ABSTRACT
High mobility group B1 (HMGB1) is a protein that is released from dying cancer cells in the context of immunogenic cell death (ICD). A recent study performed on patients with head and neck squamous cell carcinomas (HNSCC) reports that a chemoradiotherapy-induced increase in circulating HMGB1 levels predicts favorable outcome, echoing prior studies on neoadjuvant treatment of breast and rectal cancer in which the dynamics of HMGB1 plasma levels also have prognostic value. Hence, a therapy-induced rise in HMGB1 may be interpreted as a clinical sign of ICD and therapeutic response.
The induction of immunogenic cell death (ICD) in response to anticancer treatment amplifies the adjuvanticity of malignant cells, thus facilitating the chemoattraction of dendritic cells (DC) as well as the uptake, processing and presentation of tumor-associated antigens (TAAs). In essence, ICD enables the reinstatement of immunosurveillance by adaptive immune circuitries and the generation of immunological memory against TAAs, altogether supporting the long-term efficacy of anticancer therapies, an effect that can further be boosted by combination of ICD induction with subsequent immune checkpoint blockade.
Therapeutic application of radiotherapy, chemotherapeutics or targeted agents such as anthracyclines, oxaliplatin, lurbinectidin or crizotinib, all clinically approved for the treatment of a variety of cancer indications, can induce ICD. Immunogenic stress and death is characterized by the emission of danger associated molecular patterns (DAMPs) by cancer cells.Citation1,Citation2 This process follows a discrete spatial and temporal pattern, which is orchestrated by underlying cellular stress and death pathways.Citation1,Citation2 Thus, the chemotactic metabolite ATP is released by tumor cells via autophagy-associated lysosomal secretion. The chaperone calreticulin (CALR) translocates from the lumen of the endoplasmic reticulum to the plasma membrane surface during the integrated stress response (ISR). The cytoplasmic protein annexin A1 (ANXA1) and the nuclear, nonhistone chromatin-binding protein high mobility group box 1 (HMGB1) are both liberated when cell death is fully executed and the plasma membrane becomes permeabilized.
Altogether, the activation of full-blown TAA-specific adaptive anticancer immunity depends on the emission of immunoadjuvant DAMPs by malignant cells and their perception by antigen presenting dendritic cells (DCs). The release of ATP and ANXA1 triggers the chemoattraction into the tumor bed and close approximation toward malignant cells of DC expressing the purinergic receptor P2X7 (P2RX7, for ATP) and formyl peptide receptor 1 (FPR1, for ANXA1). TAA uptake is triggered by the interaction between CALR on the surface of stressed/dying cancer cells and LDL-receptor-related protein 1 (LRP1) on the surface of DCs. HMGB1 stimulates DC maturation and TAA processing through the engagement of toll like receptor 4 (TLR4), followed by downstream signaling via the MYD88 innate immune signal transduction adaptor, altogether facilitating MHC class I-restricted cross-presentation of TAAs by DCs.Citation2,Citation3
HMGB1 can be released by a variety of chemical agents including prototype ICD inducers such as anthracyclines or oxaliplatin but also by epigenetic modifiers including azacitidine, decitabine, and suberoylanilide hydroxamic acid (SAHA).Citation4 The absence of HMGB1 expression by malignant cells undergoing ICD compromises the DC-mediated priming of effector T cells by TAAs.Citation3 Experimentally, the lack of HMGB1 can be compensated and the immunogenicity of dying tumor cells is restored by providing an alternative TLR4 agonist such as dendrophilin.Citation5 There is ample evidence for the clinical importance of HMGB1 adjuvant signaling such as the poor prognosis of breast cancer patients that carry a TLR4 loss-of-function allele and are treated with ICD inducing radio- or chemotherapy as compared to those with the wild type allele.Citation3 Furthermore, the lack of HMGB1 expression in tumor biopsies can be employed as a prognostic biomarker to predict an increased residual risk of relapse after adjuvant chemotherapy in breast cancer.Citation6,Citation7 Consistently, the presence of circulating HMGB1 can be evaluated as a consensus maker of ICD and correlates with good prognosis in patients with rectal or breast cancer treated with neoadjuvant chemotherapy or radiation.Citation8,Citation9
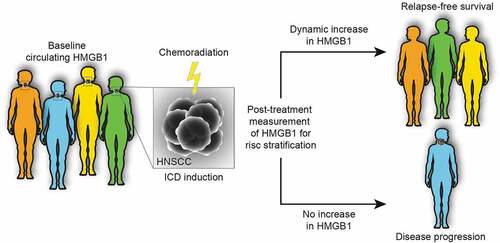
This contention is now echoed by a study evaluating the dynamic adaptation of circulating levels of HMGB1 in response to treatment with definitive chemoradiotherapy in head and neck squamous cell carcinoma (HNSCC) patients over the course of the therapy. A treatment-induced elevation of circulating HMGB1 levels predicted favorable outcome and all patients with augmented HMGB1 remained relapse-free during the course of the study.Citation10 Of note, the prognostic value of circulating HMGB1 is reflected in the amplitude of the dynamics (the difference between baseline and post-treatment levels) rather than the baseline levels, which integrate multiple comorbidities including systemic inflammation and ongoing tissue damage ().
