ABSTRACT
Gastrointestinal (GI) cancers represent a complex array of cancers that affect the digestive system. This includes liver, pancreatic, colon, rectal, anal, gastric, esophageal, intestinal and gallbladder cancer. Patients diagnosed with certain GI cancers typically have low survival rates, so new therapeutic approaches are needed. A potential approach is to harness the potent immunoregulatory properties of natural killer T (NKT) cells which are true T cells, not natural killer (NK) cells, that recognize lipid instead of peptide antigens presented by the non-classical major histocompatibility (MHC) molecule CD1d. The NKT cell subpopulation is known to play a vital role in tumor immunity by bridging innate and adaptive immune responses. In GI cancers, NKT cells can contribute to either antitumor or protumor immunity depending on the cytokine profile expressed and type of cancer. This review discusses the complexities of the role of NKT cells in liver, colon, pancreatic and gastric cancers with an emphasis on type I NKT cells.
Introduction
Natural killer T (NKT) cells are a specialized small population of T cells that play a critical role in connecting the innate and adaptive immune systems.Citation1–3 They contain various pre-formed mRNAs for cytokines such as IFN-γ, IL-4, -10 and -13 that are rapidly translated upon antigen stimulation and subsequently provide immediate immune protection and activation of downstream adaptive immune responses.Citation4–6 The name natural killer T cell originated from the discovery of a subpopulation of CD3+ T cells that express the NK cell marker, NK1.1.Citation7,Citation8 Defining NKT cells based on T cells that express NK1.1 was problematic because not all NKT cells express NK1.1 and many activated T cells express NK1.1 as an activation marker.Citation9 NKT cells differ from conventional T cells in that their receptors recognize lipids presented by the non-classical MHC I-like molecule, CD1d.Citation3,Citation9,Citation10 Like class I HLA-A, -B and -C molecules, CD1d is expressed in most nucleated cells,Citation11–13 but it is often suppressed in tumors that have escaped killing by NKT cells.Citation14–16 Currently, NKT cells are defined as any TCR+ cell that recognizes lipids presented by CD1d. An important discovery was that NKT cells (now called invariant NKT or iNKT or type I NKT cells; see below) possess a semi-invariant T cell receptor alpha (TCRα) chain, defined as Vα14-Jα18 in mice and Vα24-Jα18 in humans paired with a limited number of Vβ chains, Vβ8.2, 7, and 2 in mice and Vβ11 in humans.Citation17–20 Furthermore, α‐galactosylceramide (α-GalCer), the prototypical glycolipid derived from a marine sponge or possibly a Sphingomonas bacterium symbiotic to the sponge, is presented by CD1d and recognized by NKT cells expressing the semi-invariant TCR.Citation3,Citation6,Citation9 Compared to a subset of conventional T cells specific for any single antigen, the population of NKT cells that recognize α-GalCer is quite large with representation being 1–2% of mouse spleen cells, 15–30% of liver cells, and up to 40% of CD3+ T cells in the bone marrow.Citation1,Citation21
The discovery of NKT cells was further complicated by the studies that demonstrated the existence of a T cell population that does not possess a semi-invariant TCR and is able to recognize other lipids, but not the prototypical NKT cell agonist α-GalCer, presented by CD1d.Citation1,Citation9 As a result, the NKT cells that utilize the semi-invariant TCR were named invariant NKT (iNKT) cells or type I NKT cells, and the others that use different TCRs were called non-invariant NKT cells or type II NKT cells. The heterogeneous TCR repertoire of type II NKT cells enables them to respond to a diverse range of lipid antigens in a similar fashion as conventional T cells’ ability to recognize a large repertoire of peptide antigens. This is in contrast to type I NKT cells, which can recognize only a limited number of lipid antigens due to the utilization of a semi-invariant TCR. The discovery that type II NKT cells recognize sulfatide from the myelin sheaths of the central nervous system, and the subsequent development of sulfatide-loaded-CD1d tetramer, allowed the detection and characterization of this subset of type II NKT cells.Citation22 However, due to the diversity of type II NKT cell TCRs, it is highly unlikely that sulfatide-loaded-tetramer is capable of detecting all the subsets of type II NKT cells. The identification of additional type II NKT cell antigens is desperately needed to develop new tetramers to detect more type II NKT cell subsets. This would provide the capabilities to perform studies to further advance our knowledge about the type II NKT cell subpopulation and their function in the immune system. This review will focus on the role of NKT cells in GI cancers.
