/ml) and phorbal 12-myristate 13-acetate (PMA) (50 ng/ml) (both from Sigma-Aldrich, St. Louis, MO) for 4–6 h in the presence of 4 µl of GolgiStop (monensin) (BD Biosciences) per 6 ml media. For flow cytometric analysis, PBMC were first stained with Live/Dead Blue (Invitrogen), to exclude dead cells followed by staining with APC-Cy7-conjugated anti-CD3 and FITC-conjugated anti-Vδ2 mAbs. The cells were then washed, fixed, and permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences) and then intracellularly stained with either PE-conjugated anti-IFN-γ or anti-granzyme B mAbs. Cytokine and cytotoxic protein levels in Vδ2 T cells were then assessed by flow cytometry.
Expression of inhibitory receptors on Vγ2Vδ2 T cells
PBMC from LP.25 were stimulated using pulse zoledronic acid and cultured with IL-2 as detailed above. At various times after stimulation, the expression of inhibitory receptors was measured by staining with APC-Cy7-anti-CD3, FITC-anti-Vδ2, Live/Dead Blue, and PE-, APC-, or PE-Cy7-conjugated mAbs to the various inhibitory receptors. To assess inhibitory receptor expression on previously cryopreserved expanded Vγ2Vδ2 T cells, PC-3 cells were first treated with mitomycin C (100 μg/ml) for 1 h at 37°C in PBS. The PC-3 cells were then washed and cultured in FK-12 medium with 200 μM of pamidronate overnight. After culture, PC-3 cells were then washed twice. For restimulation, 5 × 105 pulsed PC-3 cells were co-cultured with 1 × 106 freshly thawed purified Vγ2Vδ2 T cells from LP.25 in 2 ml of C-media with IL-2 per well of a 24-well plate at 37°C in a 5% CO2 incubator. The co-cultured cell mixture was harvested at day 0, 1, 3, 5, 7, and 10, and stained with FITC-anti-Vδ2 and PE-anti-PD-1, PE-anti-CTLA-4, PE-anti-LAG-3, PE-anti-TIM-3, or PE-anti-TIGIT on ice for 30 min. Hoechst staining was used to gate out of dead cells. Surface expression of the inhibitory receptors was analyzed using an LSR II flow cytometer. Data were analyzed using Flowjo software and plotted using bi-exponential scaling with the linear scale optimized for display.
Effect of Vγ2Vδ2 T cells and IFN on PD-L1 expression on PC-3 cells
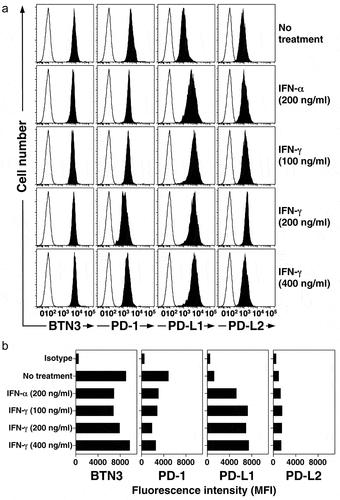
PC-3 cells were cultured without or with 200 µM pamidronate overnight. After washing, unpulsed or pamidronate-pulsed PC-3 cells were cultured with purified Vγ2Vδ2 T cells at an E:T ratio of 10:1 and added either to the inner well or to the outer well of a Transwell where the wells are separated by a 0.4 µm polycarbonate membrane (Transwell 24-well plate, Corning, Corning, NY). Culture supernatants were harvested after 48 h and IFN-γ levels measured by DuoSet sandwich ELISA (R&D Systems, Minneapolis, MN). Cells were harvested from the outer wells, washed, and then stained with anti-PD-L1 mAb and analyzed by flow cytometry gating on PC-3 cells. To determine the effects of interferon on PD-L1 expression on PC-3 cells, PC-3 cells were cultured with either 100 (500 IU), 200 (1,000 IU), or 400 (2,000 IU) ng/ml IFN-γ (ThermoFisher Scientific (Waltham, MA)) or 200 ng/ml (1,000 IU) IFN-α (human hybrid IFN-α A/D, PBL Assay Science (Piscataway, NJ)) for 48 h. To assess BTN3, PD-1, PD-L1, and PD-L2 levels, PC-3 cells were stained with the appropriate mAb and analyzed by flow cytometry.
Human prostate cancer xenograft and adoptive transfer of Vγ2Vδ2 T cells with PD-1 checkpoint blockade in immunodeficient mice
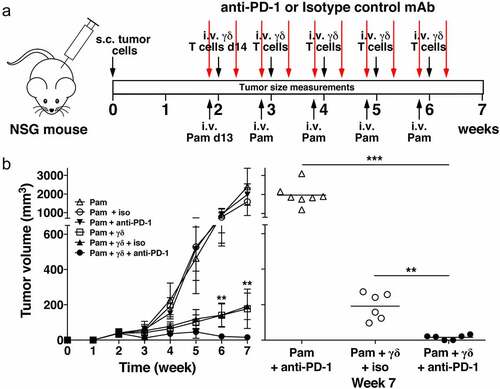
To assess the anti-tumor activity of adoptively transferred Vγ2Vδ2 T cells, human PC-3 cancer cells were xenotransplanted into immunodeficient mice using a model developed by the Scotet laboratoryCitation46 except that treatment was started one dayearlier (day 13 rather than day 14) and purified Vγ2Vδ2 T cells were used. Five-week-old female NOD.Cg-Prkdcscid Il2rgtmCitation1Wjl/SzJ (NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and used at six weeks of age. For tumor xenotransplantation, 1 × 107 human PC-3 prostate cancer cells were suspended in 200 μl of sterile PBS and inoculated subcutaneously into the right flank of NSG mice on day 0. Each treatment group consisted of eight mice. Note that due to husbandry problems that have been reported with NSG mice,Citation47 there were sporadic mouse deaths in some experiments due to commensal infections leading to lower group sizes. These deaths were independent of tumor size or treatment and did not affect the overall results. On day 13 (when tumor diameter had reached >5 mm), mice were injected i.v. with pamidronate (50 μg/kg). The mice weighed 17 to 19 g and therefore received 0.85 to 0.95 μg of pamidronate. For PD-1 checkpoint blockade, anti-PD-1 or isotype control mAbs were administrated intraperitoneally at 200 μg/mouse on day 13 after tumor inoculation. This was followed on day 14 with the i.v. injection of 1 × 106 (or the indicated cell number) freshly thawed purified Vγ2Vδ2 T cells. Two days later, mAb injections were repeated. Treatments were repeated weekly until week 6 for a total of 5 treatments. For each experiment, cryopreserved Vγ2Vδ2 T cells from the same donor and lot were used. Transferred cell numbers were based on the number of thawed Vγ2Vδ2 T cells that remained one day after culture in C-media to correct for T-cell loss due to cryopreservation (between 30% and 40% loss). Note that purified Vγ2Vδ2 T cells were used to avoid αβ T cell outgrowth that can occur in immunodeficient mice even with low levels (3–5%) of αβ T cell contamination.Citation48 Although the purified Vγ2Vδ2 T cells in these experiments were contaminated with ~1–2% αβ T cells, 50-fold fewer T cells were given per transfer compared with our previous study decreasing the probability of αβ T cell outgrowth.Citation48 Control mice received only pamidronate treatments with or without mAbs. Note that in this model, pamidronate by itself only minimally inhibits the growth of PC-3 tumors.Citation46 Tumor size was assessed once weekly by external measurement of the longitudinal and transverse tumor diameter using a digital vernier caliper. Tumor volume was calculated using the modified ellipsoidal formula where tumor volume (mm3) = (y × xCitation2)/2, where “y” is the longitudinal length and “x” is the transverse width. All experiments involving animals, including their housing and care in pathogen-free conditions and the experimental protocols used, were conducted in accordance with relevant laws and institutional guidelines, and were approved by the local IACUC committee.
Statistical analyses
For statistical analyses, the nonparametric Mann-Whitney U test was used as indicated with p < .05 considered statistically significant. Statistical analyses were done in Prism version 7.0a (GraphPad Software, La Jolla, CA).
