Abstract
Necrosis is a type of cell death often caused by cell injury and is linked to human diseases including neuron degeneration, stroke, and cancer. Cells undergoing necrosis are engulfed and degraded by engulfing cells, their predators. The mechanisms by which necrotic cells are recognized and removed remain elusive. Here we comment on our recent findings that reveal new molecular mechanisms of necrotic-cell recognition. Through studying the C. elegans touch neurons undergoing excitotoxic necrosis, we identified a receptor/ligand pair that enables engulfing cells to recognize necrotic neurons. The phagocytic receptor CED-1 is activated through interaction with its ligand phosphatidylserine (PS), exposed on the surface of necrotic cells. Furthermore, against the common belief that necrotic cells have ruptured plasma membrane, we found that necrotic C. elegans touch neurons actively present PS on their outer surfaces while maintaining plasma membrane integrity. We further identified 2 mechanisms governing the presentation of PS, one of which is shared with cells undergoing apoptosis, a “cell suicide” event, whereas the other is unique to necrotic neurons. The influx of Ca2+, a key necrosis-triggering factor, is implicated in activating a neuronal PS-scramblase for PS exposure. We propose that the mechanisms controlling PS-exposure and necrotic-cell recognition by engulfing cells are likely conserved from worms to humans.
Introduction
Necrosis and apoptosis are 2 morphologically distinct types of cell death events. Whereas apoptotic cells shrink in size and condense in chromatin, necrotic cells swell to many folds of their original sizes.Citation1,2 Necrosis is most frequently observed during cell injury, and is closely associated with diseases such as stroke, neurodegeneration, chronic inflammation, and cancer.Citation3–7 Recent discoveries made in multiple organisms demonstrated that cells possess genetic pathways that specifically trigger necrosis in response to extracellular or intracellular stimuli.Citation8–11 Unlike apoptosis, known necrosis-triggering pathways appear to be independent of the activities of caspases, which are cysteine proteases.Citation10,12 Despite the different mechanisms of death, both necrotic and apoptotic cells are engulfed by phagocytes and degraded inside phagosomes.Citation13,14 Efficient clearance of necrotic cells from animal bodies helps to resolve the wounded area, and is further essential for reducing harmful inflammatory and auto-immune responses induced by the contents of necrotic cells.Citation14,15
Pioneering researchers have established the nematode Caenorhabditis elegans as an effective model system for investigating the mechanisms of apoptosis and necrosis.Citation10,16,17 In C. elegans, a number of mutations in certain ion channel subunits belonging to the DEG/ENaC superfamily and referred to as degenerins, in the acetylcholine receptor, in trimeric GTPases, and in a few other proteins induce specific neurons to undergo necrotic cell death that mimics the excitotoxic necrosis, which occurs during stroke, trauma, and neurodegenerative disorders in humans.Citation10 In particular, dominant (dm) mutations in mec-4, which encodes a core subunit of a multimeric, mechanically gated sodium channel specifically expressed in the touch neurons, trigger the necrosis of 6 mechanosensory (touch) neurons required for sensing gentle mechanical stimuli along the body wall.Citation18–20 In mec-4(dm) mutants, these dying neurons swell to many times their original sizes and are easily distinguishable from living or apoptotic cells under Differential Interference Contrast (DIC) optics by their giant sizes ().Citation13,18 Unlike apoptosis, mec-4(dm)-induced cell death does not require CED-3 caspase activity.Citation21 Instead, the MEC-4(dm) mutations result in hyperactive channel conductivity of Na+ and Ca2+ and induce touch neurons to undergo necrosis.Citation19,22
Figure 1. Phosphatidylserine (PS) is actively presented on the surface of necrotic neurons and attracts engulfing cells (A-C) DIC and epi-fluorescence images of ced-1(e1735); mec-4(e1611) mutant embryo (A) and L1 larvae (B and C). Arrows mark apoptotic cells. Arrowheads mark necrotic touch neurons PLML and PLMR. Scale bars indicate 5 μm (A) and 10 μm (B and C), respectively. (D) Diagram depicting our proposed mechanisms of PS exposure out of a necrotic neuron. See text for detailed explanation of the models. The cylinders represent various types of ion channels, which are made constitutively open by dominant mutations in certain subunits. A dominant mutation of the Gα subunit of the trimeric G-protein also induces neuronal necrosis. Heat and other necrosis inducing stress are also indicated. Ca2+ influx is a prominent trigger that activates the PS-exposure mechanisms through inducing Ca2+ release from the ER and probably also through other unknown mediators. In addition, there might be other intracellular signals (short, solid arrows) triggered by the necrotic stress. The thickness of the long solid lines bearing solid arrows represents the relative contribution of each of CED-7 and ANOH-1 to the overall PS exposure activity. The solid line bearing an open arrow indicates activation of ANOH-1 by Ca2+. The dashed line bearing open arrows indicate Ca2+ might activate CED-7. Although CED-8 participates in necrotic cell-removal, whether it acts to facilitate the PS-exposure is unknown (represented by the dashed straight line bearing a solid arrow). All three proteins should function on the plasma membrane.
