ABSTRACT
Cytosolic calcium is an important factor during fertilization, development and differentiation. Hence, the control of cytosolic calcium levels has been studied extensively for several decades. Numerous calcium channels have been identified and their mechanism of action elucidated. However, the mode of calcium channel regulation remains elusive. Here we discuss our recent findings regarding the role of syndecans in the regulation of cytosolic calcium levels. Syndecans are transmembrane proteoglycans present in both vertebrates and invertebrates that interact with extracellular ligands resulting in the activation of several downstream signaling pathways. We identified a previously unappreciated role of syndecans in cytosolic calcium regulation in mammals that is conserved in C. elegans. We concluded that calcium regulation is the basic, evolutionarily conserved role for syndecans, which enables them to be integral for multiple cellular functions.
Redefining the role of syndecans in C. elegans biology
Ever since the first proteoglycans were identified, multiple functions have been ascribed to these molecules including major roles in cell adhesion, cell migration, immune responses, signaling and development.Citation1,2,3 However, there is a lack of understanding as to the common underlying mechanism through which proteoglycans function in multicellular organisms. Proteoglycans carry differentially sulfated sugar chains called glycosaminoglycans (GAGs) through which they interact with a large group of ligands, such as extracellular matrix proteins, growth factors and cytokines.Citation4,5 One group of transmembrane proteoglycans expressed in all members of Bilateria is the syndecans. The vertebrate syndecan family is comprised of four syndecans, named syndecan-1–4. Syndecan-4 is ubiquitously expressed, whereas syndecans 1–3 have more tissue-specific expression patterns.Citation6 Syndecans are comprised of a cytoplasmic domain, a transmembrane domain and an extracellular domain, which is susceptible to shedding through the action of metalloproteinases.Citation7 Syndecans-1 and -3 can possess both heparan sulfate and chondroitin sulfate sugar chains on their extracellular domain, whereas syndecan-2 and -4 are predominantly substituted with heparan sulfate chains.Citation5 All syndecans form homodimers, if not multimers, creating a cloud of negative charge on the cell surface enabling them to interact with a large group of positively charged ligands.Citation8 Vertebrate syndecans were initially identified as co-receptors that gather ligands to work in collaboration with other receptors.Citation9,10 However, their independent role in signaling was later identified.Citation11
In contrast to the four syndecans encoded in the genomes of vertebrates, C. elegans encodes a single syndecan called SDN-1. A number of publications have reported that syndecans are involved in the development of the nervous system in C. elegans. For instance, Rhiner et al.Citation12 showed that SDN-1 functions autonomously to control neural migration and axon guidance. In wild type worms, the HSN left (L)/right (R) motor neurons migrate during embryonic development from the posterior (tail region) to the mid-body region. In sdn-1 mutant worms, the HSNs have 2 marked defects where they either fail to exit the tail or stop prematurely before reaching their normal position near the vulva. Similarly, it was shown that the PVQL and PVQR axons in sdn-1 mutant animals exhibit axon guidance defects where axons inappropriately cross the ventral midline boundary. It is also known that the heparan sulfate chain modifications on SDN-1 along with heparan sulfate chains on other proteoglycans are involved in the regulation of these processes.Citation12 Our recently published work used a number of model systems, including C. elegans, to show that the underlying theme through which syndecans control cell behavior is through the regulation of calcium.Citation13
Figure 1. Schematic of HSN and PVQ neurons in sdn-1 and trp mutants. In wild type worms, the HSN neurons migrate from the posterior to mid-body during embryogenesis. Axon extension occurs during larval development where the HSN neurons extend axons ventrally and around vulva before entering the ventral nerve cord. A hypodermal ridge separates HSNL and HSNR neurons. In sdn-1 (zh20) mutants, the HSN cell bodies exhibit defective migration and their axons are misguided. However, abolishing trp-1 and trp-2 in sdn-1 mutant animals partially restores HSN development to wild type. Similarly, the PVQ neurons extend anteriorly directed axons from the tail. The axons are separated by the hypodermal ridge and extend to the nerve ring with minimal crossover events. PVQ axons are misguided in sdn-1 mutant animals where they exhibit frequent crossovers to the contralateral side of the ventral nerve cord. These defects could be ameliorated by removal of trp-1 and trp-2.
