ABSTRACT
Nearly 40% of the advanced cancer patients will present brain metastases during the course of their disease, with a 2-year life expectancy of less than 10%. Immune system impairment, including the modulation of both STAT3 and PD-L1, is one of the hallmarks of brain metastases. Liquid biopsy could offer several advantages in brain metastases management, such as the possibility of noninvasive dynamic monitoring. Extracellular vesicles (EVs) have been recently proposed as novel biomarkers especially useful in liquid biopsy due to their secretion in biofluids and their role in cell communication during tumor progression. The main aim of this work was to characterize the size and protein cargo of plasma circulating EVs in patients with solid tumors and their correlation with newly diagnosed brain metastases, in addition to their association with other relevant clinical variables. We analyzed circulating EVs in the plasma of 123 patients: 42 patients with brain metastases, 50 without brain metastases and 31 healthy controls. Patients with newly diagnosed brain metastases had a lower number of circulating EVs in the plasma and a higher protein concentration in small EVs (sEVs) compared to patients without brain metastases and healthy controls. Interestingly, melanoma patients with brain metastases presented decreased STAT3 activation and increased PD-L1 levels in circulating sEVs compared to patients without central nervous system metastases. Decreased STAT3 activation and increased PD-L1 in plasma circulating sEVs identify melanoma patients with brain metastasis.
Introduction
One out of 10 patients with solid tumors will develop brain metastasesCitation1, 40% of patients with metastatic tumors,Citation1,Citation2 during the course of their disease. The main tumor types with higher incidence of brain metastasis are lung cancer (40–50% of cases), breast cancer (15–25%) and melanoma (5–20%).Citation2 Despite the current multimodal strategies, central nervous system (CNS) metastases are associated with poor outcomes (less than 10% of the patients are alive after 2 years from the diagnosis) and detrimental quality of life of the patients.Citation3 Differential factors involved in brain metastases dissemination could justify the failure of systemic therapies such as the presence of the blood-brain barrier (BBB) or evolutionary divergences found in cell clones localized in the CNS compared to extracranial metastases.Citation4,Citation5
The induction of an immunosuppressive state is another factor involved in CNS metastatic spread.Citation6 Out of many factors involved, the signal transducer and activator of transcription 3 (STAT3) is particularly relevant.Citation7 Its activity has been shown to increase in brain metastases compared to primary tumors in melanoma, lung or breast cancer.Citation7–10 Moreover, STAT3-activated reactive astrocytes within brain metastases have been shown to inhibit CD8+ T lymphocyte activation through the expression of programmed death-ligand 1 (PD-L1) and the secretion of other immunosuppressive molecules, favoring tumor growth.Citation11 Indeed, STAT3 is involved in immune cell evasion aiding tumor progression and metastasis.Citation12 Similarly, PD-L1 expression has also been reported in different brain stromal cells with immunosuppressive consequences, such as reactive astrocytes,Citation11 microglial cellsCitation13 or infiltrating peripheral neutrophils.Citation14
Small extracellular vesicles (sEVs) represent a subtype of extracellular vesicles (EVs).Citation15,Citation16 In malignant diseases, EVs play an important role as intercellular communicators in the establishment of the pre-metastatic nicheCitation17,Citation18 and brain metastasis.Citation17,Citation19–21 EVs represent one of the last elements to be included in liquid biopsy studies;Citation22,Citation23 plasma samples are the main source analyzed so far. In recent years, secretion of PD-L1 in EVs has been pointed out as a potential mechanism of resistance to the treatment with immune checkpoint inhibitors.Citation24,Citation25 For example, exogenous administration of tumor cell-derived EVs, expressing PD-L1 on their surface, increased the ability of metastatic dissemination and primary tumor growth.Citation26 Plasma circulating PD-L1 from plasma decreased the activation of CD8+ lymphocytes T.Citation27 On the other hand, the secretion of STAT3 in plasma-circulating sEVs has not been reported. Considering the role of both PD-L1 and phospho-STAT3 (pSTAT3) in the biology of brain metastases, we wanted to characterize the presence of these molecules in plasma circulating sEVs in patients with newly diagnosed brain metastases to identify their relevance as minimally invasive biomarkers for the CNS metastatic dissemination.
Materials and methods
Study design, population and sample collection
Observational, single academic center study. Plasma samples were collected prospectively from February 2017 to December 2020.
Two different sets were considered:
a) Patients diagnosed with metastatic solid tumors (lung, breast, kidney cancer or melanoma, the most frequent sources known to spread to the CNS). They were prospectively included with either confirmed progressive disease state or a recent diagnosis of their metastatic tumor. Patients were not receiving any potential systemic therapy interfering with the analyses.
The assessment of the brain metastatic status was performed according to usual practice, using standard imaging tests such as brain computed tomography (CT) or brain magnetic resonance imaging (MRI). Those patients with brain metastases had to present a recent (de novo) diagnosis of their CNS involvement at the time of sample collection; patients with previous history of brain dissemination were excluded. Patients considered to have no brain metastases were also assessed by radiological tests in order to confirm their status.
b) Healthy controls. Healthy volunteers and patients with a previous diagnosis of any solid tumor radically treated and without evidence of local or distant relapse during at least 5 years of follow-up (cured controls) were included.
The clinical/pathological variables collected are detailed in Supplementary Table 1.