Results
Neither increasing the number of adoptively transferred Vγ2Vδ2 T cells nor changing the route of transfer improves anti-tumor immunity to human prostate cancer cells in immunodeficient NSG mice
Vγ2Vδ2 T cells after cryopreservation produced granzyme B and IFN-γ at levels and frequencies similar to those of freshly purified Vγ2Vδ2 T cells (Supplemental ). However, their effectiveness in mediating in vivo tumor immunity was slightly less. Tumor immunity by cryopreserved Vγ2Vδ2 T cells was assessed against prostate cancer tumors in an adoptive transfer model in immunodeficient NSG mice, adapted from the Scotet laboratory,Citation46 that we previously used to test fresh Vγ2Vδ2 T cells.Citation45 In this model, human PC-3 prostate cancer cells are subcutaneously implanted in the flanks of immunodeficient NSG mice. After 13 days, pamidronate is intravenously injected followed 1 day later by the adoptive transfer of purified Vγ2Vδ2 T cells. This treatment is repeated five times ().
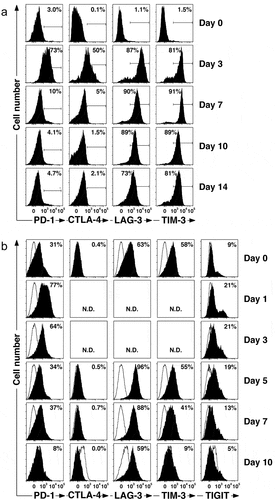
Figure 2. Vγ2Vδ2 T cells express inhibitory receptors after bisphosphonate stimulation. (a) Inhibitory receptors are expressed on Vγ2Vδ2 T cells during in vitro expansion. PBMC from a normal donor were pulsed for 4 h with 100 μM zoledronic acid, washed, and then cultured in C-media with IL-2 for 14 days to expand Vγ2Vδ2 T cells. Surface expression of various inhibitory receptors was assessed by flow cytometric analysis at the times indicated. (b) PD-1 inhibitory receptors are re-expressed on Vγ2Vδ2 T cells after stimulation by bisphophonate-treated PC-3 prostate cancer cells. Mitomycin C-treated PC-3 cells were cultured overnight with 200 μM pamidronate, washed, and then cultured with thawed purified Vγ2Vδ2 T cells in C-media with IL-2 for 10 days. Surface expression of inhibitory receptors was assessed by flow cytometric analysis at the times indicated, analyzed using FloJo software, and plotted with bi-exponential scaling. N.D. = not determined. Solid histograms show the specific antibody staining while open histograms show control mAb staining
Previously cryopreserved Vγ2Vδ2 T cells inhibited the growth of PC-3 tumors slightly less than freshly expanded Vγ2Vδ2 T cells that were previously studied.Citation45 Cryopreserved Vγ2Vδ2 T cells inhibited tumor growth such that tumor volume was 29% () and 7.4% () of control tumors at week 7. However, in our previous study, freshly purified Vγ2Vδ2 T cells inhibited the growth of PC-3 tumors more effectively to 11.2% and 6.1% of control tumors at week 7,Citation45 with little tumor growth from week 5 to week 7. Thus, Vγ2Vδ2 T cells retain most of their in vivo anti-tumor activity after cryopreservation allowing their use in the preclinical studies reported here and suggesting that they could be used for clinical treatments.
In murine studies, transferring higher numbers (10–100-fold more) of CD8 αβ T cells from Pmel-1 TCR transgenic mice can overcome potency differences between T cell subsets to provide equivalent immunity to murine B16 melanoma cells.Citation49 To determine if human Vγ2Vδ2 T cells behave similarly, the number of adoptively transferred Vγ2Vδ2 T cells was increased 31- and 62-fold. Despite these increases, tumor immunity did not improve further () with similar mean tumor volumes between the different groups (, right panel). Also, there was no significant difference between Vγ2Vδ2 T cells that were intraperitoneally injected compared with those that were intravenously injected (). Thus, unlike CD8 αβ T cells, there was no improvement in tumor immunity with the transfer of higher number of Vγ2Vδ2 T cells. These findings and the fact that even fresh Vγ2Vδ2 T cells cannot shrink the size of established PC-3 prostate tumors suggest that the tumor microenvironment may be preventing further control of tumor growth.
Expanded Vγ2Vδ2 T cells express inhibitory receptors
One of the major mechanisms by which established solid tumors evade tumor-specific T cells is through their expression of ligands for inhibitory receptors expressed by T cells. To characterize the expression of inhibitory receptors on Vγ2Vδ2 T cells, their levels were measured during ex vivo expansion. Three previous studies have demonstrated similar kinetics and level of expression of PD-1 during Vγ2Vδ2 expansion with levels peaking between days 2 to 4 with an average of 75% of Vγ2Vδ2 T cells (range 40–90%) expressing PD-1 and with levels returning to near baseline at 14 days on all 15+ donors tested.Citation50–52 We therefore tested the donor previously used in our in vivo studies for expression of PD-1 as well as other inhibitory receptors. The surface expression of PD-1, CTLA-4, LAG-3, and TIM-3 were measured at day 0, 3, 7, 10, and 14 of culture (). Vγ2Vδ2 T cells expressed inhibitory receptors during expansion. PD-1, CTLA-4, LAG-3, and TIM-3 were all upregulated by day 3 with 73% of the Vγ2Vδ2 T cells expressing PD-1 (). By day 14, Vγ2Vδ2 T cells had lost expression of PD-1 and CTLA-4 (). In contrast, LAG-3 and TIM-3 continued to be expressed at high levels by most Vγ2Vδ2 T cells (>73%). Thus, expression of PD-1 on Vγ2Vδ2 T cells from our donor showed identical kinetics and similar expression levels that are typical for healthy adult donors from previous studies and was well suited for additional in-vitro and in vivo studies.
Cryopreserved Vγ2Vδ2 T cells retained expression of LAG-3 and TIM-3 upon thawing (). When restimulated through their TCRs by exposure to pamidronate-pulsed PC-3 cells, Vγ2Vδ2 T cells rapidly upregulated expression of PD-1 and TIGIT (another inhibitory receptor) by day 1 which were lost by day 10. CTLA-4 was not expressed. TIM-3 expression decreased after day 5 until lost by day 10 whereas LAG-3 expression was maintained on 59% of cells on day 10. Thus, PD-1 can be reexpressed after TCR stimulation by exposure to pamidronate-pulsed PC-3 tumor cells. PD-1 and other inhibitory receptors, such as TIGIT, LAG-3, and TIM-3 on expanded Vγ2Vδ2 T cells makes their anti-tumor activity subject to inhibition upon interaction with ligands for these inhibitory receptors in the tumor microenvironment.
PC-3 prostate cancer cells express PD-L1 and PD-L2 ligands for PD-1
As shown above, adoptively transferred Vγ2Vδ2 T cells express checkpoint receptors that could inhibit their anti-tumor activity. To determine whether checkpoint receptors expressed by Vγ2Vδ2 T cells alter their activity in vivo, expression of inhibitory and stimulatory ligands as well as other adhesion ligands by PC-3 prostate cancer cells was determined. PC-3 cells expressed high levels of PD-L1 and PD-L2 ligands for the PD-1 checkpoint receptor (). PC-3 cells also express nectin-2/CD112 and PVR/CD155 that are the ligands for the inhibitory receptors, TIGIT and CD96 (binds only CD155), and the stimulatory receptor, DNAM-1. Note that a previous study found that PC-3 cells do not express the CTLA-4 ligands, B7-1 (CD80) and B7-2 (CD86), nor do they express the LAG-3 ligand, MHC-class II unless induced by IFN-γ.Citation53 One TIM-3 ligand, phosphatidylserine, would be expected to be present on apoptotic but not viable PC-3 cells.Citation54,Citation55
Figure 3. PC-3 prostate cancer cells express PD-L1, PD-L2, and other receptor ligands. PC-3 cancer cells were cultured in F12K medium for at least one passage and then washed and reacted with various mAbs. Surface expression was then assessed by flow cytometry, analyzed using FloJo software, and plotted with bi-exponential scaling. Solid histograms show the specific antibody staining while open histograms show control mAb staining
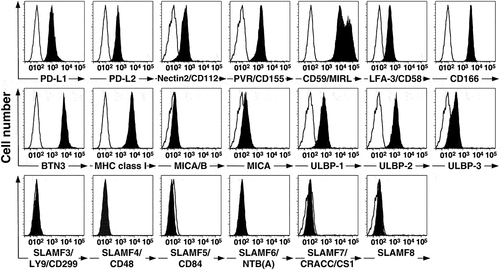
PC-3 cells express CD59 and LFA-3/CD58 that are ligands for the CD2 adhesion molecule expressed by Vγ2Vδ2 T cellsCitation56 as well as CD166/ALCAM, the ligand for the CD6 adhesion molecule that is also expressed by Vγ2Vδ2 T cells and that functions as an alternative to LFA-1/ICAM-1 adhesion.Citation57 PC-3 cells also express high level of BTN3 as well as MHC class I proteins (). Vγ2Vδ2 T cells express the NKG-2D/CD314 C-type lectin that functions to enhance stimulation by aminobisphosphonates and prenyl pyrophosphates.Citation58,Citation59 PC-3 cells express a variety of ligands for NKG2D including MICA/B, ULBP-1, −2, and −3 (). PC-3 cells did not express SLAMF3/CD299, SLAMF4/CD48, SLAMF5/CD84, SLAMF6/NTB(A), SLAMF7/CRACC, or SLAMF8. Thus, PC-3 prostate cells do not express ligands for CTLA-4 but could display the phosphatidylserine ligand for TIM-3 if undergoing apoptosis and the MHC class II ligand for LAG-3 if exposed to IFN-γ. They do express PD-L1 and PD-L2 ligands for PD-1 at high levels.