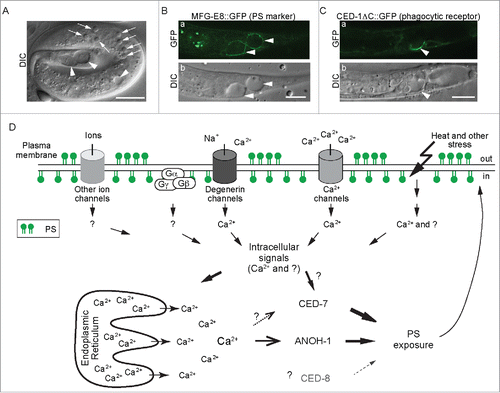
Despite dying through different mechanisms, the engulfment of both necrotic and apoptotic cells by their neighboring cells requires the functions of the same 7 ced (cell death defective) genes,Citation23 indicating the presence of certain common dying cell-removal mechanisms. On the other hand, the distinct cellular features observed during macrophage engulfment of necrotic mammalian cellsCitation24 imply that unique pathways exist to clear necrotic and apoptotic cells.
Phosphatidylserine (PS), a membrane phospholipid, is known as an “eat me” signal presented on the surface of apoptotic cells and is recognized by phagocytic receptors such as C. elegans CED-1, Drosophila Draper, and mammalian Tim4 and BAI1, leading to the initiation of their engulfment.Citation25–29 In living cells, PS is almost exclusively localized to the inner leaflet of the plasma membrane, at least partially due to an ATP-dependent aminophospholipid translocase activity that selectively returns PS from the outer to the inner leaflet.Citation30–32 During the early stage of apoptosis, PS is detected on the outer leaflet, suggesting a process of trans-bilayer redistribution.Citation30,31 Phospholipid scramblases, by catalyzing the random, bi-directional “flip-flop” of phospholipids across the membrane bilayer, could potentially counter the aminophospholipd translocase activity.Citation33 Indeed, recently, it was found that the mouse Xk-related protein 8, a phospholipid scamblase, and CED-8, its C. elegans homolog, mediate PS exposure during apoptosis.Citation34,35 Both Xk8 and CED-8 were found to be activated by caspase cleavage.Citation34,35 On the other hand, the mouse transmembrane protein 16F (TMEM16F), which was found to act as a novel Ca2+-activated phospholipid scramblase,Citation36 does not seem to be involved in PS exposure on apoptotic cell surfaces.Citation37 These results suggest that different phospholipid scramblases might function in different cell types and respond to different stimuli. In addition to the scramblases, the mammalian ATP-binding-cassette transporter A1 (ABCA1) has been implicated in the translocation of PS from the inner to the outer leaflet,Citation38,39 although evidence to the contrary also exists.Citation40
Previously, using a GFP-tagged, secreted PS reporter (MFG-E8::GFP), we have detected the presentation of PS specifically on the surface of apoptotic cells during C. elegans development.Citation41 We have further identified 2 alternative mechanisms that promote PS exposure in apoptotic somatic and germ cells, respectively.Citation41 The PS exposure on apoptotic cell surface during embryonic development, which is necessary for their engulfment, relies on the function of C. elegans CED-7, a homolog of mammalian ABCA1 transporters.Citation41 Recently, we investigated how necrotic touch neurons in C. elegans are recognized and engulfed.Citation42 Specifically, we addressed the following questions:
Do necrotic and apoptotic cells share common “eat me” signal molecules and are they recognized by the same phagocytic receptors?
Do necrotic cells actively externalize the “eat me” signal molecules to their surface? And, if the answer is yes, is (are) the PS-externalization mechanism(s) similar to that discovered in apoptotic cells?
Do different PS-exposure mechanisms work together to facilitate the removal of dying cells?
Are the PS-exposure mechanisms general or specific responding to different death triggering signals and in different cell types?
In this commentary, we will discuss our findings in the context of current understanding of necrotic cell clearance. We propose that Calcium-dependent PS exposure is a necrosis-specific mechanism employed by multiple types of cells. Moreover, multiple molecular mechanisms cooperate to regulate PS exposure in response to necrosis signals.