The protocol of this study was reviewed and approved by the Committees for Ethical Research (identification number 17/052), as indicated by the Spanish legislation. All samples were obtained after signing informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Once the inclusion criteria mentioned above were confirmed, blood samples (approximately 10 ml) were collected in EDTA tubes and centrifuged for 10 min at 500 x g at room temperature using a swing out bucket rotor. The plasma fractions (supernatant) were poured into collection tubes and immediately frozen at −80°C.
sEV isolation
Purification of sEVs from plasma was performed after thawing the samples at 37°C for 3–5 min. First, samples were centrifuged at 3,000 × g for 20 min, followed by further centrifugation of the supernatants at 12,000 × g for 20 min. The sEVs were subsequently harvested by centrifugation of the supernatant fraction at 100,000 × g for 70 min. The sEV pellet was resuspended in 3.5 ml of PBS and collected by a second ultracentrifugation at 100,000 × g for 70 min. All centrifugations were performed at 10°C using a Beckman OptimaX100 centrifuge with a Beckman 70.1Ti rotor.
sEVs were resuspended in 100 µl PBS and the protein content was measured by bicinchoninic acid (BCA) assay (Pierce). Particle number was measured from an aliquot of 1 μl of plasma sEVs diluted in 1 ml of PBS using NTA (NanoSight; Malvern) equipped with a violet laser (405 nm).
Sample preparation for proteomic analysis
Plasma samples were diluted 1:10 with 50 mM Tris-HCl and the protein content of plasma-derived exosomes was resuspended in Laemmli buffer. Proteins (10 µg) were reduced and alkylated (15 mM TCEP, 30 mM CAA) 20 min in the dark at room temperature. Then, samples were digested following the single-pot solid-phase enhanced sample preparation (SP3) protocolCitation28 with some modifications. Briefly, ethanol was added to the samples to a final concentration of 70% and proteins were incubated for 15 minutes with the beads at a bead:protein ratio 10:1 (wt/wt). Beads were washed using 70% EtOH (twice) and 100% ACN. Proteins were digested with trypsin in 50 mM TEAB (protein:enzyme ratio 1:50, overnight at 37°C; Promega). Resulting peptides were acidified, vaccum-dried and resuspended in 0.1%FA.
Mass spectrometry
For the proteomic analysis, LC-MS/MS was carried out by coupling an Ultimate 3000 RSLCnano System (Dionex) with a Q-Exactive Plus mass spectrometer (ThermoScientific). Peptides were loaded into a trap column (Acclaim PepMapTM 100, 100 µm x 2 cm, ThermoScientific) over 3 min at a flow rate of 10 µl/min in 0.1% FA. Then peptides were transferred to an analytical column (PepMapTM RSLC C18, 2 µm, 75 µm x 50 cm, ThermoScientific) and separated using a 60 min effective linear gradient (buffer A: 0.1% FA; buffer B: 100% ACN, 0.1% FA) at a flow rate of 250 nL/min. The gradient used was: 0–3 min 2% B, 3–5 min 6% B, 5–36 min 17.5% B, 36–60 min 25% B, 60–63 min 33% B, 63–65 min 45% B, 65–70 min 98% B, 70–80 min 2% B. The peptides were electrosprayed (1.5 kV) into the mass spectrometer through a heated capillary at 320°C and a S-Lens RF level of 50%. The mass spectrometer was operated in a data-dependent mode, with an automatic switch between the MS and MS/MS scans using a top 15 method (minimum AGC target 3E3) and a dynamic exclusion time of 25 sec. MS (350–1400 m/z) and MS/MS spectra were acquired with a resolution of 70,000 and 17,500 FWHM (200 m/z), respectively. Peptides were isolated using a 2 Th window and fragmented using higher-energy collisional dissociation (HCD) at 27% normalized collision energy. The ion target values were 3E6 for MS (25 ms maximum injection time) and 1E5 for MS/MS (45 ms maximum injection time). Samples were analyzed twice.
Proteomic data analysis
Raw files were processed with MaxQuant (v 1.6.10.43) using the standard settings against a human protein database (UniProtKB/Swiss-Prot, January 2022, 20,387 sequences) supplemented with contaminants. Label-free quantification was done with match between runs (match window of 0.7 min and alignment window of 20 min). Carbamidomethylation of cysteines was set as a fixed modification whereas methionine oxidation and N-term acetylation were variable protein modifications. The minimal peptide length was set to 7 amino acids and a maximum of two tryptic missed-cleavages were allowed. The results were filtered at 0.01 FDR (peptide and protein level).
The human Exocarta database (http://exocarta.org) was considered the reference set list to evaluate the protein content in exosome markers.
Data availability
Public proteomic data set on human plasma and plasma-derived sEVs used was deposited to the ProteomeXchange Consortium via the PRIDECitation29 partner repository with the dataset identifiers PXD032767. Source data have been provided as Source Data files. All other data supporting the findings of this study are available from the corresponding author on reasonable request
Immunoblotting
sEVs were lysed in Laemmli buffer at 95°C for 5 min, and 10 µg of the protein extracts were resolved by SDS-PAGE and probed using antibodies against STAT3 (1:1,000, #9139S; Cell Signaling), Phospho-STAT3 (1:2,000, #9145S; Cell Signaling), PD-L1 (1:1,000, #13684S; Cell Signaling), β-actin (1:10,000, #A5441; Sigma- Aldrich), CD81 clone 5A6 (Santa Cruz), CD9 clone VJ1/20 (kindly provided by Dr. María Yáñez-Mo), TSG101 (BD #612697). Peroxidase-conjugated AffinityPure donkey anti-rabbit or anti-mouse (1:5,000; Jackson ImmunoResearch) were used as secondary antibodies. Signal was detected using ECL Western Blotting Substrate kit (GE Healthcare). The intensity of the immunoreactive bands was quantified by densitometry using ImageJ software (National Institutes of Health).