Stimulation of Vγ2Vδ2 T cells increases PD-L1 surface expression by PC-3 cells
After adoptive transfer, tumor-specific CD8 αβ T cells first accumulate at the periphery of the tumor where their migration is arrested upon encountering antigen-expressing tumor cells.Citation60 However, cytokines secreted by the T cells penetrate further into the tumor interior. The expression of PD-L1 and PD-L2 by PC-3 cells suggests a potential mechanism by which tumors can inhibit tumor immunity by Vγ2Vδ2 T cells. To determine the effects of cytokines produced by Vγ2Vδ2 T cells on surrounding tumor cells after stimulation of the T cells by bisphosphonate-treated tumor cells, purified Vγ2Vδ2 T cells were stimulated in the inner well of a Transwell where they were separated from PC-3 cells in the outer well by a 0.4 µm membrane. Stimulation of Vγ2Vδ2 T cells with pamidronate-treated PC-3 cells in the inner well increased PD-L1 expression by PC-3 cells located in the outer well to similar levels as when the cells were co-cultured in the outer well (). This suggests that a soluble cytokine mediates this increase.
Figure 4. Co-culture with activated Vγ2Vδ2 T cells increases surface expression of PD-L1 on PC-3 cells through a soluble cytokine. PC-3 cells were cultured overnight with or without pamidronate (200 μM) and then washed twice. Treated PC-3 cells were cultured with or without purified Vγ2Vδ2 T cells in either the inner or outer wells in a Transwell plate separated by a 0.4 μm membrane under the indicated conditions for 48 h. PC-3 cells in the outer well were harvested, stained with anti-PD-L1 mAb, and the surface expression of PD-L1 assessed by flow cytometry. Culture supernatants were harvested after 48 h and assayed for IFN-γ levels by ELISA. (a) PD-L1 expression by PC-3 cells cultured under the indicated conditions. Representative of two experiments. Solid histograms show the specific antibody staining while open histograms show control mAb staining. (b) Expression of PD-L1 (MFI) on PC-3 cells in the outer well and supernatant IFN-γ levels after co-culture with Vγ2Vδ2 T cells and PC-3 cells (either untreated or treated with pamidronate) for 48 h
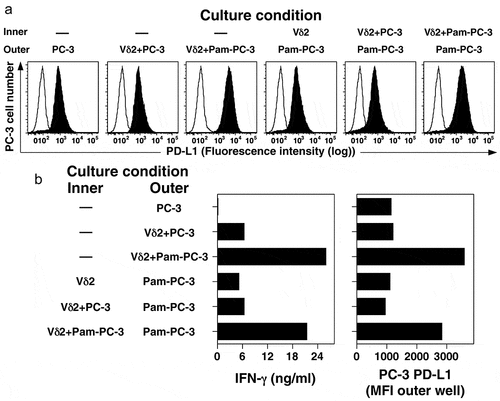
The increase in PD-L1 on PC-3 cells correlated with elevated IFN-γ levels in the cultures (, left panel). To directly assess the effects of interferon on PD-L1 levels, PC-3 cells were cultured with either IFN-α or IFN-γ for 48 h. and the surface levels of BTN3, PD-1, PD-L2, and PD-L2 were measured. Treatment with either IFN-α or IFN-γ significantly increased the expression of PD-L1 (MFI increased 4.3-fold for IFN-α and 6.2-fold for IFN-γ) but had a lesser effect on PD-L2 (MFI increased 1.3-fold for IFN-α and 1.6-fold for IFN-γ) (). The surface expression of BTN3 was not affected by either treatment while PD-1 levels decreased (MFI decreased up to 2.6-fold) (). These results suggest that IFN-γ or other cytokines released by Vγ2Vδ2 T cells upon tumor recognition can further increase PD-L1 levels on surrounding tumor cells potentially decreasing tumor immunity.
Figure 5. IFN-γ and IFN-α increase the surface expression of PD-L1 on PC-3 prostate cancer cells. PC-3 cells were treated with IFN-γ or IFN-α at the indicated concentrations for 48 h. Cells were then stained with various mAbs and surface expression of BTN3, PD-1, PD-L1, and PD-L2 assessed by flow cytometric analysis. (a) Representative histograms of surface expression of various proteins by PC-3 cells cultured under the indicated conditions. Representative of two experiments. Solid histograms show the specific antibody staining while open histograms show control mAb staining. (b) Expression of PD-L1 (MFI) on PC-3 cells after treatment with IFN-γ or IFN-α for 48 h
Combination therapy with PD-1 checkpoint blockade and adoptive transfer of Vγ2Vδ2 T cells significantly improves tumor control in a preclinical NSG mouse model of prostate cancer
Given that PC-3 cells express PD-L1 and that Vγ2Vδ2 T cells rapidly upregulate PD-1 upon reactivation, the effect of combining the adoptive transfer of human Vγ2Vδ2 T cells with PD-1 checkpoint blockade on immunity to PC-3 tumors was tested in immunodeficient NSG mice. Treatment with an anti-PD-1 mAb or an isotype control was begun at day 13 along with pamidronate followed by the adoptive transfer of Vγ2Vδ2 T cells one day later (day 14). A second dose of mAb was administered on day 16 (). This cycle of treatment was repeated weekly for a total of five cycles. We found that the addition of anti-PD-1 mAb or isotype control mAb treatment to pamidronate-treated mice had no effect on PC-3 tumor growth (). Treatment with pamidronate and adoptively transferred Vγ2Vδ2 T cells slowed PC-3 tumor growth but did not diminish tumor size. However, the addition of the anti-PD-1 mAb to adoptively transferred Vγ2Vδ2 T cells, significantly reduced PC-3 tumor volume to near zero as compared to the tumor volume in mice treated with an isotype control mAb or left untreated (). This treatment was not curative because tumor growth was noted by 1.5 weeks after treatment ended. In conclusion, the addition of PD-1 checkpoint blockade to adoptively transferred Vγ2Vδ2 T cells with pamidronate treatment significantly enhanced anti-tumor immunity shrinking tumor volumes to near zero.
