ABSTRACT
Preclinical experimentation revealed that established cancers treated with the immunogenic cell death (ICD) inducer oxaliplatin are sensitized to immune checkpoint inhibitors targeting PD-1. In contrast, no such sensitizing effect is observed when cisplatin, a non-immunogenic cell death inducer is used. Two randomized phase III clinical trials targeting unresectable gastric and gastro-esophageal junction carcinomas apparently validate this observation. Thus, oxaliplatin-based chemotherapy (together with capecitabine or 5-fluorouracil plus leucovorin) favorably interacted with nivolumab, yielding improved outcome. In contrast, the outcome of cisplatin-based chemotherapy (together with capecitabine or 5-fluorouracil) failed to be improved by concomitant treatment with pembrolizumab. These clinical findings underscore the importance of choosing appropriate ICD-inducing cytotoxicants for the development of chemoimmunotherapeutic regimens. Unfortunately, the FDA and EMA have approved PD-1 blockade in combination with “platinum-based chemotherapy” without specifying the precise nature of the platinum-containing drug. This is a non sequitur. Based on the available clinical data, such approvals should be restricted to the use of oxaliplatin.
KEYWORDS:
Main text
Platinum-based antineoplastic drugs are among the most widely used chemotherapeutic agents employed for the treatment of solid tumors including but not limited to lung, colorectal, gastric, and head and neck cancersCitation1. Cisplatin is a first-generation platinum drug initially approved by the FDA for testicular and ovarian cancers and has been, and still is, one of the most employed chemotherapeutic agents in clinical routine.Citation2 Despite the landmark success during the dawn of chemotherapy in the 1970s, major limitations of cisplatin are the (inevitable) occurrence of drug resistance as well as considerable side effects.Citation3 Since then, new generation analogues with equivalent or increased antitumor activity and decreased risk of adverse effects have been developed and introduced into clinical oncology.Citation4 Oxaliplatin, a second generation anticancer agent, turned out to be as efficient as cisplatin in the treatment of gastric cancers. Systematic meta-analysis of clinical trials in advanced gastric cancer comparing oxaliplatin-based treatment regimens with cisplatin-mediated effects revealed equivalent or superior antineoplastic effects of oxaliplatin that were coupled to a favorable safety profile associated with less neutropenia and fewer thromboembolic events, but with increased neurotoxicity.Citation5–10 Of note, accumulating evidence suggests that the improved anticancer efficacy of oxaliplatin depends at least in part on the induction of immunogenic cell death (ICD),Citation11–13 which stimulates potent antitumor immune responses.
ICD is a functionally unique form of regulated cell death that is accompanied by the exposure and release of damage-associated molecular patterns (DAMPs), which are recognized by pattern recognition receptors (PRRs) expressed on antigen presenting cells (APC) such as dendritic cells (DCs).Citation14 ICD-associated DAMPs include ATP and annexin A1 (ANXA1), the secretion of which enable the recruitment and chemotaxis of DCs; the surface exposure of calreticulin (CALR) that serves as an “eat-me” signal facilitating the phagocytosis of dying cells by DCs; and the release of nuclear DNA-binding protein high mobility group box 1 (HMGB1) by the tumor that promotes DC maturation and stimulates tumor antigen cross-presentation.Citation15–19 Moreover, type I IFN secreted by the tumor in the context of ICD triggers autocrine and paracrine circuitries that result in the release of chemokine (C-X-C motif) ligand 10 (CXCL10), which mediates chemotactic and immunostimulatory effects.Citation20Citation21–Citation22 Altogether, the ICD-associated emission of DAMPs confers robust adjuvanticity, which in turn stimulates tumor antigen-specific immune responses and the generation of long-term immunological memory.Citation23 Such immunological consequences are not strictly linked to the chemical structure of the employed chemotherapeutic agent. Thus, tumor cells undergoing ICD in response to oxaliplatin have the ability to trigger protective immune responses when injected into immunocompetent animals without any adjuvants,Citation11 whereas cells succumbing in response to cisplatin fail to induce such a vaccinating effect, providing an explanation for the observation that cisplatin is less efficient in controlling cancer than oxaliplatin in several preclinical models.Citation11,Citation24