Type I NKT cells in tumor immunity
The ability of NKT cells to function as a bridge between the innate and adaptive immune system plays a critical role in tumor immunity. The anti-tumor functions of type I NKT cells primarily depend on their ability to secrete the Th1 cytokine IFN-γ and TNF-α.Citation23,Citation24 This is in contrast to the role of type I NKT cells in protection against autoimmunity, which depends on Th2 cytokines IL-4 and IL-13.Citation23–25 The role of type I NKT cells in tumor immunity was initially discovered when it was shown that α-GalCer has anti-tumor activity and could potently activate type I NKT cells and induce IFN-γ production.Citation26 NKT cells also have the ability to secrete lytic granules and directly lyse CD1d-positive tumors.Citation27 However, many tumors have suppressed CD1d expression by the time they are clinically detectable.Citation14–16 Perhaps as an escape mechanism from NKT cell-mediated tumor immunosurveillance. In addition, other studies have revealed a mechanism that involves NKT cell-mediated killing in vivo through Fas–FasL interaction.Citation28
The studies using Jα18 KO mice, which lack type I NKT cells, further supported the importance of type I NKT cells in tumor immunity. In the absence of α-GalCer stimulation, the protection against murine tumors mediated by low-dose IL-12 treatment was dependent on type I NKT cells, as this protection was lost in Jα18 KO mice.Citation29–31 The immunosurveillance against spontaneous methylcholanthrene-induced sarcomas or in transgenic mice expressing the HER2 oncogene was deficient in Jα18 KO mice and demonstrates the importance of type I NKT cells in rejecting tumors.Citation32 This study also showed that the tumor immunosurveillance and protection elicited by exogenous IL-12 were dependent on type I NKT cells. This discovery was corroborated by the findings that NKT cells can also promote tumor immunity by inducing dendritic cells (DCs) to produce IL-12, which potently stimulates NK cells and IFN-γ production.Citation33 Furthermore, the adoptive transfer studies revealed that protection in Jα18 KO mice against methylcholanthrene-induced sarcomas and lung metastasis could be restored by the transfer of type I NKT cells from WT mice.Citation34
Although NKT cells do express granzymes and perforin and can directly kill tumor cells, the primary mechanism of tumor protection is mediated through the production of IFN-γ and downstream activation of effector NK and CD8+ T cells.Citation34 In fact, experiments using mice that lack NK, NKT, or T and B cells demonstrated that the sequential production of IFN-γ by NKT cells followed by NK cell production of IFN-γ was necessary for α-GalCer-mediated protection.Citation35,Citation36
The activation of type I NKT cells by α-GalCer also induces IL-12 production by DCs and plays a critical role in protection against tumors.Citation33 The mechanism of IL-12 production by DCs induced by α-GalCer was dependent on the direct interaction between NKT cells and DCs through CD40-CD40L binding.Citation37 In addition to stimulating IL-12 expression, NKT also has the ability to induce maturation of DCs, subsequently enhancing antigen-presenting capabilities and costimulatory molecule expression, and thus activation of CD4+ and CD8+ T cells.Citation38 The studies by the Metelitsa lab revealed that human type I NKT cells exert anti-tumor functions in a neuroblastoma model by directly killing CD1d+ tumor-associated macrophages.Citation39 Interestingly, NKT cells killed up to 90% of myeloid cells pulsed with tumor lysate, which exceeded the levels of cytotoxicity against myeloid cells pulsed with α-GalCer or the lysate from normal PBMCs.Citation39 The levels of cytotoxicity against myeloid cells pulsed with α-GalCer or the lysate from normal PBMCs were similar. This indicated that type I NKT cells do not have lytic activity against antigen-presenting cells complexed with CD1d and α-GalCer. This contradicts previous studies that showed human type I NKT cell clones have cytotoxicity against monocyte-derived DCs pulsed with α-GalCer.Citation40 Type I NKT cells also play a role in the reprogramming of pro-tumor M2 macrophages into anti-tumor M1 macrophages.Citation41 Thus, type I NKT cells can regulate the function of myeloid cells by multiple mechanisms and thus play a critical role in tumor immunity.
The main mechanism of protection provided by type I NKT cells was originally thought to be solely due to the production of IFN-γ. An exception to this paradigm was found when our lab discovered a new class of type I NKT cell agonist called beta-mannosylceramide (β-ManCer).Citation42 This lipid agonist differs from α-GalCer not only in structure, but also in its mechanism of protection, which is dependent on TNF-α and nitric oxide synthase (NOS) instead of IFN-γ.Citation43 Although β-ManCer stimulated lower levels of cytokine production than α-GalCer, it did not induce long-term anergy of NKT cells and provided protection against lung metastasis.Citation42 This was a potentially important discovery and has clinical relevance because β-ManCer can be administered repeatedly, unlike α-GalCer which induces long-term functional anergy. Interestingly, α-GalCer and β-ManCer-loaded tetramers conjugated with different fluorochromes stained the same type I NKT cell population, indicating that they were not acting through different cells.Citation44 Furthermore, it was shown that combinatorial treatment with suboptimal doses of α-GalCer and β-ManCer can synergize and provide superior protection against tumors compared to treatment with either alone.Citation43 The unique properties of β-ManCer may warrant its clinical development, especially in cases where α-GalCer is not effective.