Figure 6. PD-1 checkpoint blockade enhances immunity by adoptively transferred Vγ2Vδ2 T cells against PC-3 prostate tumors. (a) Schema of treatment protocol used to evaluate the anti-tumor efficacy of Vγ2Vδ2 T cells with PD-1 checkpoint blockade. Human PC-3 prostate cancer cells were injected s.c. into NSG mice on day 0. On day 13, pamidronate (50 μg/kg) was given i.v. with either an anti-PD-1 or an isotype control mAb (200 μg/mouse) injected i.p. On day 14, 1 × 106 purified Vγ2Vδ2 T cells were inoculated i.v. Two days later, mAb injections were repeated. Treatments continued until week 6. Longitudinal and transverse diameters of the tumors were measured weekly. (b) (left panel) Combination of PD-1 checkpoint blockade and adoptive transfer of Vγ2Vδ2 T cells significantly reduces prostate tumor volume in NSG mice compared with adoptive transfer only. Mean PC-3 tumor volume ± SD is shown for 6–8 mice per group treated with either pamidronate alone (open triangles), pamidronate with purified Vγ2Vδ2 T cells (open squares), pamidronate with control mAb (open circles), pamidronate with anti-PD-1 mAb (closed inverted triangles), pamidronate with purified Vγ2Vδ2 T cells and control antibody (closed triangles), or pamidronate with purified Vγ2Vδ2 T cells and anti-PD-1 mAb (closed circles). **p < .01, mean tumor volume of mice treated with anti-PD-1 mAb and adoptive transfer of Vγ2Vδ2 T cells versus adoptive transfer of Vγ2Vδ2 T cells alone using the Mann-Whitney U test. (right panel) Tumor volume at week 7 of individual mice treated with pamidronate and anti-PD-1 mAb (open triangles), pamidronate with purified Vγ2Vδ2 T cells and control mAb (open circles), or pamidronate with purified Vγ2Vδ2 T cells and anti-PD-1 mAb (closed circles). Bars represent mean values. **p < .01, ***p < .001 using the Mann-Whitney U test
Discussion
Adoptive transfer of Vγ2Vδ2 T cells for cancer immunotherapy has been proven safe in clinical trials but needs to be more effective. In this study, various approaches were tried to improve Vγ2Vδ2 T cell therapy. We first sought to determine if increasing the number of transferred Vγ2Vδ2 T cells would enhance tumor immunity in NSG mice. Despite increasing Vγ2Vδ2 T cells by 62-fold, no improvement in tumor immunity was observed. This finding suggested that the inability of Vγ2Vδ2 T cells to stop tumor growth was not simply due to insufficient numbers of Vγ2Vδ2 T cells. Instead, the tumor microenvironment might play a key role in determining treatment effectiveness. The expression of ligands for checkpoint receptors is one way that tumors protect themselves from T cells. Therefore, we examined the expression of checkpoint receptors by Vγ2Vδ2 T cells during the 14-day ex vivo expansion period used in clinical trials. During expansion, Vγ2Vδ2 T cells rapidly upregulated PD-1 and CTLA-4 followed by high-level expression of TIM-3 and LAG-3 checkpoint receptors. Cryopreserved Vγ2Vδ2 T cells continued to express TIM-3 and LAG-3 upon thawing and re-expressed PD-1 upon stimulation with bisphosphonate-treated PC-3 cells. These findings suggested that engagement of these receptors on Vγ2Vδ2 T cells might inhibit their tumor immunity. The potential importance of the PD-1/PD-L1 interaction was demonstrated by the fact that checkpoint blockade with anti-PD-1 mAbs enhanced Vγ2Vδ2 T cell tumor immunity to effect near complete remissions of established PC-3 prostate tumors in mice. Taken together, these findings provide support for the addition of PD-1 checkpoint blockade to adoptive immunotherapy with Vγ2Vδ2 T cells to increase tumor immunity. However, further studies with other tumors are required to establish the broad applicability of checkpoint therapies in adoptive Vγ2Vδ2 T cell therapy.
To facilitate the development of Vγ2Vδ2 T cell therapies, it would be useful if expanded Vγ2Vδ2 T cells could be cryopreserved. This would allow for the use of centralized facilities where Vγ2Vδ2 T cells could be expanded under good manufacturing practices and costs and efficiency optimized. Because Vγ2Vδ2 T cells show no alloreactivity,Citation61 cancer patients can be treated, even if their Vγ2Vδ2 T cells cannot be expanded, by using allogeneic Vγ2Vδ2 T cells as an off-the-shelf treatment. Such an approach has been used to successfully treat a patient with cholangiocarcinomaCitation31 and to improve the survival of patients with liver and lung cancer.Citation32 We therefore evaluated cryopreserved Vγ2Vδ2 T cells for their functional activity. We used standard laboratory cryopreservation techniques using a 10% DMSO/90% FCS cryopreservation solution for freezing both the original leukopac PBMC and the purified Vγ2Vδ2 T cells. Although cryopreserved Vγ2Vδ2 T cells could control tumor growth in vivo, they were not as potent as freshly expanded Vγ2Vδ2 T cells.Citation45 These findings are similar to those of others who have noted slightly reduced or similar activity on cryopreservation of T and NK cells.Citation62 Decreasing the concentration of DMSO to 5–7.5% might improve Vγ2Vδ2 T cell tumor immunity given that freezing αβ T cells twice in 10% DMSO diminishes their cytotoxic activity but no decreases were noted with 5% DMSO.Citation63 Although cryopreservation in 5% DMSO caused subtle damage to CAR T cells, freezing did not diminish their clinical effectivenessCitation64 and cryopreserved CAR T cells showed similar activity to freshly prepared CAR T cells in a mouse model.Citation65 The use of 6% pentastarch with 4% human serum albuminCitation64 or a Plasma-Lyte/human serum albumin/dextrose/dextran solution (used by the June laboratory)Citation66 might improve activity and is compatible with direct use in patients. Finally, the use of a controlled rate freezer during the freezing process would be preferable for consistency and thawing in 5% human serum albumin has been shown to decrease Vγ2Vδ2 T cell death.Citation67
Studies with murine CD8 αβ T cells have demonstrated that anti-tumor immune responses correlate with the number of transferred T cells such that increasing the number of tumor-specific CD8 αβ T cells improves tumor control and prolongs survival.Citation49 Although certain subsets control tumor growth more efficiently, even inefficient T cell subsets control tumor growth if higher numbers are transferred.Citation49 In contrast, we found that increasing the number of transferred Vγ2Vδ2 T cells, even by 62-fold, does not lead to better tumor control. These results suggested that the tumor microenvironment might be limiting the effectiveness of their tumor immunity.
One way that tumors inhibit T cell immunity is to express ligands for inhibitory receptors. In our earlier study, Vγ2Vδ2 T cells controlled the growth of established PC-3 tumors but could not shrink them.Citation45 Vγ2Vδ2 T cells activation at the periphery of the tumor might limit the killing of tumor cells located more centrally. Here, we demonstrate one possible mechanism. Vγ2Vδ2 T cells stimulated by pamidronate-pulsed tumor cells secreted IFN-γ leading to PD-L1 upregulation on bystander PC-3 cells (). If this occurs in vivo, this increase in PD-L1 expression could protect PC-3 tumor cells from attack by Vγ2Vδ2 T cells. The 6.2-fold increase in PD-L1 on PC-3 cells with IFN-γ treatment is consistent with studies detailing the responsiveness of PC-3 cells to IFN-γ treatment and the effects of IFN-γ treatment on PD-L1 expression on other tumor cells. The PC-3 cell line is responsive to IFN-γ as evidenced by upregulaton of HLA class I and HLA-DR molecules after IFN-γ treatment.Citation53 IFN-γ treatment increased PD-L1 an average of 4.9-fold on 28/30 tumor cell lines of various lineages.Citation68 The increased PD-L1 levels were noted at the earliest time-point tested (24 h) with an EC50% of 0.5–1.0 ng/ml and maximal levels at 10 ng/ml IFN-γ.Citation68 This level is below the IFN-γ levels measured in our transwell experiments. Thus, the 6.2-fold increase in PD-L1 by PC-3 cells after IFN-γ treatment is similar to other tumor cell lines. Upregulation of PD-L1 requires IFN-γ binding to the type II interferon receptor activating the JAK1/JAK2/STAT1/STAT2/STAT3/IRF1 axis as described for melanoma cellsCitation69 and other tumors.Citation68,Citation70,Citation71 Thus, PD-L1 could be inhibiting anti-tumor immunity by Vγ2Vδ2 T cells if they express PD-1.
Consistent with this possibility, Vγ2Vδ2 T cells express PD-1 as well as other inhibitory receptors during expansion for adoptive transfer and when restimulated by bisphosphonate-treated tumor cells (). During the two-week expansion period typically used in clinical trials, PD-1, CTLA-4, LAG-3, and TIM-3 were rapidly upregulated on Vγ2Vδ2 T cells by day 3. The expression of PD-1 is consistent with previous studies demonstrating increased surface PD-1 expression during ex vivo expansion of Vγ2Vδ2 T cells on all donors tested.Citation50–52,Citation72,Citation73 While surface expression of PD-1 and CTLA-4 was lost by day 14, high-level expression of LAG-3 and TIM-3 persisted.