In the past decade immune checkpoint inhibitors (ICI), such as monoclonal antibodies targeting programmed cell death protein 1 (PD-1), have become a frontline therapy for many types of cancer, including those with a high mutational burden and mismatch repair deficiency.Citation25 However, in many cases ICI monotherapy fails to confer sufficient benefit due to the lack of pre-existing immune priming.Citation26–28 Therefore, ICD-inducing regimens that are capable of stimulating adaptive anticancer immunity appear as particularly promising combinations for generating synergistic effects with ICIs. The concept of combining immunogenic chemotherapies with ICIs is supported by several preclinical studies generally employing ICD inducers to prime an adaptive antitumor immune response several days or weeks before the administration of ICIs ().Citation31,Citation54 Considerable research efforts have been dedicated to combination therapies consisting of oxaliplatin-based immunogenic chemotherapies and PD-1/PD-L1 blocking antibodies in orthotopic models of fibrosarcoma,Citation31 lung,Citation24,Citation29,Citation32 colon,Citation33–37 liver Citation55 and gastric cancer.Citation38 Importantly, these preclinical studies not only described synergistic interactions between oxaliplatin and PD-1/PD-L1 blockade, but also unraveled the role of ICD in reshaping the tumor microenvironment. Pfirschke and colleagues employed oxaliplatin together with cyclophosphamide on a Kirsten rat sarcoma viral oncogene homolog (KRAS) mutated and tumor suppressor p53 (TP53) deficient (KP) non-small cell lung cancer (NSCLC) model. This model exhibits an extremely poor infiltration by CD3+ T cells at baseline and thus resists all current monotreatment options including PD-1 blocking antibodies.Citation29 This study revealed that oxaliplatin plus cyclophosphamide induced potent ICD in the KP model, accompanied by a significant infiltration of tumor nodules by CD3+ T cells, as well as a rise of CD8+ cytotoxic T lymphocytes (CTL) over regulatory T cells (Tregs). Altogether, the treatment with oxaliplatin reestablished cancer immunosurveillance and hence sensitized KP lung cancers to subsequent immunotherapy with PD-1 and CTLA-4 blockade.Citation29 This finding has been confirmed in additional lung cancer models,Citation24,Citation32 as well as other cancer types,Citation30,Citation34,Citation38 which all exhibited increased tumor infiltrating T cells and synergistic effects of oxaliplatin with PD-1/PD-L1 ICI. Interestingly, Shivani et al. demonstrated the recruitment of CAR-T cells into murine lung cancers treated with oxaliplatin,Citation56 thus sensitizing those treatment-resistant tumors to anti-PD-L1, which makes oxaliplatin an attractive companion treatment for adoptive T cell transfer, particularly when combined with T cell-targeting ICIs. Moreover, in addition to CTLs, other immune cells, such as nature killer (NK) Citation24,Citation57 and dendritic cells (DC) Citation35 can be recruited into tumors that are treated with oxaliplatin, underscoring the immunostimulatory effect of the agent.
Table 1. Preclinical studies combining ICD inducers and PD-1/PD-L1 blockade.
Apart from enriching the tumor infiltrate with immune effectors, oxaliplatin also depletes immunosuppressive cells, including tumor-associated macrophages (TAM) and myeloid-derived suppressive cells (MDSC),Citation38,Citation58 thus favorable remolding the tumor microenvironment. The expression level of PD-L1 in cancer cells is an important prognostic parameter to predict the efficacy of PD-1/PD-L1 blockade. Interestingly, a direct consequence of the induction of ICD by oxaliplatin is the upregulation of PD-L1 expression in many types of cancer and myeloid cells.Citation32,Citation33,Citation38 This provides yet another rationale for the combination of oxaliplatin with PD-1/PD-L1 ICIs. Of note, oxaliplatin-mediated synergistic effects with ICIs may also be linked to its capacity to induce systemic antitumor immune responses, which occur in both preclinical mouse models Citation59 and high-risk rectal cancer patients.Citation60 Taken together, these studies underline the notion that ICD induction with oxaliplatin alters the tumor immune microenvironment, converting ‘cold’ into ‘hot’ tumors, while in parallel affecting systemic immune regulation, eventually resulting in the sensitization to subsequent ICI immunotherapies ().