Endpoints
We aim to characterize plasma-circulating sEVs from patients with recently diagnosed brain metastases and compare them to those from metastatic patients without CNS involvement and healthy controls. Specifically, particle number in plasma, protein concentration and expression of pSTAT3, STAT3 and PD-L1 by Western blot in plasma-circulating sEVs were quantified and correlated with clinically relevant characteristics, especially the recent diagnosis of brain metastases. The type of response achieved with the immediate treatment received after plasma sample collection and overall survival of patients were also assessed and correlated with the parameters analyzed in plasma-circulating sEVs.
Statistical analysis
GraphPad Prism (version 8.1.1) was used for all statistical analyses. Baseline patient characteristics were compared between the study groups (concretely, patients with vs without brain metastases) using t tests, Mann-Whitney test (quantitative variables) or two-tailed Fisher´s exact test and Chi-square test (qualitative variables). The differences for the parameters analyzed were assessed by parametric tests (one-way ANOVA test, unpaired Student´s test) and nonparametric tests (Kruskal-Wallis test, Mann-Whitney test). Overall survival (OS) analyses were performed using the Kaplan-Meier method; differences were evaluated using the Log-Rank test. Controlled subgroup analyses were performed to assess the influence of potential confounding factors. Statistical significance was established for a p value < .05.
Results
Analysis of circulating EVs in plasma
A total of 123 patients who met the pre-specified inclusion criteria were finally included: 42 metastatic patients with brain metastases, 50 patients without brain metastases and 31 healthy controls (). Specifically for metastatic patients with or without brain metastasis, no statistically significant differences were found in relation to the number or type of previous systemic therapies received between these groups. See Supplementary Tables 2 and 3 for further details. Metastatic patients with CNS involvement had a shorter OS (time from diagnosis of metastatic disease to death due to any cause) (median OS 25.20 months) compared to those without brain metastases (median OS 55.80 months) (Hazard ratio 1.99; 95% confidence interval 1.11–3.57) ()). A consistent trend was observed in all histological types considered, with a worse outcome for those patients with CNS dissemination (Supplementary Figures 1A-1D).
Figure 1. Overall survival analysis of patients included in the study and characterization of plasma-circulating sEVs. a. Survival analysis and graphical representation using Kaplan-Meier curves showing the cumulative survival probability in the study population according to the absence (CNS-) or presence (CNS+) of central nervous system (CNS) metastases. * p value 0.02. Differences were assessed using the Log-Rank test. b. Representative image of the particle content (x108) by NTA analysis of a plasma sample from a melanoma patient. c. Representative electron microscopy imaging of sEVs from the same patient´s plasma. d. Representative Western blot of the analysis of exosome markers CD9, CD81, TSG101 and in sEVs isolated from the plasma of three different melanoma patients. Ponceau staining was used as loading control (see Supplementary Figure 4A). e. Proteomic analysis of sEVs and plasma paired samples derived from melanoma patients. Venn diagrams showing an enrichment of Exocarta markers (green) and a reduction albumin and apoliproteins (red) in sEVs (left diagram) compared to proteins detected in plasma samples (right diagram).
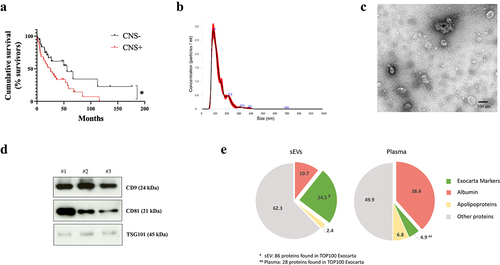
Table 1. Clinical characteristics of the patients included in the study at the time of sample collection. General characteristics of the main groups of patients included in the study are shown. CNS: Central nervous system
In order to characterize plasma circulating EVs, we measured their number using nanoparticle track analysis (NTA) ()). sEV integrity was confirmed by electron microscopy ()). We verified the enrichment of typical sEV markers such as CD9, CD81 and TSG-101, in our preparationsCitation15 ()). In order to analyze the relative abundance of sEV markers in our sEV preparation, we performed mass spectrometry in matched samples of plasma and sEVs from melanoma patients. We found that 86 out of TOP 100 proteins defined in exocartaCitation30 were present in our sEV samples (24,5%) as compared with only 26 in plasma samples (4,9%), demonstrating an enrichment of 5-fold sEV markers in our samples. Moreover, we found a reduction of potential contaminants in our sEV samples since albumin was reduced by 3.5 fold and apolipoproteins were reduced by 2.8 fold in sEV preparations compared to plasma ()).
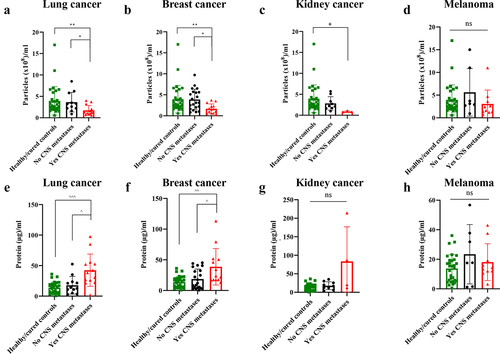
The analysis of particle number in plasma samples showed that lung, breast and kidney cancer patients with CNS metastases presented a decreased number of particles compared to those patients without brain metastasis ()). In melanoma patients we did not find significative differences among groups ()). On the other hand, CNS metastasis was associated with an increased protein concentration in plasma-circulating sEVs from lung cancer and breast cancer patients compared to those without brain metastases ()). Although not statistically significant, there was also a trend for an increased protein concentration in the circulating sEVs of kidney cancer patients with CNS metastases ()). Regarding melanoma patients, no differences were found in relation to sEV protein concentration according to the CNS status (). See Supplementary Tables 4 and 5 for further details.