PS is an “eat me”signal exposed on the surface of necrotic touch neurons and recognized by the phagocytic receptor CED-1
Using the secreted MFG-E8::GFP reporter, a high-affinity PS-binding protein that specifically detects PS on the outer surface of cells, we detected strong PS signal on the surface of necrotic but not live touch neurons in the mec-4(dm) mutant background ().Citation42 Using a live-cell imaging protocol that we established for touch neurons, we monitored the accumulation of the PS signal on surface of necrotic touch neurons. We found that the exposure of PS was an early event detectable approximately 30–90 min after the initiation of cell morphology change was visible under the DIC optics.Citation42 The fact that both necrotic and apoptotic cells expose PS on their surfaces implies that PS might serve as a common “eat me” signal to attract engulfing cells to cells that die of different mechanisms. Supporting this theme, we discovered the novel function of phagocytic receptor CED-1 in recognizing necrotic cells in addition to apoptotic cells ().Citation42 The extracellular domain of CED-1 (CED-1Ex) is capable of attaching to the surface of necrotic cells in vivo, suggesting an extracellular ligand-receptor interaction. Furthermore, we have detected direct and selective in vitro interaction between CED-1Ex and acidic phopsholipids, including PS. Most importantly, as shown by Chung et al.Citation23 and confirmed by us,Citation42 loss-of-function mutations of ced-1 result in severe defect in the removal of necrotic touch neurons as well as apoptotic cells. As a result, these kinds of dying cells are retained in the body. Together, the above results strongly indicate that CED-1 acts as a phagocytic receptor that recognizes both apoptotic and necrotic cells.
CED-1 belongs to a family of homologous transmembrane proteins that include Drosophila Draper, mouse Jedi and mEGF10, and human mEGF10 and 11.Citation43–45 Draper, like CED-1, directly associates with PS exposed to the surface of apoptotic cells.Citation29 Draper acts as a phagocytic receptor for both apoptotic cells and for injured or pruned neuronal processes.Citation46 Likewise, mouse Jedi and mEGF10 as well as human mEGF10 also participate in the recognition of apoptotic cells.Citation47,44,48 CED-1 and its homologs thus play conserved roles as phagocytic receptors in different organisms. Our discovery of CED-1 as a phagocytic receptor for necrotic cells implies that the homologs of CED-1 might also recognize necrotic cells.
As an “eat me” signal, PS is also known to attract phagocytic receptors via an indirect mechanism. Secreted bridging molecules such as mouse MFG-E8 bring dying and engulfing cells together by interacting simultaneously with both PS and phagocytic receptors.Citation49 C. elegans TTR-52, a transthyretin-like secreted protein, was proposed to act as a bridging molecule that links PS on apoptotic cells to CED-1 on engulfing cell surfaces.Citation50 We propose that the direct and indirect interactions between PS and CED-1 provide 2 distinct and probably complementing molecular mechanisms to promote the effective recognition of dying cells by CED-1.
PS is actively presented on the surface of necrotic touch neurons through 2 distinct mechanisms
Necrotic touch neurons do not seem to rupture
According to a long-existing notion, injured cells undergo necrosis and lose plasma membrane integrity.Citation51–53 Thus comes the general assumption that the PS detected on necrotic cell surfaces is a result of the rupture of necrotic cell membranes.Citation53 In the recent years, accumulating evidence has demonstrated that in addition to cell injury, necrosis is induced by genetic programs.Citation8 In short, multiple molecular mechanisms exist that induce and execute necrosis;Citation8,12 thus all necrotic cells do not necessarily lose plasma membrane integrity. In addition, the observed loss of membrane integrity of necrotic cells in culture, where they are not attacked by phagocytes, does not necessarily represent what happens inside animal bodies, where engulfing cells target dying cells at early stages of their death.Citation13,42 Previously, those necrosis events that occur inside animal bodies were rarely examined for plasma membrane integrity. We monitored the subcellular localization of particular GFP or mRFP reporters expressed either inside necrotic cells or outside necrotic cells as secreted proteins, as well as propidium iodide, a small molecule dye that is not plasma membrane permeable, within a 36-hr observation period starting 3 hrs after necrosis was initiated. We did not observe any internalization of the secreted GFP molecules expressed from cells neighboring the necrotic touch neurons or of propidium iodide.Citation42 We also failed to observe any externalization of necrotic cell-expressed GFP or mRFP signals.Citation42 These results indicate that the touch neurons undergoing excitotoxic necrosis maintain plasma membrane integrity during a long period of time - throughout embryonic and larval development. Our observation is consistent with electron microscopic studies of rat brains and C. elegans touch neurons, which reported the swelling of necrotic cells and the presence of surrounding phagocytes, yet no loss of plasma membrane integrity.Citation54,13 These results indicate that the common notion that necrotic cells lose plasma membrane integrity is not necessarily true for all kinds of necrotic cells, in particular in the developmental context, in which the dying cells are swiftly engulfed. They further imply that in order for PS to be present on the surface of necrotic touch neurons, an active PS exposure mechanism(s) must exist. Our genetic studies revealed at least 2 separate PS-exposure activities that promote PS exposure on the surface of necrotic cells.