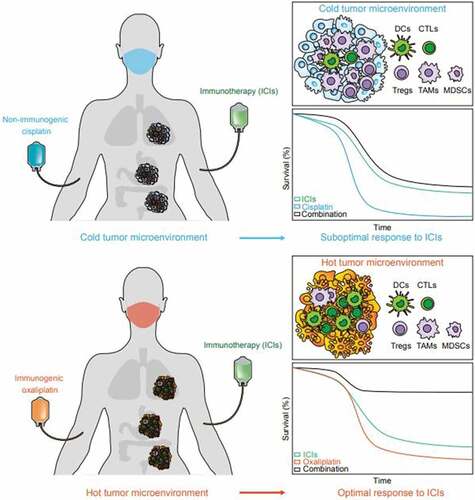
Figure 1. Synergistic effect of immunogenic chemotherapies and immune checkpoint inhibitors. Cisplatin (CDDP) is a non-immunogenic cell death (ICD)-inducing chemotherapeutic that fails to prime adaptive immunity in tumors, forming a “cold” immune microenvironment that consists more immune suppressive cells like tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDCSs), and regulatory T cells (Tregs), but less antigen presenting cells such as dendritic cells (DCs) or effector cells such as cytotoxic T lymphocytes (CTLs). Thus, CDDP cannot synergize with PD-1 targeting immune checkpoint inhibitors (ICIs). Oxaliplatin (OXA) induces ICD and establishes a primed “hot” tumor immune microenvironment that favors the infiltration and accumulation of DCs and CTLs over immunosuppressive cells, thus sensitizing to the immunotherapeutic effects of PD-1 targeting antibodies.
Several clinical trials recently confirmed that the pretreatment with ICD-inducing oxaliplatin sensitizes to immunotherapy with ICIs targeting the PD-1/PD-L1 pathway and yields an improved control of advanced gastric carcinomas, known for their particularly poor prognosis. A Phase I b trial in patients with advanced gastric or esophagogastric junction cancer confirmed the tolerability and efficacy of oxaliplatin-based chemotherapy in combination with PD-1 blocking antibodies employed as a first-line treatment.Citation61 Moreover, systematic reviews of gene expression profiles in patient biopsies revealed the importance of immune infiltration as an indicator for patient prognosis and a predictive factor for immunotherapy in gastric cancers.Citation62,Citation63 Consistent with the aforementioned preclinical studies, the positive effects of oxaliplatin-based immunogenic (neoadjuvant) chemotherapy on the immune microenvironment has been confirmed by the meta-analysis of in silico data, as well as by multiplex immunostaining and next-generation sequencing (NGS) of gastric cancer biopsies,Citation64 altogether showing an elevated level of CTLs in the tumor immune infiltrate of patients treated with oxaliplatin, correlating with improved objective response rates. More direct proof for the synergistic effect of oxaliplatin and ICI therapy comes from the recent clinical trial CheckMate 649 Citation65 targeting unresectable gastric and gastro-esophageal junction carcinomas. This trial apparently validates the observation that oxaliplatin exerts beneficial synergistic effects with PD-1 blockade, while in another comparable study (Keynote 062, Citation66) the non-ICD inducing platinum agent cisplatin failed to do so. As shown in , the studied arms in each trial were well balanced for all prognostic factors, which were comparable among these two trials. In the CheckMate 649 trial, the combination of oxaliplatin-based chemotherapy with PD-1 blockade led to a significant improvement of overall survival (OS) and progression-free survival (PFS) as compared to chemotherapy alone. In contrast, the improvement of survival was much less profound when PD-1 blockade was added to cisplatin-based chemotherapy in the KEYNOTE–062 study. Specifically, the median OS of patients (PD-L1 CPS ≥ 1) in the chemotherapy alone group was equivalent in the KEYNOTE-062 and CheckMate 649 studies (11.1 and 11.3 months, respectively). However, substantial benefits from additional combination of PD-1 blockade was exclusively found in the CheckMate 649 study in which oxaliplatin (median OS: 11.3 vs 14.0 months without vs with Nivolumab, HR = 0.77, P < 0.0001) was used rather than cisplatin as in KEYNOTE-062 (median OS: 11.1 vs 12.5 months without vs with Pembrolizumab, HR = 0.85, P = 0.05). Notably, the objective response rate (ORR) of patients in both arms was numerically higher in the CheckMate 649 study compared with that in the KEYNOTE-062, indicating that oxaliplatin is associated with greater response in gastric cancer either with or without PD-1 blocker. These results suggest that oxaliplatin-based chemotherapy is more likely to synergize with PD-1 antibody in the treatment of HER-2 negative gastric cancers, leading to greater survival benefits as compared to cisplatin.