Figure 1. Dynamic monitoring of circulating HMGB1 levels as a predictive marker for therapy outcome. The dynamic changes in circulating levels of high mobility group box 1 (HMGB1) in response to the induction of immunogenic cell death (ICD) by chemoradiation can be considered as a predictor of therapeutic efficacy. It is important to note that the prognostic value is reflected in the difference between baseline and post-treatment levels rather than the baseline level, which may be affected by several confounders such as systemic inflammation or tissue damage
In conclusion, dynamic monitoring of circulating HMGB1 levels might allow to detect the onset of ICD in response to oncological interventions and predict therapeutic outcome in other instances of solid malignancies.Citation10 It remains to be seen whether the difference in circulating HMGB1 concentrations before and after antineoplastic interventions will be solely affected by therapeutic efficacy or whether it may be affected as well by nosocomial infections and treatment-associated toxicities. Moreover, confirmatory studies validating the favorable prognostic impact of HMGB1 elevations are urgently awaited.
Disclosure statement
GK and OK are cofounders of Samsara Therapeutics. GK is a cofounder of Therafast Bio.
Additional information
Funding
References
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337. doi:10.1136/jitc-2019-000337.
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–3. doi:10.1038/nri.2016.107.
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi:10.1038/nm1622.
- Liu P, Zhao L, Loos F, Iribarren K, Lachkar S, Zhou H, Gomes-da-silva LC, Chen G, Bezu L, Boncompain G, et al. Identification of pharmacological agents that induce HMGB1 release. Sci Rep. 2017;7:14915. doi:10.1038/s41598-017-14848-1.
- Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi:10.1038/cdd.2013.72.
- Ladoire S, Penault-Llorca F, Senovilla L, Dalban C, Enot D, Locher C, Prada N, Poirier-Colame V, Chaba K, Arnould L, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11:1878–1890. doi:10.1080/15548627.2015.1082022.
- Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penault-Llorca F, Arnould L, et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy. 2016;12:864–875. doi:10.1080/15548627.2016.1154244.
- Bains SJ, Abrahamsson H, Flatmark K, Dueland S, Hole KH, Seierstad T, Redalen KR, Meltzer S, Ree AH. Immunogenic cell death by neoadjuvant oxaliplatin and radiation protects against metastatic failure in high-risk rectal cancer. Cancer Immunol Immunother. 2020;69:355–364. doi:10.1007/s00262-019-02458-x.
- Exner R, Sachet M, Arnold T, Zinn‐Zinnenburg M, Michlmayr A, Dubsky P, Bartsch R, Steger G, Gnant M, Bergmann M, et al. Prognostic value of HMGB 1 in early breast cancer patients under neoadjuvant chemotherapy. Cancer Med. 2016;5:2350–2358. doi:10.1002/cam4.827.
- Clasen K, Welz S, Faltin H, Zips D, Eckert F. Dynamics of HMBG1 (high mobility group box 1) during radiochemotherapy correlate with outcome of HNSCC patients. Strahlenther Onkol. 2021. doi:10.1007/s00066-021-01860-8.