The upregulation of inhibitory receptors on Vγ2Vδ2 T cells after TCR stimulation is very similar to their upregulation on human CD8 αβ T cells.Citation74 Three days after activation by anti-CD3 and anti-CD28 mAbs, human CD8 αβ T cells upregulate PD-1, CTLA-4, LAG-3, and TIM-3, as well as BTLA.Citation41 The inhibitory receptors are expressed on a homogeneous, activated, blast population leading the authors to conclude that the expression of inhibitory receptors does not always mark dysfunctional exhausted T cells, but expression can also be tightly linked to activation and differentiation of normal T cells.Citation41
Further insights into how the expression of the inhibitory receptors is controlled have been reported for murine CD8 T cells. Like in humans, naive murine CD8 αβ T cells stimulated with anti-CD3/CD28 upregulate multiple inhibitory receptors (PD-L1, LAG-3, CTLA-4, TIM-3, and TIGIT) that can be strongly enhanced by IL-27, a cytokine in the IL-12 family.Citation75 The main difference is that PD-1 is not upregulated in these naive CD8 αβ T cells and some inhibitory receptors were only expressed on a small proportion of the cells without IL-27. Expression of the inhibitory receptors increases with the strength of anti-CD3/CD28 signaling. With weak TCR stimulation, inhibitory receptor expression either requires or can be strongly enhanced by IL-27. Thus, like in humans, murine CD8 αβ T cells upregulate inhibitory receptors after TCR activation although with an increased requirement for IL-27. They did not test memory T cells that may require less IL-27 signaling.Citation75
IL-27 links inhibitory receptor expression on normal T cells due to activation by TCR and CD28 with inhibitory receptor expression on “exhausted” T cells found in chronic viral infections and cancer where inhibitory receptors are constitutively expressed on non-blastic T cells. “Exhausted” murine TIL cells isolated from melanoma tumors, express a module of co-inhibitory receptors (PD-1, LAG-3, TIM-3, and TIGIT).Citation38 These receptors are part of a larger co-inhibitory gene program that is shared in several physiological contexts and driven by IL-27. The transcription factors, PRDM1 and c-MAF, function as cooperative regulators of the module.Citation38 We propose that strong TCR stimulation of Vγ2Vδ2 T cells, such as is afforded by exposure to bisphosphonate-treated tumor cells, partially activates the co-inhibitory module due to induction of PRDM1 and/or c-MAF perhaps through IL-27- or TCR-signaling. A similar array of inhibitory receptors is expressed by Vγ2Vδ2 T cells as is expressed by activated CD8 αβ T cells and exhausted TIL cells including PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT. Unlike the situation with exhausted T cells, co-inhibitory module expression is transient and not chronic. This may serve to limit Vγ2Vδ2 T cells activity to prevent tissue damage.
The expression of inhibitory receptors has conventionally been associated with the “exhaustion” of T cells that have been chronically stimulated. “Exhausted” Vγ2Vδ2 T cells may exist given that PD-1 is expressed on 20% of anergic bone marrow Vγ2Vδ2 T cells in patients with multiple myeloma.Citation76 Our findings demonstrate that coordinate inhibitory receptor expression on Vγ2Vδ2 T cells also occurs during their activation and differentiation. Because our expansion protocol closely follows those used in clinical trials and because PD-1 expression has been observed on activation of Vγ2Vδ2 T cells for all donors tested,Citation50–52 expression of inhibitory receptors on Vγ2Vδ2 T cells might play a role in limiting their clinical effectiveness.
If expanded Vγ2Vδ2 T cells lack expression of PD-1 after cryopreservation, how does PD-1 checkpoint blockade enhance their in vivo tumor immunity? We propose that this effect is due to the transient expression of PD-1 following activation of Vγ2Vδ2 T cells in vivo. Systemic bisphosphonate (pamidronate) treatment of the tumor-bearing immunodeficient mice inhibits FDPS in PC-3 cells resulting in increases in IPP levels that persist for 2–3 days and that are sensed by BTN3A1.Citation46 Vγ2Vδ2 T cells adoptively transferred 1 day after pamidronate treatment will encounter stimulatory PC-3 cells that activate Vγ2Vδ2 T cell through their TCRs for killing and cytokine release, including IFN-γ. Activation upregulates PD-1 and other inhibitory receptors on the Vγ2Vδ2 T cells as observed in vitro () and the IFN-γ released upregulates PD-L1 on tumor cells. PD-1 binding to PD-L1 on PC-3 cells inhibits tumor immunity by the infiltrating Vγ2Vδ2 T cells. This inhibition can be reversed by PD-1 checkpoint blockade explaining the increased tumor immunity observed.
Given that PD-1 upregulation requires TCR stimulation, we hypothesize that this delay in expression coupled with strong TCR stimulation and the lack of upregulation of PD-L1 on the tumor targets makes it difficult to see the effects of anti-PD-1 mAbs in short-term in vitro assays. In contrast, in vivo tumor immunity likely requires repeated tumor cell killing over days after TCR activation allowing for PD-1 upregulation on Vγ2Vδ2 T cells as well as upregulation of PD-L1 on tumor cells. We propose that this is why anti-PD-1 mAbs show activity in vivo whereas it is difficult to demonstrate activity in vitro. Our findings are consistent with studies reported by the Minato lab.Citation50 They could only demonstrate PD-1-mediated inhibition with weak TCR stimulation by Daudi cells that overexpress PD-L1. PD-L1 overexpression on Daudi cells treated with zoledronic acid, only slightly inhibited cytotoxicity and anti-PD-L1 mAbs had no effect on cytotoxicity or CD107a epxression by PD-1+ Vγ2Vδ2 T cells cultured with various tumor cell lines treated with zoledronic acid (six were tested including PC-3 cells). Similarly, anti-PD-1 mAbs had no effect on Vγ2Vδ2 T cell lysis of three AML tumor cell lines treated with zoledronic acid or on CD107a expression stimulated by four different tumor cells treated with zoledronic acid.Citation72
How do these results with Vγ2Vδ2 T cells relate to T cells from cancer patients? In a study by Gestermann et al.,Citation77 TIL cells were isolated from melanoma patients and expanded in vitro (many TILs were Melan-A-specific). Expanded TILs (10–14 days after anti-CD3+IL-2 stimulation) upregulate PD-1 by day 2 and continue to express PD-1, LAG-3, TIM-3, and BTLA at day 5.Citation77 Moreover, autologous melanoma cells, co-cultured with TILs, upregulate PD-L1 as well as MHC class I and class II.Citation77 Despite the fact that the TILs express inhibitory receptors, mAbs against these receptors did not enhance TIL IFN-γ production in short-term 5-hour assays. However, if the co-culture period was extended to 5 days, anti-LAG-3 and anti-LAG-3 plus anti-PD-1 mAbs enhanced IFN-γ production.Citation77 Further supporting our findings, anti-PD-1 mAbs helped to boost natural- and antibody-dependent cellular cytotoxicity by Vγ2Vδ2 T cells against B cell lymphoma cells treated with anti-CD20 mAbs in short-term preclinical mouse studies.Citation78 Thus, the findings observed with Vγ2Vδ2 T cells closely parallel those with CD8 αβ TILs.