Table 2. Results from the Keynote-062 study and the Checkmate 649 study.
Intriguingly, another trial, Keynote-811, evaluated the benefit of combining pembrolizumab (PD-1 blocking monoclonal antibody) with chemotherapy (investigators’ choice) plus trastuzumab (HER2 blocking monoclonal antibody) in the treatment of HER-2 positive gastric or GEJ adenocarcinoma.Citation67 Researchers found that adding PD-1 blockade to trastuzumab and chemotherapy led to a significant 22.7% improvement (from 51.9% to 74.4% without vs with PD-1 blockade, P = 0.00006) in ORR. It should be noted that the majority of patients (>86%) in this study received oxaliplatin-based chemotherapy as compared to 100% of cisplatin in the KEYNOTE-062 trial. More importantly, the ORR improvement was more profound in patients receiving oxaliplatin (24.3%) than in those treated with cisplatin (11.8%), again supporting the superiority of oxaliplatin over cisplatin.
Other ongoing clinical trials that combine oxaliplatin or cisplatin with PD-1/PD-L1 blockade are summarized in . It is increasingly acknowledged that platinum drugs differ in their capacity to induce ICD and only those that trigger ICD are able to synergize with ICI. Oxaliplatin is used as the backbone of chemotherapy for gastric cancer in the majority of ongoing-phase II/III trials. Nonetheless, a substantial number of trials still include cisplatin in their chemotherapy regimen as equivalent or substitute for oxaliplatin in a few phase II studies. Similarly, there are no specifications on which type of platinum agent to combine with PD-1 blockade for first-line treatment of gastric cancer in the FDA approval, which vaguely refers to ‘platinum-based chemotherapy’ without distinguishing between cisplatin and oxaliplatin.
Table 3. List of ongoing clinical trials that evaluate the combination of PD-1/PD-L1 blockade with either cisplatin or oxaliplatin in gastric cancer.
In sum, it is still not fully appreciated by regulatory instances including FDA and EMA that different platinum drugs have distinct immunogenic properties and that it is crucial to use ICD inducing agents such as oxaliplatin when the purpose of combinational treatment is to trigger anti-tumor immune response. At this point, we strongly recommend that future clinical studies, as well as ongoing trials that are still in the stage of recruiting patients, should consider the optimization of treatment regimes in which immunogenic chemotherapy should be used during one or few cycles at relatively low doses (to avoid the adverse effects that have been observed during high-dose monotreatment schedules) as a preconditioning of the tumor immune microenvironment for subsequent curative ICI.
Acknowledgments
OK receives funding by the DIM ELICIT initiative of the Ile de France and Institut National du Cancer (INCa); GK are supported by the Ligue contre le Cancer (équipes labellisées, Program “Equipe labelisée LIGUE”; no. EL2016.LNCC (VT/PLP)); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; INCa; Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). JC is supported by the Association pour la recherche sur le cancer (ARC) grant.
Disclosure statement
GK and OK are cofounders of Samsara Therapeutics. GK is a cofounder of Therafast Bio. AH participates on data safety monitoring or consulting and advisory boards for Amgen, BMS, Basilea, Incyte, Servier, QED Therapeutics, Tahio, and Relay Therapeutics.
Data availability statement
All data sources that have been referenced.
Additional information
Funding
References
- Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2021;21:37–9. doi:10.1038/s41568-020-00308-y.
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025.
- Cocetta V, Ragazzi E, Montopoli M. Links between cancer metabolism and cisplatin resistance. Int Rev Cell Mol Biol. 2020;354:107–164. doi:10.1016/bs.ircmb.2020.01.005.
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi:10.1038/nrc2167.
- Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–148. doi:10.1093/annonc/mdu472.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi:10.1056/NEJMoa073149.
- Chinen T, Sasabuchi Y, Matsui H, Yamaguchi H, Yasunaga H. Oxaliplatin- versus cisplatin-based regimens for elderly individuals with advanced gastric cancer: a retrospective cohort study. BMC Cancer. 2022;22(1):460. doi:10.1186/s12885-022-09581-6.