A common PS-exposure mechanism employed by both apoptotic and necrotic cells
To identify the molecular mechanism(s) that drives PS exposure, we first tested the function of C. elegans CED-7, a member of the ABC transporter family.Citation55 CED-7 regulates PS exposure on the surface of apoptotic cells.Citation41 Mouse ABCA1 was also reported to participate in PS redistribution during apoptotic cell clearance.Citation38,56 We found that inactivation of ced-7 greatly reduces the frequency as well as intensity of the PS signal detected on necrotic cell surface.Citation42 Furthermore, engineered tissue-specific expression of ced-7 in touch neurons but not in engulfing cells rescues this defect, indicating that CED-7 functions cell-autonomously to promote PS exposure.Citation42 This discovery, together with the previous one revealing a key role of CED-7 in promoting PS exposure on the surface of apoptotic cells,Citation41 further demonstrate that the presentation of the “eat me” signal relies on conserved mechanism(s) during different types of cell death. We further discovered that CED-7 has 2 distinct functions, one in necrotic and the other in engulfing cells, both of which contribute to the efficient removal of necrotic touch neurons.Citation42
How CED-7 acts in necrotic cells to promote PS exposure needs to be further elucidated. CED-7 is ubiquitously expressed.Citation55 There thus must be dying cell-specific mechanisms that activate CED-7. Whether the CED-7 activation mechanisms are common or distinct in necrotic and apoptotic cells remains unknown. Moreover, the engulfing cell-specific function of CED-7 is a mystery and requires further investigation. Previous research suggests that engulfing cells might also externalize PS and that ABC transporters might be involved in this event.Citation38,57 The function of this event awaits clarification.
A distinct, touch neuron-specific, PS-exposure mechanism
In ced-7 null mutants, PS was still detectable, albeit at lower levels, on the surface of necrotic touch neurons, indicating the presence of an additional PS exposure-promoting activity.Citation42 We found that inactivating anoh-1 (anoctamin homolog 1), which encoded the C. elegans homolog of mammalian TMEM16F, greatly reduced the PS signal on necrotic cell surface.Citation42 We further found that ANOH-1 functioned in necrotic touch neurons to promote PS exposure.Citation42 Consistently, ANOH-1 is primarily expressed in neurons, including touch neurons.Citation42,58
The vertebrates TMEM16 family of proteins, also known as anoctamins, are divided into 2 subfamilies based on 2 distinct Ca2+-dependent biochemical activities: Cl− channels and phospholipid scramblases.Citation37,59 Remarkably, TMEM16F possesses both biochemical activities.Citation36,60–64 Mammalian TMEM16F promotes cellular PS exposure in response to Ca2+ ionophoreCitation60,62–64 yet not to apoptotic stimuli.Citation37 Similarly, we failed to observe any defect in the removal of apoptotic cells in C. elegans embryos and adult gonads in anoh-1 null mutants,.Citation42 This observation suggests either that anoh-1 is not involved in promoting PS exposure on the surface of apoptotic cells, or that anoh-1 is involved but only makes a minor contribution. In the latter case, in anoh-1 mutants, PS level on the surface of apoptotic cells might be reduced yet is still sufficiently high to promote engulfment.
On the other hand, the Ca2+-activated phospholipid scramblase activity of TMEM16F provides an important clue toward revealing a necrosis-specific PS-exposure mechanism. As an evolutionarily conserved feature, Ca2+ influx is known to be an effective trigger of the excitotoxic death of neurons in metazoan organisms including C. elegans and mammals.Citation65–67 Particularly in C. elegans touch neurons, the dominant mutation in MEC-4 leads to Ca2+ influx, resulting in the excitotoxic necrosis.Citation19,22 We propose that during Ca2+-activated necrosis of touch neurons, Ca2+ directly activates the PS-exposure activity of ANOH-1. Xu et al discovered that necrotic cell death under a number of excitotoxic conditions requires the release of Ca2+ from the endoplasmic reticulum (ER).Citation65 ER is a known calcium storage pool in cells.Citation68,69 Moreover, the influx of Ca2+ from the extracellular environment is known to further induce the release of Ca2+ from the lumen of the ER to the cytoplasm.Citation70,71 We propose that, like mec-4(dm)-induced necrosis, the mec-4(dm)-induced PS exposure on touch neuron surface also requires the Ca2+-release from the ER, an event that further increases the concentration of Ca2+ in the cytoplasm and facilitates the activation of ANOH-1 (). Further development based on our findings will establish a novel Ca2+-dependent mechanism leading to the exposure of the “eat me” signal molecules and the recognition of necrotic touch neurons.