There are several limitations to this study. First, there are significant differences in the microenvironment in immunodeficient NSG mice and in human patients. Immunodeficient mice do not have regulatory T cells so these are not present in the tumor microenvironment. The mice lack CD4 and CD8 T cells as well as NK cells and B cells so there is no recruitment of other T, NK, or B cells to the tumor. Also, murine ligands for co-inhibitory, co-stimulatory, and adhesion receptors do not always bind to human receptors potentially limiting interactions between murine and human cells. Mice lack BTN3A1 and BTN2A1 molecules that are essential for stimulation by prenyl pyrophosphates and bisphosphonate such that Vγ2Vδ2 T cells cannot be stimulated through their TCRs by murine cells. Despite these restrictions, murine PD-L1 does bind to human PD-1 with similar affinity to allow checkpoint blockade activity.Citation76 Thus, the PC-3 model does allow one to test the effect of anti-PD-1 treatment in the setting of infiltrating murine stromal and other cells making up the tumor microenvironment. Although these murine cells cannot present phosphoantigens, these cells can express murine PD-L1 molecules that are capable of binding to human PD-1 and transmitting negative signals to the Vγ2Vδ2 T cells.76
Second, because only one prostate cancer cell line was tested in this study, assessing additional tumors from different lineages would be important in future studies to extend the applicability of our results. The PC-3 prostate cancer line used was established from a bony metastasis from a patient treated with castration and diethylstilbestrol (a synthetic estrogen analog). There were no malignant cells in the prostate on autopsy indicating that cancer at the site of its origin was cured.Citation79 PC-3 cells have characteristics of prostatic small-cell carcinoma. These tumors are of neuroendocrine origin and lack androgen receptors and PSA.Citation80 Although de novo prostatic small-cell carcinomas are rare (0.5–2% of prostate tumors),Citation81 treatment-emergent small-cell neuroendocrine prostate cancers (which was likely to have been the case with PC-3) are present in nearly 20% of patients with metastatic castration-resistant prostate cancer.Citation82 Both diseases have poor prognoses. The PC-3 prostate cell line is an important cell line for the study of prostate cancer and has been cited in numerous publications (6,678 publication on PubMed) underscoring its importance as a major model cell line for human prostate cancer. The PC-3 mouse model is a well-established model for assessing Vγ2Vδ2 T cell tumor immunity.Citation46 However, adoptive transfer of Vγ2Vδ2 T cells has not been used to treat metastatic prostate cancer so there is no clinical evidence at present supporting their use.
Third, although a number of clinical trials are ongoing, reported studies suggest that currently available checkpoint inhibitors have only limited efficacy in prostate cancer patients.Citation83–85 There are certain molecular subtypes of prostate cancer that do respond, such as those that are deficient in mismatch repair genes (dMMR/microsatellite instability-high), have high tumor mutational burdens, and tumors with biallelic CDK12 mutations, but these subsets likely constitute less than 10% of all prostate cancers.Citation86–88 The lack of response to immunotherapy in most patients is likely due to a low tumor mutational burden (7–15 times lower than melanoma or lung cancer)Citation83 and a highly immunosuppressive tumor microenvironment.Citation84
Given the lack of efficacy of checkpoint inhibition in prostate cancer, the potential differences in the tumor microenvironment in patients versus immunodeficient mice, and the fact that clinical trials with Vγ2Vδ2 T cells are targeting a number of different tumor types, it might be better to test anti-PD-1 therapy with Vγ2Vδ2 T cell treatment in a clinical trial with tumor types already approved for anti-PD-1 treatment. Then, treatment with Vγ2Vδ2 T cells would be in addition to an established, FDA-approved therapy.
The findings in this study are consistent with studies on the adoptive transfer of conventional or CAR-transduced αβ T cells. In preclinical studies, the addition of anti-PD-1 to CAR T therapy enhanced immunity against solid tumors.Citation89–92 Preclinical studies have demonstrated increased tumor immunity by human CAR T cells after PD-1 expression is disruptedCitation93,Citation94 and there are clinical trials in progress testing this approach.Citation95 In clinical studies, the adoptive transfer of neoantigen-specific tumor-infiltrating lymphocytes and PD-1 checkpoint blockade resulted in a complete remission in a patient with metastatic breast cancer.Citation96 Mesothelioma patients treated with locally delivered mesothelin-specific CAR T cells and systemic anti-PD-1 antibodies also have shown encouraging clinical responses. Similarly, successful treatment of patients with metastatic non-small cell lung cancer with TILs has included continued treatment with the anti-PD-1 mAb, nivolumab.Citation97 Thus, the combination of PD-1 checkpoint blockade and Vγ2Vδ2 T cell therapy has strong support from studies with αβ T cells.Citation42
The expression of other inhibitory receptors (CTLA-4, TIM-3, and LAG-3) by Vγ2Vδ2 T cells suggests that further improvements in tumor immunity could be achieved by combining PD-1 checkpoint blockade with antibodies to other inhibitory receptors. A recent study provides support for this idea. Guo et al. found that adding anti-TIM-3 mAbs to treatment with a bi-specific antibody for CD3 and EpCAM and Vγ2Vδ2 T cells, further increased in vivo tumor immunity to breast cancer tumors in immunodeficient mice.Citation98 LAG-3 is also highly expressed on expanded Vγ2Vδ2 T cells. Checkpoint blockade with anti-TIM-3 or anti-LAG-3 antibodies primarily serves to enhance the effector phase of tumor immunity with less potential immune-related adverse effects.Citation99 Therefore, antibodies to these receptors might be safely combined with anti-PD-1 treatment.
Conclusion
Our findings support the premise that combining PD-1 blockade with the adoptive transfer of Vγ2Vδ2 T cells is an effective strategy to control tumor growth in vivo. This could be readily tested in patients by the addition of approved anti-PD-1 mAbs to therapy with Vγ2Vδ2 T cells. The identification of additional inhibitory receptors that can be targeted suggests a potential roadmap to improve the efficacy of Vγ2Vδ2 T cell tumor therapy through further combinations. Because Vγ2Vδ2 T cells target tumors through their isoprenoid metabolism by a mechanism that is independent of their MHC expression and tumor mutational burden, Vγ2Vδ2 T cells could have wide utility in the treatment of solid tumors.
Supplemental Material
Download ()Acknowledgments
We thank Zhimei Fang for technical assistance.
Disclosure statement
YT is a co-inventor of Japanese Patent 2014-257451 on the development of the method to expand γδ T cells using PTA, a novel bisphosphonate prodrug and of Japanese Patent 2014-73475 on the development of a non-radioactive cellular cytotoxicity assay using BM-HT, a precursor of a novel Eu3+ chelate-forming compound. The other authors have no financial or non-financial conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Rast JP, Anderson MK, Strong SJ, Luer C, Litman RT, Litman GW. α, β, γ, and δ T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity. 1997;6(1):1–17. doi:10.1016/s1074-7613(00)80237-x.
- Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22(3):191–217. doi:10.1007/s002810000042.
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215(1):59–76. doi:10.1111/j.1600-065X.2006.00479.x.
- Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375(6527):155–158. doi:10.1038/375155a0.
- Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40(4):490–500. doi:10.1016/j.immuni.2014.03.003.
- Wang H, Morita CT. Sensor function for butyrophilin 3A1 in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J Immunol. 2015;195(10):4583–4594. doi:10.4049/jimmunol.1500314.
- Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, Starick L, Nöhren A, Begley CR, Berwick KA, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity. 2020;52(3):487–498 e6. doi:10.1016/j.immuni.2020.02.014.
- Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 2020;367(6478) :eaay5516. doi:10.1126/science.aay5516.
- Tanaka Y, Iwasaki M, Murata-Hirai K, Matsumoto K, Hayashi K, Okamura H, Sugie T, Minato N, Morita CT, Toi M. Anti-tumor activity and immunotherapeutic potential of a bisphosphonate prodrug. Sci Rep. 2017;7(1):5987. doi:10.1038/s41598-017-05553-0.
- Wang H, Sarikonda G, Puan K-J, Tanaka Y, Feng J, Giner J-L, Cao R, Mönkkönen J, Oldfield E, Morita CT. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187(10):5099–5113. doi:10.4049/jimmunol.1002697.
- Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein BS, Voss SD, Morrissey LW, DeMars R, Welch WJ, et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250(4985):1269–1273. doi:10.1126/science.1978758.
- Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006.
- Zheng B, Lam C, Im S, Huang J, Luk W, Lau SY, Yau KK, Wong C, Yao K, Ng MH. Distinct tumour specificity and IL-7 requirements of CD56− and CD56+ subsets of human γδ T cells. Scand J Immunol. 2001;53(1):40–48. doi:10.1046/j.1365-3083.2001.00827.x.
- Harly C, Guillaume Y, Nedellec S, Peigné C-M, Mönkkönen H, Mönkkönen J, Li J, Kuball J, Adams EJ, Netzer S, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120(11):2269–2279. doi:10.1182/blood-2012-05-430470.