Figure 2. Quantitative analysis of plasma-circulating sEVs from patients included in the study according to their central nervous system (CNS) metastases status (including healthy/cured controls). a. Particles/ml in lung cancer. b. Particles/ml in breast cancer. c. Particles/ml in kidney cancer. d. Particles/ml in melanoma. e. Proteins/ml in lung cancer. f. Proteins/ml in breast cancer. g. Proteins/ml in kidney cancer. h. Proteins/ml in melanoma. * p value < .05. ** p value < .01. ^ p value < .05. ^^ p value 0.003. ^^^ p value 0.0003. ns: not significant.
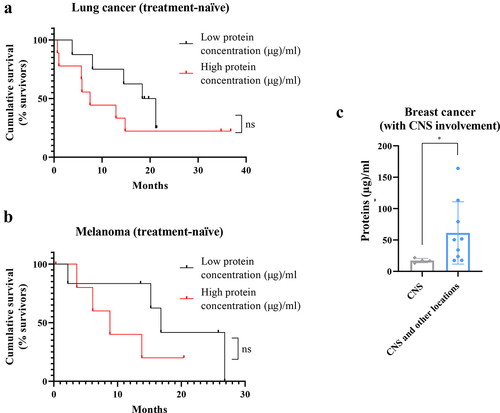
Analysis of correlation of protein concentration with patient survival showed that a high protein concentration in plasma-circulating sEVs is associated with a more aggressive clinical course and a reduced overall survival in lung cancer and melanoma patients ()). No differences were found according to the immunohistochemical subtypes for breast cancer and kidney cancer patients or according to absence/presence of previous oncological history in the healthy controls group (data not shown). Specifically in breast cancer, a higher protein concentration was found in patients with a more aggressive clinical course of their disease (tumor progression at multiple levels, including CNS and other systemic locations) compared to patients with CNS progression only ()).
Figure 3. Complementary studies for total protein concentration in patients plasma-circulating sEVs showing a correlation between a high protein amount and a worse prognosis. a. Survival analysis showing the cumulative survival probability in patients with previously untreated metastatic lung cancer according to the protein concentration in plasma-circulating sEVs (taking the median value of the group as reference). b. Survival analysis showing the cumulative survival probability in patients with previously untreated metastatic melanoma according to the protein concentration in plasma-circulating sEVs (taking the median value of the group as reference). Differences were assessed using the Log-Rank test. c. Analysis of protein concentration in circulating sEVs regarding the type of progression experienced in patients with metastatic breast cancer and known central nervous system (CNS) involvement: at CNS only or at CNS and other locations. * p value 0.02. ns: not significant.
Characterization of PD-L1 and pSTAT3 expression by Western blot in plasma-circulating sEVs
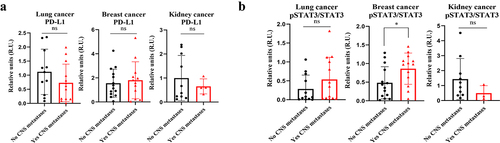
Due to the relevance of STAT3 and PD-L1 in brain metastasis progression,Citation7–11,Citation13,Citation14 we wondered if the analysis of these molecules in circulating sEVs could be modulated along metastatic progression. The comparative analysis of PD-L1 expression in plasma-circulating sEVs from patients with lung, breast or kidney cancer showed no differences regarding the CNS metastatic status ()). Regarding the analysis of STAT3, a double band at the expected weight of STAT3 was observed in most of the patients analyzed, fact that could be explained by the simultaneous presence of two isoforms (STAT3α and STAT3β as a consequence of alternative splicing)Citation31 (Supplementary Figures 2A, 2B, 3A, 4A, 4B and 4C). However, unlike in melanoma patients (see below), a double band was not clearly observed in the pSTAT3 analysis for lung, breast and kidney cancer patients (Supplementary Figures 2A, 2B and 3A); thus, the quantification of pSTAT3 expression in these cases was performed considering exclusively the band observed at the expected weight for this protein.Citation31 Patients with metastatic breast cancer and brain metastases presented an increased STAT3 activation in their plasma-circulating sEVs compared to patients without CNS involvement; in this regard, no differences were found for lung or kidney cancer patients ()).
Figure 4. Analysis of PD-L1 and pSTAT3/STAT3 expression by Western blot in plasma-circulating sEVs from patients with lung, breast or kidney cancer. a. Quantification of PD-L1 expression levels and statistical analysis of samples obtained. The data obtained for PD-L1 in densitometry were normalized to Ponceau values (see Supplementary Figure 4). b. Quantification of pSTAT3/STAT3 expression levels and statistical analysis of samples obtained. * p value 0.03. ns: not significant.
Interestingly, the analysis of PD-L1 levels in melanoma patients showed an increased expression of this protein in circulating sEVs from the patients with a recent diagnosis of brain metastases compared to those without known CNS dissemination (). In contrast to the rest of the patients considered in this series, melanoma cases without known brain metastasis presented a double band in the pSTAT3 analysis; both pSTAT3α (higher molecular weight) and pSTATβ (lower molecular weight) expressions were retained ()). In this tumor subtype, we unexpectedly observed that pSTAT3α showed a strong dephosphorylation in patients with brain metastasis ()).