Type II NKT cells in tumor immunity
In light of all the evidence of the anti-tumor functions of NKT cells, the field was surprised when it was discovered that NKT cells could also suppress tumor immunity.Citation25 The first clue was in an immunogenic fibrosarcoma tumor model in which subcutaneous tumors grew, regressed and then recurred but failed to recur in CD1d KO mice devoid of NKT cells.Citation25 This effect was due to IL-13 production by NKT cells which induced myeloid-derived suppressor cells (MDSC) to produce TGF-β and subsequently suppressing CD8+ T cell-mediated immunosurveillance.Citation45 A conundrum existed in this pathway because it was dependent on IL-13 and not IL-4, and they both signal through IL-4 Rα.Citation25 When the full mechanism was elucidated, it was found that signals from the TNF-α and IL-4Rα/STAT6 pathways synergized to upregulate IL-13Rα2, which responds to only IL-13, not IL-4 and subsequently induces the production of TGF-β.Citation46 Thus, it became clear why IL-4 was not sufficient.
The defining characteristics of type I NKT cells having anti-tumor properties and type II NKT cells suppressing tumor immunity were developed collectively among several labs using a variety of tumor models.Citation47–50 These studies used a combination of knockout (KO) mouse models, Jα18 KO mice which lack only type I NKT but not type II NKT cells, or CD1d KO mice which lack both type I and type II NKT cells. In tumor challenge experiment, the suppressive activity was maintained in Jα18 KO mice but was lost in CD1d KO mice, indicating the importance of type II NKT cells in suppressing tumor immunity. Furthermore, our lab and others have shown that sulfatide, the prototypical type II NKT cell agonist discovered by Vipin Kumar’s lab,Citation22 promotes tumor growth.Citation47,Citation49,Citation51
The relationship between type I and II NKT cells was further complicated by the observation that Jα18 KO mice were more suppressed than WT mice. This led to the finding that type I NKT cells could regulate the function of type II NKT cells, and vice versa.Citation51 Indeed, when type II NKT cells were stimulated by sulfatide, they decreased the tumor protection induced by α-GalCer-stimulated type I NKT cells.Citation51 Thus, type I and type II NKT cells form a new regulatory axis in which they cross-regulate each other and the one that dominates can set the tone for subsequent responses by conventional T cells.Citation51 This metastable balance is somewhat analogous to the axis originally discovered between Th1 and Th2 CD4+ T cells that so profoundly influenced immunology. The cross-regulation between type I and II NKT cells can be extended to other immune cells. For example, type II NKT cells enhance the tumor promoting functions of MDSCs via IL-13, while type I NKT cells suppress the pro-tumor effects of MDSCs by converting them into immunostimulatory APCs.Citation52 The contradictory effects of type I and II NKT cells in regard to CD8+ T cells in cancer are quite evident. Type I NKT cells can activate CD8+ T cells and enhance their resistance against MDSC, while type II NKT cells suppress the anti-tumor activity of CD8+ T cells.Citation34,Citation38,Citation45,Citation46,Citation53,Citation54 Because of the relationship between type I and II NKT cells, the balance between the two subsets plays a significant role in tumor immunity.
NKT cells in GI cancers
The outcome for patients diagnosed with GI cancers is generally considered poor, especially for patients with pancreatic and/or liver cancerCitation55,Citation56 because they are often diagnosed at late stages. The studies of GI cancers have revealed the importance of type I NKT cells in regulating anti-tumor immunity. The role of type I NKT cells in GI cancers is complicated and differs among the different types of cancers. For instance, type I NKT cells function to enhance tumor immunity against gastric and pancreatic cancersCitation57–60 but suppresses anti-tumor immunity in intestinal cancer,Citation61 and in liver and colon cancer it is controversial because type I NKT cells can promote or suppress tumor growthCitation62–68 (see ). A list of the studies that are directly relevant to the role of type I NKT cells in GI cancers is listed in .
Table 1. A summary of the studies demonstrating the role of type I NKT cells in GI cancers and method of identification. Selected publication criteria based on keyword search: liver, colon, intestinal, pancreatic, gastric, esophageal, cancer, and NKT cell (No such study in esophageal cancer was found.)