- Cano CE, Pasero C, De Gassart A, Kerneur C, Gabriac M, Fullana M, Granarolo E, Hoet R, Scotet E, Rafia C, et al. BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell cytotoxicity against malignant cells. Cell Rep. 2021;36(2):109359. doi:10.1016/j.celrep.2021.109359.
- Gober H-J, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–168. doi:10.1084/jem.20021500.
- Li J, Herold MJ, Kimmel B, Müller I, Rincon-Orozco B, Kunzmann V, Herrmann T. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by Vγ9Vδ2 T cells. J Immunol. 2009;182(12):8118–8124. doi:10.4049/jimmunol.0900101.
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi:10.1038/nm.3909.
- Vella M, Coniglio D, Abrate A, Scalici Gesolfo C, Lo Presti E, Meraviglia S, Serretta V, Simonato A. Characterization of human infiltrating and circulating gamma-delta T cells in prostate cancer. Investig Clin Urol. 2019;60(2):91–98. doi:10.4111/icu.2019.60.2.91.
- Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57(11):1599–1609. doi:10.1007/s00262-008-0491-8.
- Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37(8):956–968. doi:10.1016/j.exphem.2009.04.008.
- Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105(6):778–786. doi:10.1038/bjc.2011.293.
- Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60(8):1075–1084. doi:10.1007/s00262-011-1021-7.
- Sakamoto M, Nakajima J, Murakawa T, Fukami T, Yoshida Y, Murayama T, Takamoto S, Matsushita H, Kakimi K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδT cells: a phase I clinical study. J Immunother. 2011;34(2):202–211. doi:10.1097/CJI.0b013e318207ecfb.
- Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, Ariyoshi N, Goto S. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy. 2011;13(1):92–97. doi:10.3109/14653249.2010.515581.
- Izumi T, Kondo M, Takahashi T, Fujieda N, Kondo A, Tamura N, Murakawa T, Nakajima J, Matsushita H, Kakimi K. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy. 2013;15(4):481–491. doi:10.1016/j.jcyt.2012.12.004.
- Wada I, Matsushita H, Noji S, Mori K, Yamashita H, Nomura S, Shimizu N, Seto Y, Kakimi K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014;3(2):362–375. doi:10.1002/cam4.196.
- Okawaki M, Hironaka K, Yamanura M, Yamaguchi Y. Adoptive immunotherapy using autologous lymphocytes activated ex vivo with antigen stimulation for patients with incurable cancer. Kawasaki Med J. 2014;40(1):33–39. doi:10.11482/-E40(1)33.
- Yamaguchi Y, Katata Y, Okawaki M, Sawaki A, Yamamura M. A prospective observational study of adoptive immunotherapy for cancer using zoledronate-activated killer (ZAK) cells - an analysis for patients with incurable pancreatic cancer. Anticancer Res. 2016;36:2307–2313.
- Aoki T, Matsushita H, Hoshikawa M, Hasegawa K, Kokudo N, Kakimi K. Adjuvant combination therapy with gemcitabine and autologous γδ T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 2017;19(4):473–485. doi:10.1016/j.jcyt.2017.01.002.
- Alnaggar M, Xu Y, Li J, He J, Chen J, Li M, Wu Q, Lin L, Liang Y, Wang X, et al. Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7(1):36. doi:10.1186/s40425-019-0501-8.
- Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J, Yang J, Hu Y, Chen Y, Lin L, et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2020;18(2):427–439. doi:10.1038/s41423-020-0515-7.
- Kakimi K, Matsushita H, Masuzawa K, Karasaki T, Kobayashi Y, Nagaoka K, Hosoi A, Ikemura S, Kitano K, Kawada I, et al. Adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory non-small-cell lung cancer: a multicenter, open-label, single-arm, phase 2 study. J Immunother Cancer. 2020;8(2):e001185. doi:10.1136/jitc-2020-001185.
- Sato Y, Mori K, Hirano K, Yagi K, Kobayashi Y, Nagaoka K, Hosoi A, Matsushita H, Kakimi K, Seto Y. Adoptive γδT-cell transfer alone or combined with chemotherapy for the treatment of advanced esophageal cancer. Cytotherapy. 2021;23(5):423–432. doi:10.1016/j.jcyt.2021.02.002.
- Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30(2):575–579.
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi:10.1016/j.cell.2017.01.017.
- Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–1434. doi:10.1038/s41590-019-0512-0.
- Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, Nyman J, Marjanovic ND, Kowalczyk MS, Wang C, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558(7710):454–459. doi:10.1038/s41586-018-0206-z.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature13954.
- Wherry EJ. T cell exhaustion. Nat Immunol. 2010;12(6):492–499. doi:10.1038/ni.2035.
- Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front Immunol. 2013;4(455). doi:10.3389/fimmu.2013.00455.
- Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–482. doi:10.1016/j.ccell.2019.09.006.
- Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Müller S, et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2015;22(8):1969–1977. doi:10.1158/1078-0432.CCR-15-2042.
- Schepisi G, Cursano MC, Casadei C, Menna C, Altavilla A, Lolli C, Cerchione C, Paganelli G, Santini D, Tonini G, et al. CAR-T cell therapy: a potential new strategy against prostate cancer. J Immunother Cancer. 2019;7(1):258. doi:10.1186/s40425-019-0741-7.
- Nada MH, Wang H, Workalemahu G, Tanaka Y, Morita CT. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation. J Immunother Cancer. 2017;5(1):9. doi:10.1186/s40425-017-0209-6.
- Santolaria T, Robard M, Léger A, Catros V, Bonneville M, Scotet E. Repeated systemic administrations of both aminobisphosphonates and human Vγ9Vδ2 T cells efficiently control tumor development in vivo. J Immunol. 2013;191(4):1993–2000. doi:10.4049/jimmunol.1300255.
- Foreman O, Kavirayani AM, Griffey SM, Reader R, Shultz LD. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Vet Pathol. 2011;48(2):495–499. doi:10.1177/0300985810378282.
- Tanaka Y, Murata-Hirai K, Iwasaki M, Matsumoto K, Hayashi K, Kumagai A, Nada MH, Wang H, Kobayashi H, Kamitakahara H, et al. Expansion of human γδ T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci. 2018;109(3):587–599. doi:10.1111/cas.13491.
- Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17(16):5343–5352. doi:10.1158/1078-0432.CCR-11-0503.
- Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur J Immunol. 2011;41(2):345–355. doi:10.1002/eji.201040959.
- Hsu H, Boudova S, Mvula G, Divala TH, Mungwira RG, Harman C, Laufer MK, Pauza CD, Cairo C. Prolonged PD1 expression on neonatal Vδ2 lymphocytes dampens proinflammatory responses: role of epigenetic regulation. J Immunol. 2016;197(5):1884–1892. doi:10.4049/jimmunol.1600284.
- Zumwalde NA, Sharma A, Xu X, Ma S, Schneider CL, Romero-Masters JC, Hudson AW, Gendron-Fitzpatrick A, Kenney SC, Gumperz JE. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight. 2017;2(13):e93179. doi:10.1172/jci.insight.93179.
- Carlsson B, Forsberg O, Bengtsson M, Tötterman TH, Essand M. Characterization of human prostate and breast cancer cell lines for experimental T cell-based immunotherapy. Prostate. 2007;67(4):389–395. doi:10.1002/pros.20498.
- Sabatos-Peyton CA, Nevin J, Brock A, Venable JD, Tan DJ, Kassam N, Xu F, Taraszka J, Wesemann L, Pertel T, et al. Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology. 2018;7(2):e1385690. doi:10.1080/2162402X.2017.1385690.
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim Y-LE, Lee H-H, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918–1930. doi:10.4049/jimmunol.0903059.
- Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153(9):3979–3988.
- Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the activation of human γδ T cells by tumor cells sensitized with nonpeptide antigens. J Immunol. 2006;177(2):877–884. doi:10.4049/jimmunol.177.2.877.
- Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15(1):83–93. doi:10.1016/s1074-7613(01)00168-6.
- Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of Vγ9Vδ2 T Cells by NKG2D. J Immunol. 2005;175(4):2144–2151. doi:10.4049/jimmunol.175.4.2144.
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204(2):345–356. doi:10.1084/jem.20061890.
- Kabelitz D, Bender A, Schondelmaier S, da Silva Lobo ML, Janssen O. Human cytotoxic lymphocytes. V. Frequency and specificity of γδ+ cytotoxic lymphocyte precursors activated by allogeneic or autologous stimulator cells. J Immunol. 1990;145:2827–2832.