The Calcium-dependent PS exposure might be a necrosis-specific mechanism employed by multiple types of neurons and even non-neuronal cells
The Ca2+-dependent PS-exposure mechanism we discovered in touch neurons in the mec-4(dm) mutant background might apply to the necrosis induced by genetic alterations of other degenerin family channel proteins,Citation72 or of other types of channel proteins (). For instance, a dominant mutation in DEG-3, an acetylcholine-gated Ca2+ channel that displays high permeability to Ca2+, results in the necrosis of touch cells.Citation20,73 The constitutive Ca2+ influx in the deg-3 mutant background could activate ANOH-1 for PS exposure. In support of this hypothesis, we detected the exposure of PS on the surface of necrotic touch neurons in deg-3 mutants.Citation42
In addition to touch neurons, other types of neurons are also induced to undergo excitotoxtic necrosis by hyper-influx of Ca2+. For example, certain interneurons and sensory neurons are induced to undergo necrosis by the same dominant mutations in deg-3.Citation20,73 Dominant mutation in deg-1 and unc-8, both of which encode degenerin ion channels, induce the necrosis of interneuronsCitation74 and motor neurons,Citation75 respectively. Furthermore, hyperactive channels rely on trimeric G proteins to signal downstream. Expression of an active Gα subunit (gsa-1(QL)) results in the necrosis of motor neurons.Citation76 The necrosis induced by the hyperactive mutant Gα protein requires the release of Ca2+ from ER,Citation65 implying increased Ca2+ concentration in the cytoplasm. Consistently, besides touch neurons, the expression of ANOH-1 is also detected in many other neurons. Combining these lines of evidence together, we speculate that multiple types of neurons rely on ANOH-1, which is activated by Ca2+, to promote PS exposure during necrosis ().
Besides excitotoxic signal generated by hyperactive channels or downstream signaling molecules, necrosis of neurons is also inducible by other kinds of stress, such as heat stroke, hypoxia, and hypo-osmotic shock.Citation77 In C. elegans, heat treatment induces widespread necrosis in many cells, including neurons.Citation78 As heat treatment was detected to increase cytoplasmic Ca2+ concentration, and as moderating Ca2+ release from intracellular organelles protects heat-induced necrosis, Kourtis et al. proposed that heat stroke might induce necrosis through increasing cytoplasmic Ca2+ concentration.Citation78 Heat-induced necrotic cells might thus also utilize a Ca2+-activated phospholipid scramblase such as ANOH-1 to expose PS on their surface (). Moreover, the study of heat-induced necrosis also suggests that the Ca2+-induced PS exposure mechanism might not be limited to neurons.Citation78 Although ANOH-1 expression is primarily observed in neurons, non-neuronal cells might utilize other putative phospholipid scramblasesCitation41 that response to necrotic signals.
Multiple molecular mechanisms cooperate to regulate PS exposure in response to necrosis signals
We discovered that the anoh-1(-) ced-7(-) double mutants display more severe defects in PS-exposure and necrotic cell-removal than each single mutant alone.Citation42 Since each mutant allele analyzed is a null allele, according to the principle of epistasis grouping analysis, a further enhanced phenotype of the double mutants over the stronger single mutant phenotype indicates that the 2 genes being tested act in separate pathways. ANOH-1 and CED-7 are likely to both contribute to the overall PS-exposure activity. We propose that they act in 2 independent and partially redundant pathways (). Our discoveries have further established that cells die of different mechanisms employ both common (ABC transporter-based) and unique (TMEM16F-like phospholipid scramblase-based) molecular activities to present a common “eat me” signal. Assuming that a certain level of PS is needed to efficiently attract phagocytic receptor molecules, since a necrotic C. elegans touch neuron possesses a surface area many times of that of an apoptotic cell, the robust and timely exposure of PS on the large surface area might rely on the cooperation of multiple molecular activities such as those represented by CED-7 and ANOH-1. Currently, it is unknown whether CED-7 or mouse ABCA1 is activated by Ca2+. The possibility exists that in necrotic neurons, Ca2+ influx might enhance the activity of CED-7. We further found that CED-8, a homolog of the mammalian phospholipid scramblase Xk8,Citation34,35 also made a modest contribution to the removal of necrotic cells: ced-8 loss-of-function mutation results in a transient delay of necrotic touch neuron removal in the L1 but not later larval stages.Citation42 Comparing to the phenotypes displayed by ced-7 or anoh-1 mutants, this phenotype is much weaker and transient. We also found that ced-8 and anoh-1 acted in 2 independent pathways to promote necrotic cell removal.Citation42 Currently, it is unknown whether CED-8 facilitates PS-exposure to the surface of necrotic cells; moreover, the functional relationship between ced-7 and ced-8 in the context of necrotic cell removal remains to be elucidated. CED-8 might represent a third pathway that is in parallel to both the CED-7 and the ANOH-1 pathways ().