- Huang J, Zhao Y, Xu Y, Zhu Y, Huang J, Liu Y, Zhao L, Li Z, Liu H, Wang Q-L, et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: a meta-analysis of randomized controlled trials. Oncotarget. 2016;7(23):34824–34831. doi:10.18632/oncotarget.9189.
- Zhang F, Zhang Y, Jia Z, Wu H, Gu K. Oxaliplatin-based regimen is superior to cisplatin-based regimen in tumour remission as first-line chemotherapy for advanced gastric cancer: a meta-analysis. J Cancer. 2019;10(8):1923–1929. doi:10.7150/jca.28896.
- Montagnani F, Turrisi G, Marinozzi C, Aliberti C, Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi:10.1007/s10120-011-0007-7.
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi:10.1038/onc.2009.356.
- Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9:1703449. doi:10.1080/2162402X.2019.1703449.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi:10.4161/onci.1.2.19026.
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:e000337. doi:10.1136/jitc-2019-000337.
- Kepp O, Bezu, L., Yamazaki, T., Di Virgilio, F., Smyth, M. J., Kroemer, G., & Galluzzi, L. ATP and cancer immunosurveillance. EMBO J. 2021;40:e108130. doi:10.15252/embj.2021108130.
- Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500. doi:10.1038/s41590-022-01132-2.
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. doi:10.1146/annurev-immunol-032712-100008.
- Kroemer G, Kepp O. Radiochemotherapy-induced elevations of plasma HMGB1 levels predict therapeutic responses in cancer patients. Oncoimmunology. 2021;10(1):2005859. doi:10.1080/2162402X.2021.2005859.
- Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24(4):311–318. doi:10.1016/j.cytogfr.2013.05.001.
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi:10.1038/nm.3708.
- Sprooten J, Garg AD. Type I interferons and endoplasmic reticulum stress in health and disease. Int Rev Cell Mol Biol. 2020;350:63–118. doi:10.1016/bs.ircmb.2019.10.004.
- Yamazaki T, Kirchmair A, Sato A, Buqué A, Rybstein M, Petroni G, Bloy N, Finotello F, Stafford L, Navarro Manzano E, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21(10):1160–1171. doi:10.1038/s41590-020-0751-0.
- Workenhe ST, Pol J, Kroemer G. Tumor-intrinsic determinants of immunogenic cell death modalities. Oncoimmunology. 2021;10(1):1893466. doi:10.1080/2162402X.2021.1893466.
- Xin M, Lin D, Yan N, Li H, Li J, Huang Z. Oxaliplatin facilitates tumor-infiltration of T cells and natural-killer cells for enhanced tumor immunotherapy in lung cancer model. Anticancer Drugs. 2022;33(2):117–123. doi:10.1097/CAD.0000000000001248.
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. doi:10.1038/s41467-020-17670-y.
- Husstegge M, Hoang NA, Rebstock J, Monecke A, Gockel I, Weimann A, Schumacher G, Bechmann I, Lordick F, Kallendrusch S, et al. PD-1 inhibition in patient derived tissue cultures of human gastric and gastroesophageal adenocarcinoma. Oncoimmunology. 2021;10(1):1960729. doi:10.1080/2162402X.2021.1960729.
- Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212–224. doi:10.1038/s41591-021-01233-9.
- Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. doi:10.1038/s41571-020-0413-z.
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi:10.1016/j.immuni.2015.11.024.
- Zhu H, Shan, Y., Ge, K., Lu, J., Kong, W., & Jia, C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr). 2020;43(6):1203–1214. doi:10.1007/s13402-020-00552-2.
- Levesque S, Le Naour, J., Pietrocola, F., Paillet, J., Kremer, M., Castoldi, F., & Pol, J. G. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology. 2019;8(11):e1657375. doi:10.1080/2162402X.2019.1657375.
- Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. journal of Receptors and Signal transduction. 2019;39(3):208–214. doi:10.1080/10799893.2019.1655050.
- Song W, Shen L, Wang Y, Liu Q, Goodwin TJ, Li J, Dorosheva O, Liu T, Liu R, Huang L, et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat Commun. 2018;9(1):2237. doi:10.1038/s41467-018-04605-x.