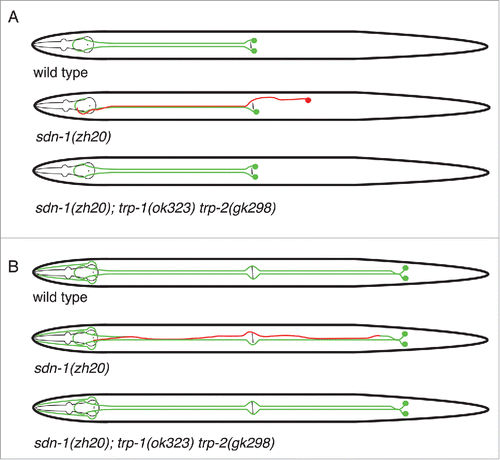
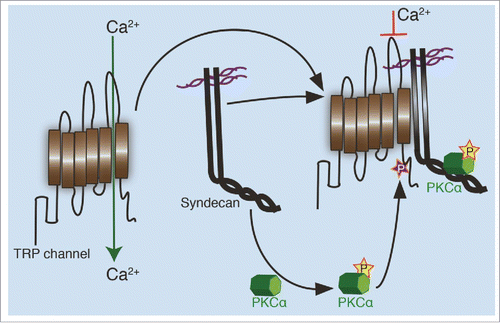
Figure 2. Regulation of TRP channels by syndecans. In an unphosphorylated state, TRP channels allow calcium transport through the plasma membrane. When aligned in close proximity with a syndecan, possibly through complex formation between the syndecan and TRP channel, an active PKCα created during the interaction, phosphorylates the channel on a conserved serine residue. This phosphorylation closes the channel formed between 5th and 6th transmembrane domain, limiting the cellular calcium influx.
Calcium in neurons
Calcium is important throughout the lifecycle of an organism - from fertilization, through development to survival. This is not surprising, as calcium influences intrinsic genetic programs, biochemical pathways, protein half-life and extracellular signals.Citation14,15,16,17 The temporary hike in calcium called transient calcium is involved in neuronal development and signaling, where it controls proliferation, differentiation migration, apoptosis, receptor release and receptor-ligand interactions.Citation18,19,20,21 Neurons show 2 distinct types of calcium transient called calcium spikes and calcium waves. Calcium spikes are rapid and widespread throughout the cell, whereas calcium waves occur in patterns and are restricted to specific compartments of the cell. Rapid calcium signals in neurons are associated with neuronal activity but also rapidly return to baseline after the completion of each activity. One such activity that makes use of calcium transients is the release of neurotransmitters.Citation22 Neurotransmitters promote signaling between neurons and between neurons and muscle at the neuromuscular junction. This process is initiated by packaging neurotransmitters into synaptic vesicles and fusion of vesicles with the presynaptic region before releasing their content into the synaptic cleft. With transient influx through voltage-gated channels,Citation23 calcium enters into the axonal terminus and promotes fusion of the vesicles with presynaptic region. Calcium can influence not only the fusion but also the release of the contents of the synaptic vesicles, which may require a higher transient calcium concentration. In addition to voltage-gated channels, other channel types are present in the pre- and post-synaptic region, not to mention the channels that coordinate calcium release from intracellular calcium stores.Citation24,25,26 In certain cases, such as in spinal neurons during early development, it has been shown that calcium spikes are caused by release of calcium from the intracellular stores. In addition, abolishing calcium spikes by depleting the intracellular stores results in a decrease in the number of GABAergic neurons.Citation27
Calcium is an important signaling molecule not only during neurotransmission but also in neuronal development and differentiation.Citation19,28 This includes the generation of specific subtypes of neurons that enable an organism to process information and perform specific behaviors. Calcium plays an important role in the regulation of growth and guidance of axons and dendrites during development. It controls such events by activating enzymes, regulating cytoskeletal dynamics and controlling transcription factor activation/repression.Citation29,30,31 In contrast to ‘experience-driven’ calcium signaling during synaptic activity,Citation32 calcium transients during development are considered to be more spontaneous.Citation33 The transient calcium during development appears to occur as calcium waves or calcium spikes where their spatiotemporal features may be important to provide functional specificity to neurons. For instance, blocking of calcium spikes using chemical inhibitors significantly reduces the number of GABAergic neurons,Citation34 whose formation is a result of calcium-mediated neurotransmitter specification. A number of publications have covered the role of calcium during neuronal development, which is beyond the scope of this commentary. Importantly however, a number of neurodegenerative diseases, such as Amyotrophic lateral sclerosis, Huntington's disease, Cerebellar ataxia, Alzheimer's disease, Parkinson's disease and Familial hemiplegic migraine, (see Citation35 for details) are associated with dysregulated calcium signaling, cementing the importance of the study of calcium in the nervous system. Even with a plethora of studies published about the regulation of calcium transients in neurons, the entire mechanism of neuronal transient calcium regulation is still poorly understood. Here, we add a crucial piece to the puzzle, where a mechanism for regulating the cytosolic calcium in neurons and other cell types by syndecans was described for the first time.