Figure 5. Study of pSTAT3/STAT3 and PD-L1 expression by Western blot in plasma-circulating sEVs from patients with melanoma. a. Western blot image of the expression of different proteins (indicated on the right) in sEVs from melanoma patients according to the absence (No) or presence (Yes) of central nervous system (CNS) metastases. Ponceau staining was used as loading control (see Supplementary Figure 4 F). b. Quantification of PD-L1 expression levels and statistical analysis of samples considered in A. The data obtained for PD-L1 in densitometry were normalized to Ponceau values (see Supplementary Figure 4). c. Quantification of pSTAT3α/STAT3α band expression levels and statistical analysis of samples considered in A. d. Quantification of PD-L1 expression levels and statistical analysis of the whole population of this study, including the healthy/cured controls group. The data obtained for PD-L1 in densitometry were normalized to Ponceau values (see Supplementary Figure 4). No CNS: Absence of CNS metastases. Yes CNS: Presence of CNS metastases. ^ p value 0.03. * p value 0.02. **** p value < .0001.
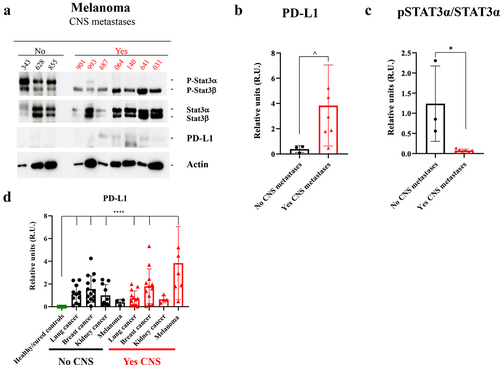
Strikingly, some patients with low pSTAT3/STAT3 sEVs expression, regardless of their tumor subtype or CNS status, were repeatedly associated with an increased PD-L1 level (Supplementary Figures 2A, 2B and 3A). Of note, sEVs purified from the plasma of healthy patients showed very low levels of pSTAT3 (Supplementary Figure 3B) and a complete absence of PD-L1 expression (Supplementary Figure 3C).
In the global analysis of PD-L1 expression ()), a statistically significant absence of this protein was found in the healthy controls group; this quantification also showed that there is a significant increase of PD-L1 in circulating sEVs regardless of the patients group analyzed except in melanoma patients with no dissemination to the CNS and kidney cancer patients with dissemination to the CNS. These data suggest that patients with no active cancer have undetectable levels of PD-L1 in plasma circulating sEVs.
Discussion
Tests using biological fluids for detecting tumor material are a noninvasive approach complementary or even alternative to tissue biopsies. Circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) are considered the cornerstones of liquid biopsy-based diagnosis.Citation32 However, in addition to CTCs and ctDNA, the analysis of tumor-secreted EVs have been of increasing interest in recent years. Although there remains debate about the benefits of circulating EVs compared to other circulating biomarkers, there have been several studies demonstrating their utility and benefits in diagnostics using specific biomarkers including DNA, RNA and protein biomarkers.Citation33,Citation34
Several studies support an association between an increased number of plasma particles and a worse outcome or aggressive features in oncological patients with colonCitation35 or head and neck cancer,Citation36 among others. However, other studies have found no differences in the number of circulating EVs analyzed in plasma or other biological fluids from melanoma patients according to the tumor stage or the extent of loco-regional lymph node involvement,Citation37,Citation38 similar to the results here presented for this specific subtype.
These differences could probably by explained by the heterogeneity of the series considered, as well as the absence of a standardized methodology for sample handling or the divergent biology of the different tumor types.Citation37 In our series, we observed that the number of circulating particles was reduced in breast, lung and kidney cancer patients with brain metastases. Although the reasons behind these changes are hard to interpret, we observed that, concomitantly with these changes, breast and lung cancer patients with brain metastases showed increased amount of protein associated to circulating sEVs. Although scarce, data from different studies regarding the value of total protein concentration in circulating sEVs point to an association between a higher protein concentration in circulating sEVs and a worse clinical course.Citation37–40 In our series, the analysis of the correlation between the total protein content in sEVs and patient survival suggests a potential prognostic value. Measurement of protein concentration in sEVs could be useful for the assessment of tumor dissemination to the CNS; nevertheless, since we did not reach statistical significance in these cohorts, analyses in larger cohorts are needed. Similarly, since total protein in sEVs may indicate systemic changes (e.g. EVs derived from circulating platelets, immune cells, etc …) the source of this material as well as the biological relevance needs to be solved.
Tumor-secreted EVs have the intrinsic ability to breach biological barriers such as the BBB.Citation41 Brain metastases, result from the dissemination of tumor cells to the brain, most commonly from lung cancer, melanoma, and breast cancer.Citation1,Citation2 EVs contribute to different stages of brain metastasis increasing BBB permeability,Citation21,Citation41 reprogramming of brain metastatic nichesCitation20,Citation42 and increasing brain metastatic organotropism of tumor cells.Citation18,Citation19 Overall, these studies demonstrate that EVs can drive bidirectional cross talk between tumor cells and their microenvironment promoting metastasis formation in the brain. However, the analysis of EVs in clinical samples is unreported, to the best of our knowledge, this study constitutes the first approach to characterize the potential clinical value of a noninvasive technique, liquid biopsy using plasma-circulating sEVs, in patients with different solid tumors and recently diagnosed brain metastases.
To perform these analyses the selection of plasma samples was carried out following well-established criteria to ensure the homogeneity of the selected group and to limit possible confounding factors common in clinical practice. It is noteworthy that none of the oncological patients were under active systemic treatment potentially influencing the results at the time of sample collection; in addition, the group of patients with CNS involvement consisted of patients with no previously known brain disease (de novo diagnosis). This series of patients consisted of three distinct groups of subjects. The healthy controls, when compared with the other two oncological groups, allowed us to characterize features inherent to any oncological disease. Specifically, the presence of two defined groups of metastatic patients, which differed only in the presence of CNS involvement, allowed to establish associations between the results obtained and the metastatic brain dissemination process. As previously described in the literature, and showed in our series, CNS metastatic disease constitutes a negative prognostic factor for OS.Citation3,Citation43 In the studies considering each histological subtype separately, small sample sizes limited the power of statistical analyses and no statistically significant difference was reached.