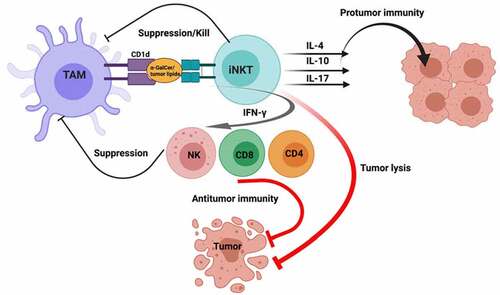
Figure 1. Type I NKT cell antitumor and protumor functions. Initially, tumor-associated macrophages (TAMs) presenting α-GalCer or tumor-derived lipids activate type I NKT cells. In antitumor immunity, activated NKT cells can directly lyse tumor cells and/or secrete TH1 cytokines such as IFN-γ to induce NK, CD4 and CD8 T cell antitumor functions. In contrast, activated NKT cells can secrete TH2 cytokines such as IL-4 and IL-10 and Th17 cytokine IL-17 which contribute to protumor immunity. Created with BioRender.com
NKT cells in liver cancer
The studies investigating the role of NKT cells in liver cancer have been controversial and difficult to dissect, because there are studies that show hepatic NKT cells playing both pro-tumorigenic and anti-tumorigenic roles.Citation63,Citation64,Citation67–69 To add another layer of complexity, there are major differences between human and murine hepatic NKT cells. The percentage of hepatic type I NKT cells in adult mice is large (10–30% of liver lymphocytes) compared to the percentages found in humans which is quite variable and ranges from 0.05 to 1%.Citation70 Furthermore, the studies by the Derek Doherty lab showed that human hepatic type I NKT cells from normal and tumor-bearing livers produced IFN-γ but not IL-4 when stimulated with α-GalCer.Citation70 In contrast, murine hepatic NKT cells stimulated with α-GalCer have the ability to produce both Th1 and Th2 cytokines.Citation71–73
In liver cancer, the distinction that stimulated CD4+ type I NKT cells secrete higher levels of Th2 cytokines and have lower cytolytic activity than CD4− type I NKT cells is evident. In patients with liver cancer, the proportion of tumor infiltrating CD4+ type I NKT cells is increased compared to healthy donor liver.Citation65 Furthermore, the number of CD4− type I NKT cells are decreased in liver cancer.Citation65 This supports other studies that showed CD4− type I NKT cells are potent producers of IFN-γ and suppress tumor growth.Citation70,Citation74,Citation75 Interestingly, the combination of intratumoral type I NKT cells and IFN-γ are potential independent prognostic factors, for which the levels are inversely proportional to the risk of recurrence and proportional to survival after curative resection in patients with HCC.Citation66 The effect of type I NKT cells on hepatic tumor cells is site specific. When mice are treated with α-GalCer, the growth of disseminated BNL 1MEA.7 R.1 (BNL) liver cancer cells in the liver of BALB/c mice were suppressed but the treatment had no effect on the growth of subcutaneous BNL tumors.Citation69 In the same study, it was shown that hepatic NKT cells were rapidly activated after α-GalCer administration compared to splenic NKT cells. Moreover, in a preclinical setting the adoptive transfer of splenic NKT cells stimulated by DCs presenting HCC-derived antigens resulted in complete resolution of subcutaneous Hep3B hepatic tumors.Citation76 This suggests that splenic and hepatic NKT cells are functionally different, and the sources of stimulation are critical factors in liver cancer. The studies from the Tim Greten lab have also demonstrated the importance of the microbiome in regulating NKT cell-mediated anti-tumor responses in liver cancer. They demonstrated that primary bile acids upregulated the chemokine CXCL16 in the liver, which is recognized by CXCR6 expressed on NKT cells. Thus, the interaction between CXCL16 and CXCR6 led to the accumulation of liver NKT cells and suppression of liver tumor growth. Microbes that converted primary to secondary bile acids abrogated this activity.Citation67
NKT cells in colon cancer
Similar to the role of NKT cells in liver cancer, the studies investigating the role of NKT cells in colon cancer have demonstrated both pro-tumor and anti-tumor roles. For example, in a study with tissues from 103 patients with colorectal cancer (CRC) it was shown that higher Vα24+ NKT cell tumor infiltration in colorectal carcinomas is an independent prognostic factor for favorable prognosis.Citation62 In contrast, it has been demonstrated that high peripheral blood CD16+ NKT-like (CD3+CD56+) cells are associated with shorter disease-free survival of patients with CRC.Citation77,Citation78 It is important to note that the CD3+CD56+ cell population can include both NKT and conventional T cells, because activated T cells can also express CD56.Citation77 Together, these studies would suggest that it is the non-NKT activated CD3+ T cells expressing CD56 and CD16 in PBMCs that correlate with poor prognosis, not the type I NKT cells themselves.
The yin and yang nature of NKT cells in colon cancer are also evident in mouse models. In two models of hypercholesterolemia, the ApoE KO mouse and the C57BL/6 mouse fed a high cholesterol diet, the number of colorectal tumors was increased after treatment with the potent carcinogen, azoxymethane.Citation79 In this study, hypercholesterolemia inhibited the differentiation of hematopoietic stem cells into terminally differentiated type I NKT cells in the thymus, in the colon submucosa and at the early stages of tumorigenesis, subsequently impairing immunosurveillance against colorectal neoplasia.Citation79 Additionally, in support of the anti-tumor functions of type I NKT cells, a subcutaneous mouse model of colon cancer demonstrated that the anti-tumor effect of whole-body hyperthermia was increased after treatment with α-GalCer.Citation80 In contrast, recent studies have revealed that IL-10-producing type I NKT cells accumulate in pre-cancerous colon polyps.Citation64 Moreover, the IL-10 producing NKT cells were identified as a unique subset expressing low levels of the transcription factor Yin Yang 1 (YY1), which is required for the full function of the transcription factor, PLZF.Citation64
The ability of type I and type II NKT cells to cross-regulate each other is apparent in colon cancer. The studies in our lab have shown that both Tregs and type II NKT cells can suppress tumor immunity in a subcutaneous CT26 colon cancer mouse model.Citation51 In wild-type (WT) mice, type I and type II NKT cells cancel each other’s functions, so Tregs are the primary regulator of tumor immunity, similar to the situation in CD1d KO mice (lack both type I and type II NKT cells).Citation51 Interestingly, in the absence of type I NKT cells (Jα18 KO mice), blocking Tregs did not reduce tumor growth because now type II NKT cells could suppress instead of the Tregs.Citation51 This demonstrates the ability of type II NKT cells to suppress protection against colon cancer, as well as their regulation by type I NKT cells that regulate the regulators.