Ca2+ homeostasis is closely associated with neuron degeneration conditions in both C. elegans and mammals.Citation69,77 Our findings have a broader implication in the physiological role of the clearance of many kinds of degenerative neurons resulted from pathological conditions or aging.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by NIGMS grant GM104279.
References
- Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 2007; 32:37–43; PMID:17141506; http://dx.doi.org/10.1016/j.tibs.2006.11.001
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16:3–11; PMID:18846107; http://dx.doi.org/10.1038/cdd.2008.150
- Jacobson MD, Bergeron L. Cell death in the nervous system, in Apoptosis, the molecular biology of programmed cell death. MD Jacobson, N. McCarthy, Editors. 2002, Oxford University Press; 278–301.
- Yamashima T. Ca2+-dependent proteases in ischemic neuronal death: a conserved 'calpain-cathepsin cascade' from nematodes to primates. Cell Calcium 2004; 36:285–93; PMID:15261484; http://dx.doi.org/10.1016/j.ceca.2004.03.001
- Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol Ther 2009; 8:1791–7; PMID:19770591; http://dx.doi.org/10.4161/cbt.8.19.9762
- Challa S, Chan FK. Going up in flames: necrotic cell injury and inflammatory diseases. Cell Mol Life Sci 2010; 67:3241–53; PMID:20532807; http://dx.doi.org/10.1007/s00018-010-0413-8
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 2010; 72:19–44; PMID: 20148665; http://dx.doi.org/10.1146/annurev.physiol.010908.163111
- McCall K. Genetic control of necrosis - another type of programmed cell death. Curr Opin Cell Biol 2010; 22:882–8; PMID:20889324; http://dx.doi.org/10.1016/j.ceb.2010.09.002
- Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 2010; 35:434–41; PMID:20346680; http://dx.doi.org/10.1016/j.tibs.2010.03.001
- Vlachos M, Tavernarakis N. Non-apoptotic cell death in Caenorhabditis elegans. Dev Dyn 2010; 239:1337–51; PMID:20108319; http://dx.doi.org/10.1002/dvdy.22230
- Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol 2014; 35:14–23; PMID:25087983
- Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 2014; 35:24–32; PMID:24582829
- Hall DH, Gu G, Garcia-Anoveros J, Gong L, Chalfie M, Driscoll M. Neuropathology of degenerative cell death in Caenorhabditis elegans. J Neurosci 1997; 17:1033–45; PMID:8994058
- Krysko DV, D'Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 2006; 11:1709–26; PMID:16951923; http://dx.doi.org/10.1007/s10495-006-9527-8
- Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ 2010; 17:381–97; PMID:20019744; http://dx.doi.org/10.1038/cdd.2009.195
- Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet 1998; 14:410–416; PMID:9820030; http://dx.doi.org/10.1016/S0168-9525(98)01573-X
- Driscoll M, Gerstbrein B. Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat Rev Genet 2003; 4:181–94; PMID:12610523; http://dx.doi.org/10.1038/nrg1018
- Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 1981; 82:358–70; PMID:7227647; http://dx.doi.org/10.1016/0012-1606(81)90459-0
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 1991; 349:588–93; PMID:1672038; http://dx.doi.org/10.1038/349588a0
- Treinin M, Chalfie M. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron 1995; 14:871–7; PMID:7718248; http://dx.doi.org/10.1016/0896-6273(95)90231-7
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell 1986; 44:817–29; PMID:3955651; http://dx.doi.org/10.1016/0092-8674(86)90004-8
- Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 2004; 7:1337–44; PMID:15543143; http://dx.doi.org/10.1038/nn1347
- Chung S, Gumienny TL, Hengartner MO, Driscoll M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol 2000; 2:931–7; PMID:11146658; http://dx.doi.org/10.1038/35046585
- Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D'Herde K, Vandenabeele P. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ 2006; 13:2011–22; PMID:16628234; http://dx.doi.org/10.1038/sj.cdd.4401900
- Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 2001b; 104:43–56; PMID:11163239; http://dx.doi.org/10.1016/S0092-8674(01)00190-8
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007; 450(7168):435–9; PMID:17960135
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007; 450(7168):430–4; PMID:17960134
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med 2010; 207:1807–17; PMID:20805564; http://dx.doi.org/10.1084/jem.20101157