Although no specific studies have been performed in patients with brain metastatic dissemination, there are data reporting an association between the EV expression of PD-L1 and pSTAT3 and a more advanced oncological disease, more aggressive clinical course and, even, mechanisms of treatment resistance.Citation24,Citation25,Citation44
STAT3α, the predominant STAT3 splice form in several cell types, typically shows rapid phosphorylation and nuclear translocation following cytokine stimulation, but their differential role is still a matter of discussion.Citation45 We have observed that plasma sEVs from melanoma patients with brain metastases showed reduced pSTAT3α levels in addition to an increased PD-L1 expression compared to those from melanoma patients without CNS dissemination, characterized by higher levels of pSTAT3 together with the absence of PD-L1. The fact that pSTAT3α disappears from circulating sEVs suggest that it could be executing its actions intra-nuclearly in immune and/or tumor cells preventing its shedding in plasma-circulating sEVs. Indeed, activated STAT3 in immune cells results in inhibition of immune mediators and promotion of immunosuppressive factorsCitation46 as well as melanoma metastatic behavior.Citation47 Moreover, STAT3 inhibition reduced melanoma metastasis to brainCitation48 and has recently been hypothesized to be considered a potential target for immunotherapy.Citation49 Interestingly, a comparative study between lung cancer patients with and without CNS metastases also showed a higher concentration of PD-L1+ myeloid cells, immunosuppressive myeloid cells and regulatory T lymphocytes in the peripheral blood of patients with brain involvement, all of them considered as systemic immunosuppression markers.Citation50 In summary, we observed a decrease of pSTAT3α levels and increased PD-L1 expression in patients with CNS dissemination suggesting that these changes are a consequence of the systemic immunosuppression observed in melanoma brain metastatic patients. These data suggest that the analysis of these molecules in plasma-circulating sEVs in melanoma patients with brain metastasis undergoing immunotherapy or in the combination with STAT3 inhibitors could be useful to monitor treatment response. Nevertheless, we have to be careful in the interpretation of these results due to the limited samples analyzed in this study.
On the other hand, no significant differences were found for the expression of PD-L1 and pSTAT3 in plasma-circulating sEVs from lung, breast or kidney cancer patients depending on the presence or absence of newly diagnosed brain metastases, suggesting that these diseases depend on other molecular pathways. However, complementary analyses in some of these tumor types suggested an association between a pattern consisting of a reduced pSTAT3 or an increased PD-L1 expression (similar to that of melanoma patients with brain metastases) and a more aggressive clinical course.
Noteworthy, we observed an inverse correlation between the pSTAT3 and PD-L1 expressions in plasma-circulating sEVs in most of the tumor types considered. Further mechanistic studies are warranted to explain these findings highlighting the relationship between EV expression and cellular activation of these proteins. Our findings also support the potential value of sEV-derived PD-L1 as a diagnostic marker of active oncological disease in several types independently of brain metastasis (e.g. breast cancer and lung cancer). It was previously reported that plasma-derived sEVs from the healthy controls group were characterized by the absence of PD-L1 expression and that PD-L1 secreted in plasma sEVs from melanoma patients is useful to identify patients responding to immunotherapies.Citation24,Citation51 Importantly, we found that increase of PD-L1 levels was specific in melanoma patients with brain metastasis suggesting a strong immunosuppressive systemic change. It would be interesting to monitor PD-L1 in the plasma of melanoma patients with brain metastasis as a potential biomarker of response to therapy in this specific subtype. However, the study of plasma EVs is limited by the uncertainty of the cellular origin of these vesicles. Previous studies have suggested that most of the material found in blood would have an immunological origin.Citation52,Citation53 Unfortunately, in our study we could not identify the origin of STAT3 and PD-L1 in circulation, the identification of the source of these molecules would be important in future studies to determine whether they reflect systemic or tumor changes along metastatic progression.
Considering the role of EVs in the pre-metastatic niche conformation, the analysis of these vesicles using blood, the main route of neoplastic dissemination to the brain, could inform molecular features with potential to predict the risk of brain metastases. However, this study has several limitations. Despite the total number of the series exceeds 120 patients, the analyses according to the tumor subtypes are limited by a low sample size, which only allows the establishment of hypotheses for future studies. Nevertheless, the inclusion of different types of solid tumors favors the generalization of these results.
The samples were only collected at the time of the recent diagnosis of CNS dissemination; this fact makes it difficult the interpretation of the findings between true molecular risk factors predisposing to brain metastases or the systemic expression of already established brain lesions. Dynamic follow-up studies, including sample collections at different points during the clinical course (before and after the diagnosis of brain involvement) will be useful for the interpretation of the results here presented. Moreover, the intrinsic limitations of the imaging techniques currently used for the diagnosis could lead to a potential selection bias difficult to assess for in this series.
Finally, the complexity of cancer biology makes it difficult to explain oncological events, such as tumor dissemination to the brain, by means of a single approach. There are genetic, environmental or immunological factors, among others, not considered in this work, which could also influence the results presented. In addition to clinical studies, in vitro and in vivo analyses are also needed to explain these results and to adequately assess their clinical significance.
Overall, this study suggests that plasma-circulating EVs present different quantitative and qualitative characteristics depending on the presence or absence of neoplastic brain dissemination, especially in melanoma patients. This information highlights the potential usefulness of EVs for the development of new biomarkers that could improve the care of oncological patients; however, functional studies defining the role of these vesicles in CNS metastases should be performed for an adequate interpretation of the data.
