ABSTRACT
Chronic inflammation drives proliferative responses, hence increasing cellular multiplication with the consequent risk of malignant transformation. Autoimmune responses against self-antigens drive chronic inflammation but may also enhance cancer immunosurveillance with the consequent reduction of tumor incidence and progression. These notions, which have been well established at the preclinical level, may explain the generally positive associations between immune-inflammatory diseases but also some negative associations, for example between breast cancer and rheumatoid arthritis or systemic lupus erythematosus, which have recently been confirmed in a study enrolling close to half a million participants from the UK Biobank.
Evermore accurate and expanding archiving of clinical and biological patient data greatly facilitates the discovery of novel epidemiological associations among different diseases. A recent study published in JAMA OncologyCitation1 exploits a large dataset involving 478 753 participants to investigate the relationship between cancers and “immune-mediated diseases”, which is a heterogeneous collection of pathological conditions. Such diseases may be either organ-specific or systemic, involve a mostly inflammatory process without the recognition of specific autoantigens (as this may apply to Crohn’s disease, psoriasis, sarcoidosis and ulcerative colitis), an overreaction to exogenous allergens (as this is the case in allergic rhinitis, asthma and celiac disease) or the unwarranted recognition of specific autoantigens with a variable contribution of autoreactive T lymphocytes and autoantibodies. If considered as one single entity, the diagnosis of such “immune-mediated diseases” slightly increases the risk of developing any kind of cancer with a multivariable hazard radio of 1.08 (95% confidence interval: 1.04–1.12). However, the study in JAMA Oncology has sufficient power to unveil more subtle associations between specific immunologically relevant diseases and distinct categories of malignancy that can be either positive or negative.Citation1
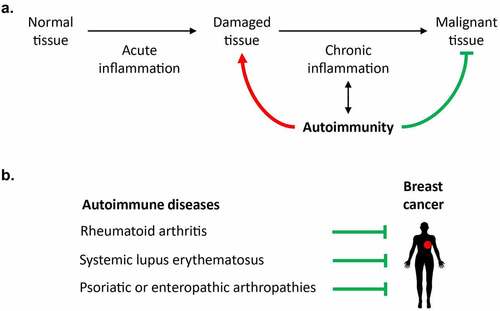
Cancer is the result of two processes, namely (i) the cell-autonomous accumulation of (epi)genetic alterations leading to the activation of oncogenes and the inactivation of tumor suppressor genes, and (ii) the systemic failure of immunosurveillance, i.e. the capacity of innate and cognate immune effectors to recognize and eliminate (pre-)malignant cells.Citation2,Citation3 Taken into account the importance of cell-autonomous (epi)genetic aberrations for carcinogenesis and tumor progression, it is not a surprise that chronic tissue damage coupled to long-term inflammatory processes augments the risk of malignant transformation, based on the well-established notion that each cellular duplication is coupled to the risk of propagating non-repaired mutations and chromosomal aberrations, hence enhancing genomic instability and the risk of developing cancers ().Citation4–7 However, there are additional possible links between ”immune-mediated disease” and enhanced cancer risk. At a general level, chronic inflammation may drive and accompany tissue senescence, thus facilitating carcinogenesis due to accelerated biological aging.Citation2 Moreover, the accumulation of inflammatory cells in inflamed tissues may subvert specific immune responses, hence ultimately abolishing immunosurveillance. For instance, macrophages and granulocytes can inappropriately differentiate into myeloid-derived suppressor cells which impede the activity of cytotoxic lymphocytes.Citation8
Figure 1. Epidemiological links between systemic autoimmune diseases and breast cancer. (a). Repeated acute inflammatory responses and autoimmune damage can cause chronic inflammation and promote carcinogenesis. By contrast, some autoimmune diseases have been associated with a reduced incidence or progression of cancer, as these pathologies can fuel the immunosurveillance of (pre)malignant cells. (b). Several studies have revealed a negative association between breast cancer and some autoimmune manifestations such as rheumatoid arthritis, systemic lupus erythematosus, and psoriatic or enteropathic arthropathies.