- Tung TT, Nagaosa K, Fujita Y, Kita A, Mori H, Okada R, Nonaka S, Nakanishi Y. Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper. J Biochem 2013; 153:483–91; PMID:23420848; http://dx.doi.org/10.1093/jb/mvt014
- Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol 2003; 65:701–34; PMID:12471163; http://dx.doi.org/10.1146/annurev.physiol.65.092101.142459
- Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res 2005; 44:207–34; PMID:15979148; http://dx.doi.org/10.1016/j.plipres.2005.05.001
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014; 344:1164–8; PMID:24904167; http://dx.doi.org/10.1126/science.1252809
- Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost 2001; 86:266–75; PMID:11487015
- Chen YZ, Mapes J, Lee ES, Skeen-Gaar RR, Xue D. Caspase-mediated activation of Caenorhabditis elegans CED-8 promotes apoptosis and phosphatidylserine externalization. Nat Commun 2013; 4:2726; PMID:24225442
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013; 341:403–6; PMID:23845944; http://dx.doi.org/10.1126/science.1236758
- Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010; 468:834–8; PMID:21107324; http://dx.doi.org/10.1038/nature09583
- Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem 2013; 288:13305–16; PMID:23532839; http://dx.doi.org/10.1074/jbc.M113.457937
- Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol 2000; 2:399–406; PMID:10878804; http://dx.doi.org/10.1038/35017029
- Alder-Baerens N, Muller P, Pohl A, Korte T, Hamon Y, Chimini G, Pomorski T, Herrmann A. Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J Biol Chem 2005; 280:26321–9; PMID:15905177; http://dx.doi.org/10.1074/jbc.M413993200
- Williamson P, Halleck MS, Malowitz J, Ng S, Fan X, Krahling S, Remaley AT, Schlegel RA. Transbilayer phospholipid movements in ABCA1-deficient cells. PLoS One 2007; 2:e729; PMID:17710129; http://dx.doi.org/10.1371/journal.pone.0000729
- Venegas V, Zhou Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell 2007; 18:3180–92; PMID:17567952; http://dx.doi.org/10.1091/mbc.E07-02-0138
- Li Z, Venegas V, Nagaoka Y, Morino E, Raghavan P, Audhya A, Nakanishi Y, Zhou Z. Necrotic cells actively attract phagocytes through the collaborative action of two distinct PS-Exposure mechanisms. PLoS Genet 2015; 11:e1005285; PMID:26061275; http://dx.doi.org/10.1371/journal.pgen.1005285
- Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun 2003; 300:619–23; PMID:12507493; http://dx.doi.org/10.1016/S0006-291X(02)02904-2
- Wu HH, Bellmunt E, Scheib JL, Venegas V, Burkert C, Reichardt LF, Zhou Z, Farinas I, Carter BD. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat Neurosci 2009; 12:1534–41; PMID:19915564; http://dx.doi.org/10.1038/nn.2446
- Kay JN, Chu MW, Sanes JR. MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature 2012; 483:465–9; PMID:22407321; http://dx.doi.org/10.1038/nature10877
- Fullard JF, Kale A, Baker NE. Clearance of apoptotic corpses. Apoptosis 2009; 14:1029–37; PMID:19291407; http://dx.doi.org/10.1007/s10495-009-0335-9
- Hamon Y, Trompier D, Ma Z, Venegas V, Pophillat M, Mignotte V, Zhou Z, Chimini G. Cooperation between engulfment receptors: the case of ABCA1 and MEGF10. PLoS One 2006; 1:e120; PMID:17205124; http://dx.doi.org/10.1371/journal.pone.0000120
- Scheib JL, Sullivan CS, Carter BD. Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk. J Neurosci 2012; 32:13022–31; PMID:22993420; http://dx.doi.org/10.1523/JNEUROSCI.6350-11.2012
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417:182–7; PMID:12000961; http://dx.doi.org/10.1038/417182a
- Wang X, Li W, Zhao D, Liu B, Shi Y, Chen B, Yang H, Guo P, Geng X, Shang Z, et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol 2010; 12:655–64; PMID:20526330; http://dx.doi.org/10.1038/ncb2068
- Denecker G, Vercammen D, Steemans M, Vanden Berghe T, Brouckaert G, Van Loo G, Zhivotovsky B, Fiers W, Grooten J, Declercq W, et al. Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell Death Differ 2001; 8:829–40; PMID:11526436; http://dx.doi.org/10.1038/sj.cdd.4400883
- Krysko DV, Brouckaert G, Kalai M, Vandenabeele P, D'Herde K. Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J Morphol 2003; 258:336–45; PMID:14584035; http://dx.doi.org/10.1002/jmor.10161
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev 2006; 20:1–15; PMID:16391229; http://dx.doi.org/10.1101/gad.1376506