The role of NKT cells in colonic pre-cancer polyps has also been studied.
In agreement with the pro-tumor functions of type I NKT cells in colon cancer, it has been recently shown that type I NKT cells increase intestinal polyp formation in a spontaneous mouse model of colon cancer using the ApcMin/+ mice.Citation61,Citation81 Additional studies identified a unique polyp type I NKT cell population (CD4+, NK1.1−, CD44int and PD-1lo) that did not express PLZF, that were identified as IL-10 and IL-17 producers that suppressed Th1 immunity and promoted regulatory T cells (Tregs).Citation61 Interestingly, α-GalCer treatment can disrupt the natural tumor promoting function of type I NKT cells and reduce polyp development in the orthotopic ApcMin/+ mouse model.Citation81 The treatment with α-GalCer induced TH1 skewing and cytokine production.Citation81
NKT cells in pancreatic cancer
Currently, there is little information about the function of NKT cells in pancreatic cancer. The role of type I NKT cells in pancreatic cancer was first revealed when studies using α-GalCer-pulsed DCs showed anti-tumor functions in C57BL/6 mice inoculated with syngeneic Panc02 tumors.Citation82 In this study, the mice treated with α-GalCer-pulsed DCs exhibited a significant expansion of IFN-γ producing type I NKT cells, which correlated with a decrease in tumor growth.Citation82 More recently, a study by Janakiram et al. demonstrated the functional importance of NKT cells in pancreatic cancer by crossing the KPT model (p48Cre/+-LSL-KrasG12D/+) with CD1d KO mice that lack both type I NKT and type II NKT cells.Citation60 The KPT-CD1d KO mice displayed an increase in pancreatic intraepithelial neoplasia (PanIN) formation associated with an increase in M2 macrophages expressing microsomal prostaglandin E synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX) compared to KPT mice. Furthermore, the loss of NKT cells resulted in a decrease in the percentage of IFN-γ producing NK and CD8+ T cells in the pancreas.Citation60
NKT cells in gastric cancer
The role of NKT cells in gastric cancer first surfaced nearly 20 y ago by a study conducted by Yanagisawa et al. that demonstrated type I NKT cells derived from PBMCs of gastric cancer patients display a much lower proliferative response to α-GalCer than those of healthy volunteers.Citation83 Furthermore, a preclinical study showed that gastric cancer cell lines and primary tumors express CD1d.Citation57 As a result, type I NKT cells had a robust anti-tumor response against CD1d-positive gastric cancer in vitro and in vivo in the presence of α-GalCer.Citation57 In the same study, cisplatin upregulated CD1d expression in gastric cancer cells and enhanced their susceptibility to NKT-mediated cytotoxicity.Citation57 Recently, a transcriptomic study involving 876 patients with gastric cancer revealed that low CD1d expression is associated with poor prognosis.Citation84
Conclusion
GI cancers are some of the deadliest cancers, and it is evident that NKT cells play an integral part in regulating tumor immunity. The role of NKT cells in GI cancers is complex and can differ depending on the type of cancer and location. For example, in liver cancer, α-GalCer treatment is effective in treating BNL 1MEA.7 R.1 tumors in the liver, but it is ineffective in treating the same tumors implanted subcutaneously.Citation69 To further complicate the role of NKT cells in GI cancers, there are data that show that NKT cells can have protumor and antitumor functions within the same type of cancer. In colon cancer, it has been demonstrated that IL-10 producing NKT cells accumulates in precancerous polypsCitation61,Citation64 and that type I NKT cells increase polyp formation in a mouse model using ApcMin/+ mice.Citation81 In contrast, in a study with colorectal cancer patient tissue samples it was shown that higher Vα24+ NKT cell tumor infiltration in colorectal carcinomas is an independent favorable prognostic factor.Citation62
Although several clinical trials with non-GI cancer patients have demonstrated that α-GalCer-based treatments are safe and can induce complete and partial clinical responses, unfortunately, the responses in the clinical trials have been inconsistent and unpredictable.Citation85–90 The contradictory roles of type I NKT cells in GI tumor immunity may exist in other types of cancers and help explain the inconsistent responses in the clinical trials using α-GalCer-based treatments.Citation85,Citation87–89,Citation91
It has been demonstrated that type I NKT cells do have the potential to suppress tumor growth, but it is highly context dependent. In order to improve α-GalCer-based treatments, the type of cancer, location of the tumor, and a patient’s baseline level and class of circulating NKT cells should be evaluated before starting treatments. Furthermore, the development and clinical testing of alternative type I NKT cell agonists that elicit a Th1-skewed response and type II NKT cell inhibitors to suppress protumor immunity is warranted and could lead to more consistent clinical responses.