A new role for syndecan
As previously discussed, syndecans are known to interact with a large group of ligands and have many roles in diverse tissues. Our previous work with mouse fibroblasts revealed that there is no significant change in gene or protein expression between wild type and syndecan-4 knockout cells. However, the localization of several proteins including NFACTc4 is defective in the absence of syndecan-4. NFATc4 is a calcium response protein,Citation36 the expression of which shifts between the nucleus and cytoplasm in response to cytosolic calcium changes. The observed change in nuclear expression of NFATc4 in syndecan-4 knockout fibroblasts compared to wild type cells indicated to us that syndecan-4 knockout cells exhibit defective calcium metabolism. Using a troponin C based calcium indicator twitch-1,Citation37 we confirmed that cytosolic calcium levels in syndecan-4 knockout cells are elevated. This led to the possibility of deregulated calcium channels in the knockout cells. To our surprise, none of the conventional channels such as L and T-type channels were involved in syndecan-mediated calcium regulation, leading us to focus on stretch-activated channels. Stretch-activated channels are present on the cell surface and they induce transient calcium changes in cells. They belong to the family of transient receptor potential (TRP) channels. In contrast to the 28 channels in the mammalian genome, C. elegans harbors 17 TRP channels. With a set of pharmacological and knockdown experiments in mammalian cells, we were able to establish that the channels regulated by syndecans belong to the canonical subgroup of TRP channels. Three genes, trp-1, trp-2 and trp-3 encode the canonical family of TRP channels in C. elegans. trp-3 is only expressed in sperm whereas trp-1 and trp-2 are widely expressed.
As our research focused on nervous system, we studied the potential syndecan-mediated regulation of TRP-1 and TRP-2 in neuronal development. A significant observation came from a set of calcium measurements in ventral motor neurons using a calcium cameleon sensor.Citation38 sdn-1(zh20) mutant animals showed an elevated level of calcium in the ventral motor neurons. Follow-up experiments showed that removing trp-1 and trp-2 in the sdn-1 null background could restore the calcium to wild type levels in ventral motor neurons of sdn-1 mutant animals. Further, we asked whether the neuronal defects observed in sdn-1 mutant animals are caused by defective calcium regulation. First, we confirmed the earlier findings that sdn-1(zh20) mutant animals show defective neuronal migration and axon guidance along with defective locomotory behavior. We subsequently found that removal of trp-1 and trp-2 partially suppressed the neurodevelopmental and locomotion defects of sdn-1 mutant animals. Taken together, we hypothesize that the developmental and behavioral defects observed in sdn-1 mutant animals are the result of disturbed calcium kinetics in C. elegans. Based on observations from multiple mammalian models and the C. elegans data, we concluded that the absence of syndecans resulted in uncontrolled opening of the TRP channels. This was validated in epithelial cells, activated keratinocytes and in vivo mouse models showing the widespread utilization of such a mechanism.
Our work suggests that syndecans form complexes with TRP channels to regulate the influx of calcium into cells. TRP channels are comprised of cytoplasmic domains, an extracellular domain and 6 transmembrane domains, where the 5th and 6th transmembrane domains act as calcium entry pore. A conserved serine residue immediately after the 6th transmembrane domain is known to be phosphorylated by protein kinases,Citation39 leading to the downregulation of calcium release. We speculate that PKCα activation through an interaction with syndecan that is downstream of ligand-driven signaling, leads to phosphorylation of a conserved serine residue on TRP channels resulting in the downregulation of channel function. The complex formation between syndecan and the TRP channel allow the two proteins to remain in close proximity, giving rise to a local transient calcium change. To test the role of PKCα on calcium regulation in wild type fibroblasts, we inhibited PKCα using pharmacological agents. As we expected, the level of calcium elevated despite the presence of at least two syndecans, syndecan-2 and -4. This confirmed our findings that active PKCα is required for TRP channel regulation and that syndecans control calcium kinetics through PKCα.