- Hajos F, Garthwaite G, Garthwaite J. Reversible and irreversible neuronal damage caused by excitatory amino acid analogues in rat cerebellar slices. Neuroscience 1986; 18:417–36; PMID:3526173; http://dx.doi.org/10.1016/0306-4522(86)90163-6
- Wu Y, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 1998a; 93:951–960; PMID:9635425; http://dx.doi.org/10.1016/S0092-8674(00)81201-5
- Rigot V, Hamon Y, Chambenoit O, Alibert M, Duverger N, Chimini G. Distinct sites on ABCA1 control distinct steps required for cellular release of phospholipids. J Lipid Res 2002; 43:2077–86; PMID:12454269; http://dx.doi.org/10.1194/jlr.M200279-JLR200
- Mapes J, Chen YZ, Kim A, Mitani S, Kang BH, Xue D. CED-1, CED-7, and TTR-52 regulate surface phosphatidylserine expression on apoptotic and phagocytic cells. Curr Biol 2012; 22: 1267–75; PMID:22727702; http://dx.doi.org/10.1016/j.cub.2012.05.052
- Wang Y, Alam T, Hill-Harfe K, Lopez AJ, Leung CK, Iribarne D, Bruggeman B, Miyamoto MM, Harfe BD, Choe KP. Phylogenetic, expression, and functional analyses of anoctamin homologs in Caenorhabditis legans. Am J Physiol Regul Integr Comp Physiol 2013; 305: R1376–89; PMID:24049119; http://dx.doi.org/10.1152/ajpregu.00303.2012
- Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 2014; 94: 419–59; PMID:24692353; http://dx.doi.org/10.1152/physrev.00039.2011
- Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A 2011; 108: 19246–51; PMID:22084121; http://dx.doi.org/10.1073/pnas.1114799108
- Yang H, Kim A, David T, Palmer D, Jin T, Tien J, Huang F, Cheng T, Coughlin SR, Jan YN, et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 2012; 151: 111–22; PMID:23021219; http://dx.doi.org/10.1016/j.cell.2012.07.036
- Malvezzi M, Chalat M, Janjusevic R, Picollo A, Terashima H, Menon AK, Accardi A. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun 2013; 4:2367; PMID:23996062; http://dx.doi.org/10.1038/ncomms3367
- Scudieri P, Caci E, Venturini A, Sondo E, Pianigiani G, Marchetti C, Ravazzolo R, Pagani F, Galietta LJ. Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J Physiol 2015; 593:3829–48; PMID:26108457; http://dx.doi.org/10.1113/JP270691
- Yu K, Whitlock JM, Lee K, Ortlund EA, Cui YY, Hartzell HC. Identification of a lipid scrambling domain in ANO6/TMEM16F. Elife 2015; 4:e06901; PMID:26057829
- Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 2001; 31:957–71; PMID:11580896; http://dx.doi.org/10.1016/S0896-6273(01)00432-9
- Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life 2008; 60:575–90; PMID:18478527; http://dx.doi.org/10.1002/iub.91
- Mano I, Driscoll M. Caenorhabditis elegans glutamate transporter deletion induces AMPA-receptor/adenylyl cyclase 9-dependent excitotoxicity. J Neurochem 2009; 108:1373–84; PMID:19054279; http://dx.doi.org/10.1111/j.1471-4159.2008.05804.x
- Paschen W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium 2001; 29:1–11; PMID:11133351; http://dx.doi.org/10.1054/ceca.2000.0162
- Mattson MP. Calcium and neurodegeneration. Aging Cell 2007; 6:337–50; PMID:17328689; http://dx.doi.org/10.1111/j.1474-9726.2007.00275.x
- Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 2000; 23:222–9; PMID:10782128; http://dx.doi.org/10.1016/S0166-2236(00)01548-4
- Verkhratsky A. Endoplasmic reticulum calcium signaling in nerve cells. Biol Res 2004; 37:693–9; PMID:15709699; http://dx.doi.org/10.4067/S0716-97602004000400027
- Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 1994; 367:467–70; PMID:7509039; http://dx.doi.org/10.1038/367467a0
- Treinin M, Gillo B, Liebman L, Chalfie M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci U S A 1998; 95:15492–5; PMID:9860996; http://dx.doi.org/10.1073/pnas.95.26.15492
- Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 1990; 345:410–6; PMID:2342572; http://dx.doi.org/10.1038/345410a0
- Tavernarakis N, Shreffler W, Wang S, Driscoll M. unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron 1997; 18:107–19; PMID:9010209; http://dx.doi.org/10.1016/S0896-6273(01)80050-7
- Korswagen HC, Park JH, Ohshima Y, Plasterk RH. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev 1997; 11:1493–503; PMID:9203577; http://dx.doi.org/10.1101/gad.11.12.1493
- Nikoletopoulou V, Tavernarakis N. Necrotic cell death in Caenorhabditis elegans. Methods Enzymol 2014; 545:127–55; PMID:25065889; http://dx.doi.org/10.1016/B978-0-12-801430-1.00006-8
- Kourtis N, Nikoletopoulou V, Tavernarakis N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 2012; 490:213–8; PMID:22972192; http://dx.doi.org/10.1038/nature11417