In specific conditions, autoimmune diseases have been linked to improved immunosurveillance, as exemplified for autoimmune thyroiditis and thyroid cancer,Citation9,Citation10 vitiligo and melanomaCitation11,Citation12 or non-cirrhotic primary biliary cholangitis and cholangiocarcinoma.Citation13 Moreover, in preclinical models, vaccination with normal epithelial cells from the intestine or the mammary gland can induce protective immune responses against colon and breast cancer, respectively, underscoring the possibility that immune responses against (by definition) non-mutated self-antigens can participate to cancer immunosurveillance.Citation14,Citation15 The development of human breast cancer becomes also more improbable when histologically normal mammary gland is infiltrated by T lymphocytes at a high CD8/FOXP3 ratio,Citation16 pleading in favor of the hypothesis that even healthy tissues are patrolled by specific T cells to avoid their cancerization.Citation17
Beyond these examples of organ-specific autoimmune responses that protect against the development or progression of cancers affecting the same organ, accumulating evidence indicates that systemic autoimmunity may reduce the incidence of cancers affecting multiple organs as well. For example, systemic lupus erythematosus (SLE) is associated with a significant reduction of several malignancies (breast, endometrial, prostate, and uterine cancers, melanoma).Citation18,Citation19 Rheumatoid arthritis is linked to significantly reduced incidence of breast, colorectal and prostate cancer.Citation1,Citation20 Breast cancer is also significantly less frequent in patients with psoriatic or enteropathic arthropathies ().Citation1 The mechanisms of these effects have not been elucidated but may involve improved immunosurveillance due to the increase in the general immune tonus. Indeed, the presence of a broad repertoire of autoantibody specificities (≥3) is related to a particularly strong reduction (by close to 60%) in breast cancer incidence.Citation21 Moreover, a cell-penetrating lupus autoantibody that binds single-stranded RNA has been shown to sensitize cancer cells to doxorubicin treatment.Citation22 Lupus-associated anti-ribosomal P autoantibodies reportedly mediate direct pro-apoptotic effects on cancer cells.Citation23 On top of these considerations, it appears intriguing that the cancer types that are negatively associated with systemic autoimmunity are under particularly strong immunosurveillance.Citation24,Citation25
Nonetheless, other explanations to this epidemiological link between systemic autoimmunity and reduced incidence of specific cancers have been suggested. Thus, anti-inflammatory medications including aspirin may improve anticancer immunosurveillance,Citation26,Citation27 contrasting with the effects of glucocorticoids, which subvert immunosurveillance.Citation28 In addition, SLE patients tend to avoid external factors that aggravate the disease (such as sunlight, which is the main trigger of melanoma)Citation29 as well as endocrine therapies (such as oral contraceptives or hormone replacement therapy),Citation30 perhaps contributing to the reduced incidence of such cancers (). Hence, it is not yet clear to which extent the relationship between autoimmunity and reduction of cancer incidence is direct (via immune effects) or indirect (via iatrogenic and behavioral effects). Future studies in suitable mouse models developing spontaneous or experimentally induced autoimmune diseases must dissipate these uncertainties and establish mechanistic links between self-reactive and anticancer immunity.
Disclosure statement
GK is a co-founder of Samsara Therapeutics, everImmune, and Therafast Bio. All other authors declare that they have no potential conflicts of interest.
Additional information
Funding
References
- He MM, Lo CH, Wang K, Polychronidis G, Wang L, Zhong R, Knudsen MD, Fang Z, Song M. Immune-Mediated Diseases Associated With Cancer Risks. JAMA Oncol. 2021. doi:10.1001/jamaoncol.2021.5680.
- Lopez-Otin C, Kroemer G. Hallmarks of Health. Cell. 2021;184(1):33–3. doi:10.1016/j.cell.2020.11.034.
- Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med . 2018;10(459):eaat7807. doi:10.1126/scitranslmed.aat7807.
- Lahouel K, Younes L, Danilova L, Giardiello FM, Hruban RH, Groopman J, Kinzler KW, Vogelstein B, Geman D, Tomasetti C, et al. Revisiting the tumorigenesis timeline with a data-driven generative model. Proc Natl Acad Sci U S A. 2020;117(2):857–864. doi:10.1073/pnas.1914589117.
- Ranta A, Kumar S. Recent advancements in role of TAM receptors on efferocytosis, viral infection, autoimmunity, and tissue repair. Int Rev Cell Mol Biol. 2020;357:1–19.
- Marroqui L, Perez-Serna AA, Babiloni-Chust I, Dos Santos RS. Type I interferons as key players in pancreatic beta-cell dysfunction in type 1 diabetes. Int Rev Cell Mol Biol. 2021;359:1–80. doi:10.1016/bs.ircmb.2021.02.011.
- Boursot P, Yonekawa H, Bonhomme F. Heteroplasmy in mice with deletion of a large coding region of mitochondrial DNA. Mol Biol Evol. 1987;4:46–55. doi:10.1093/oxfordjournals.molbev.a040421.
- Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–498. doi:10.1038/s41577-020-00490-y.
- Aydogan BI, Mutlu ABB, Yuksel S, Gullu S, Emral R, Demir O, Sahin M, Gedik VT, Corapcioglu D, Sak SD, et al. The association of histologically proven chronic lymphocytic thyroiditis with clinicopathological features, lymph node metastasis, and recurrence rates of differentiated thyroid cancer. Endocr Pathol. 2021;32:280–287. doi:10.1007/s12022-020-09653-y.