Notes on contributors
A.C-G., L.M.S., E.C.G., D.C. and G.dV. provided patient samples. P. X-E. performed mass spectrometry analyses. A.C-G, M.H-R., S.S-R., performed experimental work A.C-G., M.H-R. G.dV. and H.P. contributed to the analysis of results. A.C-G., L.M.S., M.H-R. G.dV. and H.P. conceived the original hypothesis. M.H-R., and H.P. planned the experiments. A.C-G., M.H-R., G.dV. and H.P. wrote the original manuscript. M.H-R, S.S-R. performed the experimental work for the revised version of the manuscript. H.P. directed and supervised the work. All the authors contributed to and approved the final version of the manuscript.
Statement of translational relevance
Brain metastases are critical for outcomes and quality of life in almost 50% of oncological patients, generally associated with a poor short-term prognosis. Early or preventive diagnosis of this complication represents an unmet need. There is a necessity of discovering new biomarkers that could aid to predict disease outcome.
In this study, we analyzed plasma circulating extracellular vesicles (EVs) from a cohort of 92 patients with different solid tumors (lung, breast, kidney cancer and melanoma) and found that newly diagnosed patients with brain metastases presented lower number of circulating particles and a higher protein concentration in small extracellular vesicles (sEVs) compared to patients without brain metastases and healthy controls. Out of all groups analyzed, melanoma patients with brain metastases presented decreased STAT3 activation and increased PD-L1 levels in circulating sEVs compared to patients without central nervous system metastases.
The data presented in this work suggest that circulating sEVs may represent the immunosuppressive status of newly diagnosed brain metastases characterized by the reduced phospho-STAT3 (pSTAT3) and increased PD-L1, although the origin of these molecules found in circulating sEVs remains to be uncovered.
Supplemental Material
Download Zip (16.1 MB)Acknowledgments
We thank Dr. Maria Yañez-Mó for her advice and help in sEV characterization. We are also grateful for the support of the Translational Network for the Clinical Application of Extracellular Vesicles (TeNTaCLES), RED2018-102411-T (AEI/10.13039/501100011033), the Ramón y Cajal Programme, the FERO Foundation.
The CNIO, certified as a Severo Ochoa Excellence Centre, is supported by the Spanish government through the ISCIII.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–11. doi:10.1007/s11060-004-8093-6.
- Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662.
- Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol Northwood Lond Engl. 2000;17(4):279–286. doi:10.1007/BF02782192.
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. doi:10.1158/2159-8290.CD-15-0369.
- Fischer GM, Jalali A, Kircher DA, Lee W-C, McQuade JL, Haydu LE, Joon AY, Reuben A, de Macedo MP, Carapeto FCL, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9(5):628–645. doi:10.1158/2159-8290.CD-18-1489.
- Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi:10.1016/j.ccell.2017.02.009.
- Xie T, Huang F-J, Aldape KD, Kang S-H, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66(6):3188–3196. doi:10.1158/0008-5472.CAN-05-2674.
- Singh M, Garg N, Venugopal C, Hallett R, Tokar T, McFarlane N, Mahendram S, Bakhshinyan D, Manoranjan B, Vora P, et al. STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget. 2015;6(29):27461–27477. doi:10.18632/oncotarget.4742.
- Lee H-T, Xue J, Chou P-C, Zhou A, Yang P, Conrad CA, Aldape KD, Priebe W, Patterson C, Sawaya R, et al. Stat3 orchestrates interaction between endothelial and tumor cells and inhibition of Stat3 suppresses brain metastasis of breast cancer cells. Oncotarget. 2015;6(12):10016–10029. doi:10.18632/oncotarget.3540.
- Chiu W-T, Lee H-T, Huang F-J, Aldape KD, Yao J, Steeg PS, Chou C-Y, Lu Z, Xie K, Huang S, et al. Caveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by Stat3 inhibition. Cancer Res. 2011;71(14):4932–4943. doi:10.1158/0008-5472.CAN-10-4249.
- Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Ramón Y Cajal S, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018;24(7):1024–1035. doi:10.1038/s41591-018-0044-4.
- Zhang L, Kuca K, You L, Zhao Y, Musilek K, Nepovimova E, Wu Q, Wu W, Adam V. Signal transducer and activator of transcription 3 signaling in tumor immune evasion. Pharmacol Ther. 2021;107969. doi:10.1016/j.pharmthera.2021.107969.
- Guldner IH, Wang Q, Yang L, Golomb SM, Zhao Z, Lopez JA, Brunory A, Howe EN, Zhang Y, Palakurthi B, et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell. 2020;183(5):1234–1248.e25. doi:10.1016/j.cell.2020.09.064.
- Zhang L, Yao J, Wei Y, Zhou Z, Li P, Qu J, Badu-Nkansah A, Yuan X, Huang Y-W, Fukumura K, et al. Blocking immunosuppressive neutrophils deters pY696-EZH2–driven brain metastases. Sci Transl Med. 2020;12(545):eaaz5387. doi:10.1126/scitranslmed.aaz5387.
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326.
- Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles. 2019;8(1):1648167. doi:10.1080/20013078.2019.1648167.
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi:10.1016/j.ccell.2016.10.009.
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi:10.1038/nature15756.
- Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, Kim HS, Oxley PR, Scandariato I, Casanova-Salas I, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21(11):1403–1412. doi:10.1038/s41556-019-0404-4.
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, Li P, Li M, Wang X, Zhang C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi:10.1038/nature15376.
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6(1):6716. doi:10.1038/ncomms7716.