Conclusions
To conclude, we have discovered a novel feature of syndecans that may lead to further insights into the basic role of syndecans not only in C. elegans, but also in mammals. We were able to relate multiple roles played by syndecans to cell adhesion, cell migration and development as a result of their role in cytosolic calcium regulation. Since calcium is a second messenger that functions in several important signaling pathways, it is possible the newly found syndecan-mediated calcium regulation can result in global effects in animals. In addition, downstream signaling molecules not only in syndecan-initiated mechanisms but also in other pathways could be affected by the cytosolic calcium change. The evolutionarily conserved nature of the syndecan-TRP channel axis suggests the existence of an ancient function of syndecans in calcium regulation.
Since the time of their identification, syndecans have been implicated as co-receptors in signaling with other molecules such as integrins. However, the novel role of syndecans in calcium regulation provides a new perspective, thus amending their previous status as independent receptors on the cell surface. Moreover, intracellular signals that control key functions in all types of neurons depend on calcium ion influx. With the introduction of a new regulatory mechanism, another layer of complexity has been added to the system. Both syndecans and TRP channels respond to extracellular ligands and external mechanical forces. This means that intracellular calcium can be regulated externally when the extracellular matrix is taken into consideration. Therefore the known role of the extracellular matrix in neuronal development and neuronal activity can also be a result of cytosolic calcium changes in the neurons. Now the most important question that needs to be answered concerns the role of syndecan-TRP channel axis in microdomains such as dendrites of neurons. Unlike other cells, neurons have different action potentials at different parts of the cell. There can be significant differences in calcium in the cell body and dendrites where calcium transients regulate key functions. If the syndecan-TRP axis exists in microdomains, it can control the activity of the neurons in addition to their development. Moreover, the role of calcium or expression of syndecans might not be restricted to the nervous system of C. elegans as both syndecan and TRP channels are expressed in multiple tissue types. In addition, there is a possibility that other proteoglycans such as glypicans may have similar roles by regulating other families of TRP channels. Therefore, we speculate these findings will have a significant impact in C. elegans biology and beyond.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This paper was funded by the Danish National Research Foundation, the Danish Council for Natural Sciences, the Novo Nordisk Foundation, the Lundbeck Foundation, and a grant from the European Research Council (ERC) grant no. 260807.
References
- Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 1999; 68:729-77; http://dx.doi.org/10.1146/annurev.biochem.68.1.729
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 2000; 404:725-28; PMID:10783877; http://dx.doi.org/10.1038/35008000
- Selleck SB. Proteoglycans and pattern formation. Sugar biochemistry meets developmental genetics. Trends Genet 2000; 16:206-12; PMID:10782114; http://dx.doi.org/10.1016/S0168-9525(00)01997-1
- Park PW, Reizes O & Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand–receptor encounters. J Biol Chem 2000; 275:29923-26; PMID:10931855; http://dx.doi.org/10.1074/jbc.R000008200
- Couchman JR. Transmembrane Signaling Proteoglycans. Ann Rev Cell Dev Biol 2010; 26: 89-114; http://dx.doi.org/10.1146/annurev-cellbio-100109-104126
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell 1994; 5:797-805; PMID:7812048; http://dx.doi.org/10.1091/mbc.5.7.797
- Ryu HY, Lee J, Yang S, Park H, Choi S, Jung KC, Lee ST, Seong JK, Han IO, Oh ES. Syndecan-2 functions as a docking receptor for promatrix metalloproteinase-7 in human colon cancer cells. J Biol Chem 2009; 284:35692-701; PMID:19858218; http://dx.doi.org/10.1074/jbc.M109.054254
- Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A, Couchman JR. Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J Biol Chem 2010; 285:14247-58; PMID:20154082; http://dx.doi.org/10.1074/jbc.M109.056945
- David G, Van der Schueren B, Marynen P, Cassiman JJ, Van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol 1992; 118:961-69.