Acknowledgments
We would like to thank Dr Hoyoung Maeng at the National Cancer Institute-Vaccine Branch for critically reading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–9. doi:10.1016/S0065-230X(08)00408-9.
- Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Front Immunol. 2014;5:543. doi:10.3389/fimmu.2014.00543.
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi:10.1172/JCI200423594.
- Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenbrg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100(14):8395–8400. doi:10.1073/pnas.1332805100.
- Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang Z, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069–1076. doi:10.1084/jem.20030630.
- Terabe M, Berzofsky JA. Tissue-specific roles of NKT cells in tumor immunity. Front Immunol. 2018;9:1838. doi:10.3389/fimmu.2018.01838.
- Sykes M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4-CD8- and alpha beta TCR+NK1.1+ cells. J Immunol. 1990;145:3209–3215.
- Levitsky HI, Golumbek PT, Pardoll DM. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J Immunol. 1991;146:1113–1117.
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231–237. doi:10.1038/nri1309.
- Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269(5221):185–186. doi:10.1126/science.7542402.
- Wen X, Rao P, Carreño LJ, Kim S, Lawrenczyk A, Porcelli SA, Cresswell P, Yuan W. Human CD1d knock-in mouse model demonstrates potent antitumor potential of human CD1d-restricted invariant natural killer T cells. Proc Natl Acad Sci U S A. 2013;110(8):2963–2968. doi:10.1073/pnas.1300200110.
- Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr Top Microbiol Immunol. 2007;314:113–141. doi:10.1007/978-3-540-69511-0_5.
- Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–1123. doi:10.1038/ni.3298.
- Swann J, Crowe NY, Hayakawa Y, Godfrey DI, Smyth MJ. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol. 2004;82(3):323–331. doi:10.1111/j.0818-9641.2004.01254.x.
- Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E, Gul L, Heczey A, Asgharzadeh S, Kim E, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122(6):2221–2233. doi:10.1172/JCI59535.
- Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167(6):3114–3122. doi:10.4049/jimmunol.167.6.3114.
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097–1106. doi:10.1084/jem.180.3.1097.
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–568. doi:10.1038/nri854.
- Imai K, Kanno M, Kimato H, Shigemoto K, Yamamoto S, Taniguchi M. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc Natl Acad Sci U S A. 1986;83(22):8708–8712. doi:10.1073/pnas.83.22.8708.
- Koseki H, Imai K, Ichikawa T, Hayata I, Taniguchi M. Predominant use of a particular alpha-chain in suppressor T cell hybridomas specific for keyhole limpet hemocyanin. Int Immunol. 1989;1(6):557–564. doi:10.1093/intimm/1.6.557.
- Slauenwhite D, Johnston B. Regulation of NKT cell localization in homeostasis and infection. Front Immunol. 2015;6:255. doi:10.3389/fimmu.2015.00255.
- Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi:10.1084/jem.20031389.
- Smyth MJ, Godfrey DI. NKT cells and tumor immunity–a double-edged sword. Nat Immunol. 2000;1(6):459–460. doi:10.1038/82698.
- Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180(6):3627–3635. doi:10.4049/jimmunol.180.6.3627.
- Terabe M, Matsui S, Nobel-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi:10.1038/82771.
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi:10.1126/science.278.5343.1626.
- Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Otura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59(20):5102–5105.
- Wingender G, Krebs P, Beutler B, Kronenberg M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J Immunol. 2010;185(5):2721–2729. doi:10.4049/jimmunol.1001018.
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi:10.1126/science.278.5343.1623.
- Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165(5):2665–2670. doi:10.4049/jimmunol.165.5.2665.
- Takeda K, Hayakawa Y, Atsuta M, Hong S, Van Kaer L, Kobayashi K, Ito M, Yagita H, Okumura K. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000;12(6):909–914. doi:10.1093/intimm/12.6.909.
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661–668. doi:10.1084/jem.191.4.661.
- Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)- 12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189(7):1121–1128. doi:10.1084/jem.189.7.1121.
- Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119–127. doi:10.1084/jem.20020092.
- Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99(4):1259–1266. doi:10.1182/blood.V99.4.1259.
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201(12):1973–1985. doi:10.1084/jem.20042280.
- Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199(12):1607–1618. doi:10.1084/jem.20040317.
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–279. doi:10.1084/jem.20030324.
- Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck R, Seeger R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119(6):1524–1536. doi:10.1172/JCI37869.
- Gansert JL, Kiessler V, Engele M, Wittke F, Röllinghoff M, Krensky AM, Porcelli SA, Modlin RL, Stenger S. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170(6):3154–3161. doi:10.4049/jimmunol.170.6.3154.
- Paul S, Chhatar S, Mishra A, Lal G. Natural killer T cell activation increases iNOS+CD206- M1 macrophage and controls the growth of solid tumor. J Immunother Cancer. 2019;7(1):208. doi:10.1186/s40425-019-0697-7.
- O’Konek JJ, Kato S, Takao S, Izhak L, Xia Z, Illarionov P, Besra GS, Terabe M, Berzofsky JA. beta-mannosylceramide activates type I natural killer T cells to induce tumor immunity without inducing long-term functional anergy. Clin Cancer Res. 2013;19(16):4404–4411. doi:10.1158/1078-0432.CCR-12-2169.
- O’Konek JJ, Illarionov P, Khursigara DS, Ambrosino E, Izhak L, Castillo II BF, Raju R, Khalili M, Kim HY, Howell AR, et al. Mouse and human iNKT cell agonist beta-mannosylceramide reveals a distinct mechanism of tumor immunity. J Clin Invest. 2011;121(2):683–694. doi:10.1172/JCI42314.
- Clark K, Yau J, Bloom A, Wang J, Venzon DJ, Suzuki M, Pasquet L, Compton BJ, Cardell S, Porcelli SA, et al. Structure-function implications of the ability of monoclonal antibodies against alpha-Galactosylceramide-CD1d complex to recognize beta-mannosylceramide presentation by CD1d. Front Immunol. 2019;10:2355. doi:10.3389/fimmu.2019.02355.
- Terabe M, Matsui S, Park JM, Mamura M, Nobel-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, et al. Transforming growth factor-b production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi:10.1084/jem.20022227.
- Fichtner-Feigl S, Terabe M, KitaniA, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68(9):3467–3475. doi:10.1158/0008-5472.CAN-07-5301.
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Va14Ja18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202(12):1627–1633. doi:10.1084/jem.20051381.
- Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111(12):5637–5645. doi:10.1182/blood-2007-05-092866.
- Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63(3):199–213. doi:10.1007/s00262-013-1509-4.
- Zhao J, Weng X, Bagchi S, Wang C-R. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci U S A. 2014;111(7):2674–2679. doi:10.1073/pnas.1323845111.
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179(8):5126–5136. doi:10.4049/jimmunol.179.8.5126.
- Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182(4):1818–1828. doi:10.4049/jimmunol.0802430.
- Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114(1):80–87. doi:10.1002/ijc.20669.
- Stober D, Jomantaite T, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170(5):2540–2548. doi:10.4049/jimmunol.170.5.2540.
- Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349 e15. doi:10.1053/j.gastro.2020.02.068.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
- Xu Q, Li J, Zhang N, Zhang L, Qian R. Utilization of invariant natural killer T cells for gastric cancer treatment. Future Oncol. 2018;14(20):2053–2066. doi:10.2217/fon-2017-0724.
- Peng LS, Mao,FY, Zhao YL, Wang TT, Chen N, Zhang JY, Cheng P, Li WH, Lv YP, Teng YS. Altered phenotypic and functional characteristics of CD3+CD56+ NKT-like cells in human gastric cancer. Oncotarget. 2016;7(34):55222–55230. doi:10.18632/oncotarget.10484.
- Lundgren S, Warfvinge CF, Elebro J, Heby M, Nodin B, Krzyzanowska A, Bjartell A, Leandersson K, Eberhard J, Jirström K. The Prognostic Impact of NK/NKT cell density in periampullary adenocarcinoma differs by morphological type and adjuvant treatment. PLoS One. 2016;11(6):e0156497. doi:10.1371/journal.pone.0156497.
- Janakiram NB, Mohammed A, Bryant T, Ritchie R, Stratton N, Jackson L, Lightfoot S, Benbrook DM, Asch AS, Lang ML, et al. Loss of natural killer T cells promotes pancreatic cancer in LSL-Kras(G12D/+) mice. Immunology. 2017;152(1):36–51. doi:10.1111/imm.12746.
- Wang Y, Sedimbi S, Löfbom L, Singh AK, Porcelli SA, Cardell SL. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2017. doi:10.1038/mi.2017.34.
- Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11(20):7322–7327. doi:10.1158/1078-0432.CCR-05-0877.
- Yoshioka K, Ueno Y, Tanaka S, Nagi K, Onitake T, Hanaoka R, Watanabe H, Chayama K. Role of natural killer T cells in the mouse colitis-associated colon cancer model. Scand J Immunol. 2012;75(1):16–26. doi:10.1111/j.1365-3083.2011.02607.x.