- Li R, Johnson R, Yu G, McKenna DH, Hubel A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy. 2019;21(9):943–957. doi:10.1016/j.jcyt.2019.07.004.
- Worsham DN, Reems J-A, Szczepiorkowski ZM, McKenna DH, Leemhuis T, Mathew AJ, Cancelas JA. Clinical methods of cryopreservation for donor lymphocyte infusions vary in their ability to preserve functional T-cell subpopulations. Transfusion (Paris). 2017;57(6):1555–1565. doi:10.1111/trf.14112.
- Panch SR, Srivastava SK, Elavia N, McManus A, Liu S, Jin P, Highfill SL, Li X, Dagur P, Kochenderfer JN, et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol Ther. 2019;27(7):1275–1285. doi:10.1016/j.ymthe.2019.05.015.
- Xu H, Cao W, Huang L, Xiao M, Cao Y, Zhao L, Wang N, Zhou J. Effects of cryopreservation on chimeric antigen receptor T cell functions. Cryobiology. 2018;83:40–47. doi:10.1016/j.cryobiol.2018.06.007.
- Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040–1047. doi:10.1056/NEJMoa1504542.
- Burnham RE, Tope D, Branella G, Williams E, Doering CB, Spencer HT. Human serum albumin and chromatin condensation rescue ex vivo expanded γδ T cells from the effects of cryopreservation. Cryobiology. 2021;99:78–87. doi:10.1016/j.cryobiol.2021.01.011.
- Mimura K, Teh JL, Okayama H, Shiraishi K, Kua L-F, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2017;109(1):43–53. doi:10.1111/cas.13424.
- Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. doi:10.1016/j.celrep.2017.04.031.
- Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi:10.1038/bjc.2015.101.
- Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, Pan F, Topalian SL. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7(1):305. doi:10.1186/s40425-019-0770-2.
- Hoeres T, Holzmann E, Smetak M, Birkmann J, Wilhelm M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. Oncoimmunology. 2019;8(3):1550618. doi:10.1080/2162402x.2018.1550618.
- Hsu H, Boudova S, Mvula G, Divala TH, Rach D, Mungwira RG, Boldrin F, Degiacomi G, Manganelli R, Laufer MK, et al. Age-related changes in PD-1 expression coincide with increased cytotoxic potential in Vδ2 T cells during infancy. Cell Immunol. 2021;359:104244. doi:10.1016/j.cellimm.2020.104244.
- Fuertes Marraco SA, Neubert NJ, Verdeil G, Speiser DE. Inhibitory receptors beyond T cell exhaustion. Front Immunol. 2015;6:310. doi:10.3389/fimmu.2015.00310.
- DeLong JH, O’Hara Hall A, Rausch M, Moodley D, Perry J, Park J, Phan AT, Beiting DP, Kedl RM, Hill JA, et al. IL-27 and TCR stimulation promote T cell expression of multiple inhibitory receptors. Immunohorizons. 2019;3(1):13–25. doi:10.4049/immunohorizons.1800083.
- Castella B, Foglietta M, Sciancalepore P, Rigoni M, Coscia M, Griggio V, Vitale C, Ferracini R, Saraci E, Omede P, et al. Anergic bone marrow Vγ9Vδ2 T cells as early and long-lasting markers of PD-1-targetable microenvironment-induced immune suppression in human myeloma. Oncoimmunology. 2015;4(11):e1047580. doi:10.1080/2162402X.2015.1047580.
- Gestermann N, Saugy D, Martignier C, Tillé L, Fuertes Marraco SA, Zettl M, Tirapu I, Speiser DE, Verdeil G. LAG-3 and PD-1+LAG-3 inhibition promote anti-tumor immune responses in human autologous melanoma/T cell co-cultures. Oncoimmunology. 2020;9(1):1736792. doi:10.1080/2162402X.2020.1736792.
- Rossi C, Gravelle P, Decaup E, Bordenave J, Poupot M, Tosolini M, Franchini D-M, Laurent C, Morin R, Lagarde J-M, et al. Boosting γδ T cell-mediated antibody-dependent cellular cytotoxicity by PD-1 blockade in follicular lymphoma. Oncoimmunology. 2019;8(3):1554175. doi:10.1080/2162402X.2018.1554175.
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17(1):16–23.
- Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71(15):1668–1679. doi:10.1002/pros.21383.
- Wang J, Liu X, Wang Y, Ren G. Current trend of worsening prognosis of prostate small cell carcinoma: a population-based study. Cancer Med. 2019;8(15):6799–6806. doi:10.1002/cam4.2551.
- Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, Weinstein AS, Friedl V, Zhang C, Witte ON, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492–2503. doi:10.1200/JCO.2017.77.6880.
- Venkatachalam S, McFarland TR, Agarwal N, Swami U. Immune checkpoint inhibitors in prostate cancer. Cancers (Basel). 2021;13(9):2187. doi:10.3390/cancers13092187.
- Stultz J, Fong L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021;24(3):697–717. doi:10.1038/s41391-021-00340-5.
- de Almeida DVP, Fong L, Rettig MB, Autio KA. Immune checkpoint blockade for prostate cancer: niche role or next breakthrough? Am Soc Clin Oncol Educ Book. 2020;40:e89-e106. doi:10.1200/EDBK_278853.
- Sena LA, Fountain J, Isaacsson Velho P, Lim SJ, Wang H, Nizialek E, Rathi N, Nussenzveig R, Maughan BL, Velez MG, et al. Tumor frameshift mutation proportion predicts response to immunotherapy in mismatch repair-deficient prostate cancer. The Oncologist. 2021;26(2):e270–e278. doi:10.1002/onco.13601.
- Ritch E, Fu SYF, Herberts C, Wang G, Warner EW, Schonlau E, Taavitsainen S, Murtha AJ, Vandekerkhove G, Beja K, et al. Identification of hypermutation and defective mismatch repair in ctDNA from metastatic prostate cancer. Clin Cancer Res. 2020;26(5):1114–1125. doi:10.1158/1078-0432.CCR-19-1623.
- Abida W, Cheng ML, Armenia J, Middha S, Autio KA Vargas HA, Rathkopf D, Morris MJ, Danila DC, Slovin SF, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471–478. doi:10.1001/jamaoncol.2018.5801.
- Moon EK, Ranganathan R, Eruslanov E, Kim S, Newick K, O’Brien S, Lo A, Liu X, Zhao Y, Albelda SM. Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clin Cancer Res. 2016;22(2):436–447. doi:10.1158/1078-0432.CCR-15-1070.
- Serganova I, Moroz E, Cohen I, Moroz M, Mane M, Zurita J, Shenker L, Ponomarev V, Blasberg R. Enhancement of PSMA-directed CAR adoptive immunotherapy by PD-1/PD-L1 blockade. Mol Ther Oncolytics. 2017;4:41–54. doi:10.1016/j.omto.2016.11.005.
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi:10.1158/1078-0432.CCR-13-0458.
- Hu Z, Xia J, Fan W, Wargo J, Yang Y-G. Human melanoma immunotherapy using tumor antigen-specific T cells generated in humanized mice. Oncotarget. 2016;7(6):6448–6459. doi:10.18632/oncotarget.7044.
- Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi:10.1038/s41598-017-00462-8.
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–3144. doi:10.1172/JCI83092.
- McGowan E, Lin Q, Ma G, Yin H, Chen S, Lin Y. PD-1 disrupted CAR-T cells in the treatment of solid tumors: promises and challenges. Biomed Pharmacother. 2020;121:109625. doi:10.1016/j.biopha.2019.109625.
- Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–730. doi:10.1038/s41591-018-0040-8.
- Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, Landin AM, Mullinax JE, Saller JJ, Saltos AN, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27(8):1410–1418. doi:10.1038/s41591-021-01462-y.
- Guo Q, Zhao P, Zhang Z, Zhang J, Zhang Z, Hua Y, Han B, Li N, Zhao X, Hou L. TIM-3 blockade combined with bispecific antibody MT110 enhances the anti-tumor effect of γδ T cells. Cancer Immunol Immunother. 2020;69(12):2571–2587. doi:10.1007/s00262-020-02638-0.
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001.