- Wang W, Wu L, Zhang J, Wu H, Han E, Guo Q. Chemoimmunotherapy by combining oxaliplatin with immune checkpoint blockades reduced tumor burden in colorectal cancer animal model. Biochem Biophys Res Commun. 2017;487(1):1–7. doi:10.1016/j.bbrc.2016.12.180.
- Maharjan R, Choi JU, Kweon S, Pangeni R, Lee NK, Park SJ, Chang K-Y, Park JW, Byun Y. A novel oral metronomic chemotherapy provokes tumor specific immunity resulting in colon cancer eradication in combination with anti-PD-1 therapy. Biomaterials. 2022;281:121334. doi:10.1016/j.biomaterials.2021.121334.
- Limagne E, Thibaudin M, Nuttin L, Spill A, Derangère V, Fumet J-D, Amellal N, Peranzoni E, Cattan V, Ghiringhelli F, et al. Trifluridine/Tipiracil plus Oxaliplatin Improves PD-1 Blockade in Colorectal Cancer by Inducing Immunogenic Cell Death and Depleting Macrophages. Cancer Immunol Res. 2019;7(12):1958–1969. doi:10.1158/2326-6066.CIR-19-0228.
- Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, Serre L, Jordheim LP, Matera EL, Dumontet C, et al. The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front Immunol. 2018;9:2100. doi:10.3389/fimmu.2018.02100.
- Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, et al. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology. 2021;160(3):781–796. doi:10.1053/j.gastro.2020.10.036.
- Yamazaki T, Buque A, Ames TD, Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology. 2020;9(1):1721810. doi:10.1080/2162402X.2020.1721810.
- Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, Engblom C, Rickelt S, Yamazaki T, Iribarren K, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun. 2019;10(1):1486. doi:10.1038/s41467-019-09415-3.
- Petrazzuolo A, Perez-Lanzon M, Liu P, Maiuri MC, Kroemer G. Crizotinib and ceritinib trigger immunogenic cell death via on-target effects. Oncoimmunology. 2021;10(1):1973197. doi:10.1080/2162402X.2021.1973197.
- Petrazzuolo A, Perez-Lanzon M, Martins I, Liu P, Kepp O, Minard-Colin V, Maiuri MC, Kroemer G. Pharmacological inhibitors of anaplastic lymphoma kinase (ALK) induce immunogenic cell death through on-target effects. Cell Death Dis. 2021;12(8):713. doi:10.1038/s41419-021-03997-x.
- Hossain DMS, Javaid S, Cai M, Zhang C, Sawant A, Hinton M, Sathe M, Grein J, Blumenschein W, Pinheiro EM, et al. Dinaciclib induces immunogenic cell death and enhances anti-PD1-mediated tumor suppression. J Clin Invest. 2018;128(2):644–654. doi:10.1172/JCI94586.
- Xie W, Forveille S, Iribarren K, Sauvat A, Senovilla L, Wang Y, Humeau J, Perez-Lanzon M, Zhou H, Martínez-Leal JF, et al. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology. 2019;8(11):e1656502. doi:10.1080/2162402X.2019.1656502.
- Kepp O, Zitvogel L, Kroemer G. Lurbinectedin: an FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology. 2020;9(1):1795995. doi:10.1080/2162402X.2020.1795995.
- Orecchioni S, Talarico G, Labanca V, Calleri A, Mancuso P, Bertolini F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer. 2018;118(10):1329–1336. doi:10.1038/s41416-018-0076-z.
- Xie W, Mondragón L, Mauseth B, Wang Y, Pol J, Lévesque S, Zhou H, Yamazaki T, Eksteen JJ, Zitvogel, et al. Tumor lysis with LTX-401 creates anticancer immunity. Oncoimmunology. 2019;8(7):1594555. doi:10.1080/2162402X.2019.1594555.
- Bezu L, Wu Chuang A, Sauvat A, Humeau J, Xie W, Cerrato G, Liu P, Zhao L, Zhang S, Le Naour J, et al. Local anesthetics elicit immune-dependent anticancer effects. J Immunother Cancer. 2022;10(4):e004151. doi:10.1136/jitc-2021-004151.
- Chen G, Chen Z, Wen D, Wang Z, Li H, Zeng Y, Dotti G, Wirz RE, Gu Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc Natl Acad Sci U S A. 2020;117(7):3687–3692. doi:10.1073/pnas.1917891117.