- Darcy PW, Denzin LK, Sant’Angelo DB. YY1(lo) NKT cells are dedicated IL-10 producers. Sci Rep. 2020;10(1):3897. doi:10.1038/s41598-020-60229-6.
- Bricard G, CessonV, Devevre E, Bouzourene H, Barbey C, Rufer N, IM JS, Alves PM, Martinet O, Halkic N, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182(8):5140–5151. doi:10.4049/jimmunol.0711086.
- Xiao YS, Gao Q, Xu XN, Li YW, Ju MJ, Cai MY, Dai CX, Hu J, Qiu SJ, Zhou J, et al. Combination of intratumoral invariant natural killer T cells and interferon-gamma is associated with prognosis of hepatocellular carcinoma after curative resection. PLoS One. 2013;8(8):e70345. doi:10.1371/journal.pone.0070345.
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391). doi:10.1126/science.aan5931.
- Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564. doi:10.1016/j.ccell.2014.09.003.
- Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, Jinushi M, Sugimoto Y, Sasaki Y, Hori M, Hayashi N. CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer. 2003;106(1):81–89. doi:10.1002/ijc.11163.
- Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O’Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171(4):1775–1779. doi:10.4049/jimmunol.171.4.1775.
- Bendelac A, Rivera MN, Park S-H, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15(1):535–562. doi:10.1146/annurev.immunol.15.1.535.
- Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol. 2016;13(3):337–346. doi:10.1038/cmi.2015.115.
- Hayakawa Y, Takeda K, Takeda H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166(10):6012–6018. doi:10.4049/jimmunol.166.10.6012.
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195(5):625–636. doi:10.1084/jem.20011786.
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Va24 natural killer cells. J Exp Med. 2002;195(5):637–641. doi:10.1084/jem.20011908.
- Margalit M, Shibolet O, Klein A, Elinav E, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma by transplantation of ex-vivo immune-modulated NKT lymphocytes. Int J Cancer. 2005;115(3):443–449. doi:10.1002/ijc.20889.
- Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. 2019;68(6):1011–1024. doi:10.1007/s00262-019-02343-7.
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21(11):573–583. doi:10.1016/S0167-5699(00)01735-7.
- Tie G, Yan J, Khair L, Messina JA, Deng A, Kang J, Fazzio T, Messina LM. Hypercholesterolemia increases colorectal cancer incidence by reducing production of NKT and gammadelta T cells from hematopoietic stem cells. Cancer Res. 2017;77(9):2351–2362. doi:10.1158/0008-5472.CAN-16-1916.
- Hattori T, Kokura S, Okuda T, Okayama T, Takagi T, Handa O, Naito Y, Yoshida N, Yoshikawa T. Antitumor effect of whole body hyperthermia with alpha-galactosylceramide in a subcutaneous tumor model of colon cancer. Int J Hyperthermia. 2007;23(7):591–598. doi:10.1080/02656730701708328.
- Wang Y, Sedimbi SK, Löfbom L, Besra GS, Porcelli SA, Cardell SL. Promotion or suppression of murine intestinal Polyp development by iNKT cell directed Immunotherapy. Front Immunol. 2019;10:352. doi:10.3389/fimmu.2019.00352.
- Nagaraj S, Ziske C, Strehl J, Messmer D, Sauerbruch T, Schmidt-Wolf IGH. Dendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivo. Int Immunol. 2006;18(8):1279–1283. doi:10.1093/intimm/dxl059.
- Yanagisawa K, Seino K-I, IshikawaY, Nozue M, Todoroki T, Fukao K. , . Impaired proliferative response of Valpha 24 NKT cells from cancer patients against alpha-galactosylceramide. JImmunol. 2002;168(12):6494–6499. doi:10.4049/jimmunol.168.12.6494.
- Melo AM, Conroy MJ, Foley EK, Dockry E, Breen EP, Reynolds JV, Lysaght J, Doherty DG. CD1d expression and invariant natural killer T-cell numbers are reduced in patients with upper gastrointestinal cancers and are further impaired by commonly used chemotherapies. Cancer Immunol Immunother. 2020;69(6):969–982. doi:10.1007/s00262-020-02514-x.
- Giaccone G, Punt CJA, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BME, Scheper RJ, van der Vliet HJJ, van den Eertwegh AJM, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709.
- Nicol AJ, Tazbirkova A, Nieda M. Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res. 2011;17(15):5140–5151. doi:10.1158/1078-0432.CCR-10-3105.
- Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57(3):337–345. doi:10.1007/s00262-007-0373-5.
- Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fugisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11(5):1910–1917. doi:10.1158/1078-0432.CCR-04-1453.
- Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–389. doi:10.1182/blood-2003-04-1155.
- Waldowska M, Bojarska-Junak A, Rolinski J. A brief review of clinical trials involving manipulation of invariant NKT cells as a promising approach in future cancer therapies. Cent Eur J Immunol. 2017;42(2):181–195. doi:10.5114/ceji.2017.69361.
- Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of {alpha}-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi:10.1084/jem.20042592.