- Ryu YJ, Yoon JH. Chronic lymphocytic thyroiditis protects against recurrence in patients with cN0 papillary thyroid cancer. Surg Oncol. 2020;34:67–73. doi:10.1016/j.suronc.2020.03.008.
- Verkhovskaia S, Di Pietro FR, Mastroeni S, Carbone ML, Abeni D, Morese R, Morelli FM, D’Atri S, Marchetti P, De Galitiis F, et al. Vitiligo-like leukoderma as an indicator of clinical response to immune checkpoint inhibitors in late-stage melanoma patients. J Cancer Res Clin Oncol. 2021. doi:10.1007/s00432-021-03811-3.
- Farinazzo E, Zelin E, Agozzino M, Papa G, Pizzichetta MA, Di Meo N, Zalaudek I. Regression of nevi, vitiligo-like depigmentation and halo phenomenon may indicate response to immunotherapy and targeted therapy in melanoma. Melanoma Res. 2021;31(6):582–585. doi:10.1097/CMR.0000000000000776.
- Paillet J, Plantureux C, Levesque S, Le Naour J, Stoll G, Sauvat A, Caudana P, Tosello Boari J, Bloy N, Lachkar S, et al. Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma. J Exp Med. 2021;218(10):e20200853. doi:10.1084/jem.20200853.
- Buque A, Bloy N, Perez-Lanzon M, Iribarren K, Humeau J, Pol JG, Lévesque S, Mondragon L, Yamazaki T, Sato A, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 2020;11(1):3819. doi:10.1038/s41467-020-17644-0.
- Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon L, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–931. doi:10.1038/s41591-020-0882-8.
- Semeraro M, Adam J, Stoll G, Louvet E, Chaba K, Poirier-Colame V, Sauvat A, Senovilla L, Vacchelli E, Bloy N, et al. The ratio of CD8 + /FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology. 2016;5(10):e1218106. doi:10.1080/2162402X.2016.1218106.
- Zitvogel L, Perreault C, Finn OJ, Kroemer G. Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol. 2021;18(9):591–602. doi:10.1038/s41571-021-00508-x.
- Bernatsky S, Ramsey-Goldman R, Foulkes WD, Gordon C, Clarke AE. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: a meta-analysis. Br J Cancer. 2011;104:1478–1481.
- Clarke AE, Pooley N, Marjenberg Z, Langham J, Nicholson L, Langham S, Embleton N, Wang X, Desta B, Barut V, et al. Risk of malignancy in patients with systemic lupus erythematosus: systematic review and meta-analysis. Semin Arthritis Rheum. 2021;51(6):1230–1241. doi:10.1016/j.semarthrit.2021.09.009.
- Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212. doi:10.1186/s13075-015-0728-9.
- Shah AA, Igusa T, Goldman D, Li J, Casciola-Rosen L, Rosen A, Petri M. Association of systemic lupus erythematosus autoantibody diversity with breast cancer protection. Arthritis Res Ther. 2021;23(1):64. doi:10.1186/s13075-021-02449-3.
- Hansen JE, Chan G, Liu Y, Hegan DC, Dalal S, Dray E, Kwon Y, Xu Y, Xu X, Peterson-Roth E, et al. Targeting cancer with a lupus autoantibody. Sci Transl Med. 2012;4(157):157ra42. doi:10.1126/scitranslmed.3004385.
- Gardner-Thorpe J, Ito H, Ashley SW, Whang EE. Autoantibody-mediated inhibition of pancreatic cancer cell growth in an athymic (nude) mouse model. Pancreas. 2003;27(2):180–189. doi:10.1097/00006676-200308000-00012.
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi:10.1038/nrclinonc.2017.101.
- Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–1138. doi:10.1038/nm.3944.
- Castoldi F, Humeau J, Martins I, Lachkar S, Loew D, Dingli F, Durand S, Enot D, Bossut N, Chery A, et al. Autophagy-mediated metabolic effects of aspirin. Cell Death Discov. 2020;6(1):129. doi:10.1038/s41420-020-00365-0.
- Zhang Y, Chen H, Chen S, Li Z, Chen J, Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1957605. doi:10.1080/2162402X.2021.1957605.
- Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25(9):1428–1441. doi:10.1038/s41591-019-0566-4.
- Zandman-Goddard G, Solomon M, Rosman Z, Peeva E, Shoenfeld Y. Environment and lupus-related diseases. Lupus. 2012;21(3):241–250. doi:10.1177/0961203311426568.
- Choi MY, Flood K, Bernatsky S, Ramsey-Goldman R, Clarke AE. A review on SLE and malignancy. Best Pract Res Clin Rheumatol. 2017;31(3):373–396. doi:10.1016/j.berh.2017.09.013.