- LeBleu VS, Kalluri R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer. 2020;6(9):767–774. doi:10.1016/j.trecan.2020.03.007.
- Mader S, Pantel K. Liquid biopsy: current status and future perspectives. Oncol Res Treat. 2017;40(7–8):404–408. doi:10.1159/000478018.
- Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi:10.1038/s41586-018-0392-8.
- Poggio M, Hu T, Pai -C-C, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, et al. Suppression of exosomal pd-l1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427.e13. doi:10.1016/j.cell.2019.02.016.
- Wen SW, Sceneay J, Lima LG, Wong CSF, Becker M, Krumeich S, Lobb RJ, Castillo V, Wong KN, Ellis S, et al. the biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–6827. doi:10.1158/0008-5472.CAN-16-0868.
- Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 + exosomes in plasma of head and neck cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(4):896–905. doi:10.1158/1078-0432.CCR-17-2664.
- Hughes CS, Foehr S, Garfield DA, Furlong EE, Steinmetz LM, Krijgsveld J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol Syst Biol. 2014;10(10):757. doi:10.15252/msb.20145625.
- Perez-Riverol Y, Bai J, Bandla C, García-Seisdedos D, Hewapathirana S, Kamatchinathan S, Kundu DJ, Prakash A, Frericks-Zipper A, Eisenacher M, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50(D1):D543–D552. doi:10.1093/nar/gkab1038.
- Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1(1):18374. doi:10.3402/jev.v1i0.18374.
- Ng IHW, Ng DCH, Jans DA, Bogoyevitch MA. Selective STAT3-α or -β expression reveals spliceform-specific phosphorylation kinetics, nuclear retention and distinct gene expression outcomes. Biochem J. 2012;447(1):125–136. doi:10.1042/BJ20120941.
- Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–491. doi:10.1158/2159-8290.CD-15-1483.
- Cortés-Hernández LE, Eslami-S Z, Costa-Silva B, Alix-Panabières C. Current applications and discoveries related to the membrane components of circulating tumor cells and extracellular vesicles. Cells. 2021;10(9):2221. doi:10.3390/cells10092221.
- Li S, Yi M, Dong B, Tan X, Luo S, Wu K. The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int J Cancer. 2021;148(11):2640–2651. doi:10.1002/ijc.33386.
- Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51(4):409–418. doi:10.1002/gcc.21926.
- Ludwig S, Floros T, Theodoraki M-N, Hong C-S, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23(16):4843–4854. doi:10.1158/1078-0432.CCR-16-2819.
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi:10.1038/nm.2753.
- García-Silva S, Benito-Martín A, Sánchez-Redondo S, Hernández-Barranco A, Ximénez-Embún P, Nogués L, Mazariegos MS, Brinkmann K, Amor López A, Meyer L, et al. Use of extracellular vesicles from lymphatic drainage as surrogate markers of melanoma progression and BRAF V600E mutation. J Exp Med. 2019;216(5):1061–1070. doi:10.1084/jem.20181522.
- Wang N, Song X, Liu L, Niu L, Wang X, Song X, Xie L. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. 2018;109(5):1701–1709. doi:10.1111/cas.13581.
- Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, Jakobsen KR, Meldgaard P, Folkersen BH, Rasmussen TR, Varming K, Jørgensen MM, Sorensen BS. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol. 2016;10(10):1595–1602. doi:10.1016/j.molonc.2016.10.003.
- Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, Park T-E, Ingber DE, Daisy CC, Moses MA, et al. Tumor-derived extracellular vesicles breach the intact blood–brain barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi:10.1021/acsnano.9b04397.
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor STF, Li S, Chin AR, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi:10.1038/ncb3094.
- Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, et al. Brain metastases. Nat Rev Dis Primer. 2019 Jan;5(1):5.
- Zhang Q, Liu R-X, Chan K-W, Hu J, Zhang J, Wei L, Tan H, Yang X, Liu H. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. J Exp Clin Cancer Res. 2019;38(1). doi:10.1186/s13046-019-1314-9.
- Caldenhoven E, van Dijk TB, Solari R, Armstrong J, Raaijmakers JAM, Lammers JWJ, Koenderman L, de Groot RP. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J Biol Chem. 1996;271(22):13221–13227. doi:10.1074/jbc.271.22.13221.
- Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018;415:117–128. doi:10.1016/j.canlet.2017.12.003.
- Swoboda A, Soukup R, Eckel O, Kinslechner K, Wingelhofer B, Schörghofer D, Sternberg C, Pham HTT, Vallianou M, Horvath J, et al. STAT3 promotes melanoma metastasis by CEBP-induced repression of the MITF pathway. Oncogene. 2021;40(6):1091–1105. doi:10.1038/s41388-020-01584-6.
- Kong L-Y, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA, Schmittling RJ, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(18):5759–5768. doi:10.1158/1078-0432.CCR-08-0377.
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. doi:10.1038/nri1995.
- Li YD, Lamano JB, Lamano JB, Quaggin-Smith J, Veliceasa D, Kaur G, Biyashev D, Unruh D, Bloch O. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol Immunother CII. 2019;68(9):1501–1513. doi:10.1007/s00262-019-02384-y.
- Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros M-P, Arnould L, Garrido C, Aubin F, Gobbo J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9(1):1710899. doi:10.1080/20013078.2019.1710899.
- Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–661. doi:10.1158/2159-8290.CD-13-1014.
- Shi A, Kasumova G, Michaud WA, Cintolo-Gonzalez J, Díaz-Martínez M, Ohmura J, Mehta A, Chien I, Frederick DT, Cohen S, et al. Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome. Sci Adv. 2020;6(46):eabb3461. doi:10.1126/sciadv.abb3461.