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 1992; 8:365-93; http://dx.doi.org/10.1146/annurev.cb.08.110192.002053
- Oh ES, Woods A, Couchman JR. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J Biol Chem. 1997; 272: 8133-36; PMID:9079625; http://dx.doi.org/10.1074/jbc.272.13.8133
- Rhiner C, Gysi S, Fröhli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development 2005;132: 4621-33; PMID:16176946; http://dx.doi.org/10.1242/dev.02042
- Gopal S, Søgaard P, Multhaupt HA, Pataki C, Okina E, Xian X, Pedersen ME, Stevens T, Griesbeck O, Park PW, et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J Cell Biol 2015; 210:1199-211; PMID:26391658; http://dx.doi.org/10.1083/jcb.201501060
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol 2010; 61:593-620; PMID:20192754; http://dx.doi.org/10.1146/annurev-arplant-070109-104628
- Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 2003; 4:530-38; PMID:12838336; http://dx.doi.org/10.1038/nrm1154
- Lewis SE, Anderson P, Goldspink DF. The effects of calcium on protein turnover in skeletal muscles of the rat. Biochem J 1982; 204: 257-64; PMID:6288015; http://dx.doi.org/10.1042/bj2040257
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 2003;17:2205-32; PMID:12975316; http://dx.doi.org/10.1101/gad.1102703
- Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog Brain Res 2009;175:443-52; PMID:19660672; http://dx.doi.org/10.1016/S0079-6123(09)17529-5
- Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harb Perspect Biol 2011; 3: a004259; PMID:21730044
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4:552-65; PMID:12838338; http://dx.doi.org/10.1038/nrm1150
- Berridge MJ. Neuronal calcium signaling. Neuron 1998; 21:13-26; PMID:9697848; http://dx.doi.org/10.1016/S0896-6273(00)80510-3
- Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 1998; 18:8214-27; PMID:9763467
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerveterminals. Science 2002; 295:2276-79; PMID:11910115; http://dx.doi.org/10.1126/science.1068278
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006; 52:485-96; PMID:17088214; http://dx.doi.org/10.1016/j.neuron.2006.09.033
- Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci 2003; 23:11229-34; PMID:14657182
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol 2005;15:275-81; PMID:15919193; http://dx.doi.org/10.1016/j.conb.2005.05.003
- Holliday J, Adams RJ, Sejnowski TJ, Spitzer NC. Calcium-induced release of calcium regulates differentiation of cultured spinal neurons. Neuron 1991; 7:787-96; PMID:1742025; http://dx.doi.org/10.1016/0896-6273(91)90281-4
- Carey MB, Matsumoto SG. Spontaneous calcium transients are required for neuronal differentiation of murine neural crest. Dev Biol 1999; 215:298-313; PMID:10545239; http://dx.doi.org/10.1006/dbio.1999.9433
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 1993; 260:181-86; PMID:8097060; http://dx.doi.org/10.1126/science.8097060
- Qiu Z, Ghosh A. A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 2008; 60:775-87; PMID:19081374; http://dx.doi.org/10.1016/j.neuron.2008.09.040
- Spitzer NC. Activity-dependent neuronal differentiation prior to synapse formation: the functions of calcium transients. J Physiol Paris. 2002; 96:73-80; PMID:11755785; http://dx.doi.org/10.1016/S0928-4257(01)00082-1
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol 2004; 351-63; PMID:16921405
- Tang F, Dent EW, Kalil K. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J Neurosci 2003; 23:927-36; PMID:12574421
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature 1995; 375:784-87; PMID:7596410; http://dx.doi.org/10.1038/375784a0
- Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 2014; 71:2787-814; PMID:24442513; http://dx.doi.org/10.1007/s00018-013-1550-7
- Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 1997; 11:824-34; PMID:9106655; http://dx.doi.org/10.1101/gad.11.7.824
- Thestrup T, Litzlbauer J, Bartholomäus I, Mues M, Russo L, Dana H, Kovalchuk Y, Liang Y, Kalamakis G, Laukat Y, et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat Methods 2014; 11:175-82; PMID:24390440; http://dx.doi.org/10.1038/nmeth.2773
- Smith ES, Martinez-Velazquez L, Ringstad N. A chemoreceptor that detects molecular carbon dioxide. J Biol Chem 2013; 288:37071-81; PMID:24240097; http://dx.doi.org/10.1074/jbc.M113.517367
- Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW Jr. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol Pharmacol 2005; 67:558-63; PMID:15533987; http://dx.doi.org/10.1124/mol.104.007252