- Lin A, Gorbanev Y, De Backer J, Van Loenhout J, Van Boxem W, Lemière F, Cos P, Dewilde S, Smits E, Bogaerts A, et al. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv Sci (Weinh). 2019;6(6):1802062. doi:10.1002/advs.201802062.
- Herter-Sprie GS, Koyama S, Korideck H, Hai J, Deng J, Li YY, Buczkowski KA, Grant AK, Ullas S, Rhee K, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight. 2016;1(9):e87415. doi:10.1172/jci.insight.87415.
- Zhang H, Xie W, Zhang Y, Dong X, Liu C, Yi J, Zhang S, Wen C, Zheng L, Wang H, et al. Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model. Cancer Gene Ther. 2022;29(5):456–465. doi:10.1038/s41417-021-00389-3.
- Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5):e11622. doi:10.15252/emmm.201911622.
- Kroemer G, Kepp O. Small cell lung cancer responds to immunogenic chemotherapy followed by PD-1 blockade. Oncoimmunology. 2021;10(1996686). doi:10.1080/2162402X.2021.1996686.
- Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr). 2020;43(6):1203–1214. doi:10.1007/s13402-020-00552-2.
- Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee SM, Amezquita RA, et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell. 2021;39(2):193–208 e110. doi:10.1016/j.ccell.2020.11.005.
- Siew YY, Soek-Ying Neo, Hui-Chuing Yew, Shun-Wei Lim, Yi-Cheng Ng,Si-Min Lew, Wei-Guang Seetoh, See-Voon Seow, and Hwee-Ling Koh. Oxaliplatin regulates expression of stress ligands in ovarian cancer cells and modulates their susceptibility to natural killer cell-mediated cytotoxicity. Int Immunol. 2015;27(12):621–632. doi:10.1093/intimm/dxv041.
- Gou HF, Zhou L, Huang J, Chen XC. Intraperitoneal oxaliplatin administration inhibits the tumor immunosuppressive microenvironment in an abdominal implantation model of colon cancer. Mol Med Rep. 2018;18(2):2335–2341. doi:10.3892/mmr.2018.9219.
- Stojanovska V, Prakash M, McQuade R, Fraser S, Apostolopoulos V, Sakkal S, Nurgali K. Oxaliplatin treatment alters systemic immune responses. Biomed Res Int. 2019;2019:4650695. doi:10.1155/2019/4650695.
- Kalanxhi E, Meltzer S, Schou JV, Larsen FO, Dueland S, Flatmark K, Jensen BV, Hole KH, Seierstad T, Redalen KR, et al. Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br J Cancer. 2018;118(10):1322–1328. doi:10.1038/s41416-018-0085-y.
- Xu J, Xu N, Bai Y, Liu R, Mao C, Sui H, Wang X, Jiang Q, Dou Y. Anti-PD-1 antibody HX008 combined with oxaliplatin plus capecitabine for advanced gastric or esophagogastric junction cancer: a multicenter, single-arm, open-label, phase Ib trial. Oncoimmunology. 2020;10(1):1864908. doi:10.1080/2162402X.2020.1864908.
- Zhang AZ, Yuan X, Liang WH, Zhang HJ, Li Y, Xie YF, Li JF, Jiang CH, Li FP, Shen XH, et al. Immune infiltration in gastric cancer microenvironment and its clinical significance. Front Cell Dev Biol. 2021;9:762029. doi:10.3389/fcell.2021.762029.
- Li C, Pan J, Jiang Y, Yu Y, Jin Z, Chen X. Characteristics of the immune cell infiltration landscape in gastric cancer to assistant immunotherapy. Front Genet. 2021;12:793628. doi:10.3389/fgene.2021.793628.
- Xing X, Shi J, Jia Y, Dou Y, Li Z, Dong B, Guo T, Cheng X, Li X, Du H, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in gastric cancer as determined by multiplex immunofluorescence and T cell receptor repertoire analysis. J Immunother Cancer. 2022;10(3):e003984. doi:10.1136/jitc-2021-003984.
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2.
- Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, Kudaba I, Garrido M, Chung HC, Lee J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi:10.1001/jamaoncol.2020.3370.
- Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–730. doi:10.1038/s41586-021-04161-3.
