ABSTRACT
While ample information was gathered in identifying guidance cues and their downstream mediators, very little is known about how the information from multiple extracellular cues is intracellularly to generate normal patterning. Netrin and Wnt signaling pathways play key roles in normal development as well as in malignancies. In C. elegans, as in vertebrates, dorso-ventral (D/V) graded distributions of UNC-6/Netrin and antero-posterior (A/P) graded distributions of Wnts provide instructive polarity information to guide cells and axons along their respective gradients. In this commentary, I will discuss recent findings demonstrating that these 2 signaling pathways also function redundantly to regulate polarity orthogonal to the axis of their gradation. Thus, Wnt signaling components contribute to D/V polarity, while Netrin signaling components contribute to A/P polarity and their joint action collaboratively governs migratory transitions from one axis to the other. These findings pave the way to unraveling broader roles of Wnt and Netrin signaling pathways, roles that are masked due to their redundant nature, and provide a conceptually novel view of how antero-posterior and dorso-ventral guidance mechanisms are orchestrated to establish polarity in multiple biological processes.
Migrating cells and axons can travel long distances navigating the antero-posterior (A/P) or dorso-ventral (D/V) axis, or both, by responding to a multitude of environmental cues encountered along their migratory path. These include secreted guidance cues such as Netrin and Slit known to function in guiding cells and axons along the D/V axisCitation1-5; secreted glycoproteins of the Wnt family, signaling through Frizzled and Ryk/derailed receptors to mediate A/P guidanceCitation6-11; and a variety of other cues embedded in the extra cellular matrix.Citation12-14 While considerable advances were made in identifying guidance cues and their downstream mediators, how extracellular information comprised of additive, overlapping, or opposing inputs is integrated within the cell to culminate in a defined output, is yet to be fully elucidated.
The UNC-6/Netrin guidance cue is graded along the D/V axis and functions through its receptors UNC-40/Frazzled/DCC and UNC-5 to mediate attraction or repulsion of migrating cells and growth cones. This highly conserved guidance system is critical for nervous system patterning in both vertebrates and invertebrates.Citation15-17 However, there are indications that the Netrin signaling pathway, or components thereof, could have functions that are not restricted to migration along a single axis. For example, in C. elegans UNC-40/DCC is involved in A/P migrations of Q neuroblastsCitation18,19 and A/P dendrite growth.Citation20 Furthermore, over-expression of UNC-40/DCC in the mechanosensory neurons causes A/P polarity reversals in ALM and PLM axonsCitation21,22 akin to the effects of impairing Wnt signaling in these neurons.Citation9,11 Moreover, unc-5 and unc-40 are both necessary for mediating A/P ALM axon reversals induced by vab-8, a kinesin-like protein required for posterior guidance, suggesting a possible functional overlap between D/V and A/P signaling pathways.Citation21
In a recent study, the role of Netrin signaling in A/P guidance was further tested in additional sets of cells and axons.Citation23 Similar to the mechanosensory neurons, the C. elegans distal tip cells (DTCs) migrate over long distances along both the A/P and D/V axes. The DTCs are born in the ventral mid-body of the animal and migrate in 3 sequential phases alternating between the A/P and D/V axes as they lead the elongation of anterior and posterior mirror image U-shaped hermaphrodite gonad arms. In the first migration phase (phase 1) the 2 DTCs migrate along the A/P axis in opposite directions, one migrating anteriorly while the other migrates posteriorly (). At the onset of the second migration phase (phase 2) the DTCs repolarize and reorient along the D/V axis to initiate the second migration phase, which occurs along the D/V axis from the ventral to the dorsal side. Once the DTCs reach the dorsal muscle bands they repolarize and reorient again along the A/P axis to migrate back toward the mid-body (phase 3). These cells provide an excellent model system to study cell migration along the A/P or D/V axes and the regulatory mechanisms governing transition between the 2 axes, transitions which require repolarization of the cell from one axis to the other. Many of the signaling pathways regulating DTC migration, such as Netrins, Wnts, integrins and matrix metalloproteases, are highly conserved and function to guide cell and growth cone migrations in vertebrates and invertebrates.Citation24 Thus, the information gleaned from studying these cells is relevant to both cell migration and axon guidance in vertebrates and invertebrates. Netrins and Wnts in C. elegans are known for having a graded distribution along the D/V and A/P axes, respectivelyCitation16,25; accordingly, Netrin signaling governs D/V migrations of the DTCs Citation15,26 while Wnts are involved in their A/P migrations.Citation27,28 Interestingly, simultaneous abrogation of Wnt and Netrin signaling components uncovered a redundant role for UNC-6/Netrin and its receptors, UNC-5 and UNC-40, in A/P guidance and a reciprocal role for Wnt signaling components (including Wnt regulators such as MIG-14/WntlessCitation29 or SFRP-1 Citation25) in D/V guidance, demonstrating that Wnts and Netrin also guide migrations orthogonal to the axis of their gradation.Citation23 Notably, simultaneous compromise of both Wnt and Netrin signaling components caused a nearly complete penetrance of D/V transition defects of the posterior DTC, demonstrating that the sum of Wnt and Netrin signaling fully accounts for the polarization of this cell along the D/V axis. The involvement of Wnt signaling in D/V guidance of cells (DTCs) and growth cones (see below) identifies Wnt signaling as one of the long sought mechanisms that functions in parallel to Netrin signaling to promote D/V guidance of cells and axons. Wnt and Netrin signaling also fully account for proper reorientation of the posterior DTC along the A/P axis during the third migration phase, suggesting that together Wnt and Netrin signaling govern all migratory transitions of this cell.Citation23
Figure 1. The C. elegans hermaphrodites U-shaped gonad arms are formed by 3 sequential migration phases of the anterior and posterior DTCs (marked as white crescent shaped cells), both undergoing a mirror image symmetrical pattern of migration. The DTCs are born in the mid-body, their first migration phase (phase 1) occurs along the A/P axis in opposite directions; the anterior DTC migrates toward the head while the posterior DTC migrates toward the tail. At the onset of the second migration phase (phase 2), the DTCs pause and reorient to polarize along the D/V axis. This phase 2 D/V migration is dependent on the unc-5 Netrin receptor, which is transcriptionally up-regulated at the time of the turn.Citation26 The polarity information determining the directionality along the D/V axis is provided by the UNC-6/Netrin guidance cue,Citation15,26 which is secreted from ventral sourcesCitation16 (depicted by the red gradient). UNC-5 also functions redundantly with Wnt signaling components to regulate D/V guidance.Citation23 Wnts are distributed along the A/P axis. Some Wnts (like EGL-20) are secreted from posterior sources, while others (like CWN-1 and CWN-2) are secreted from more anterior sources. Gradients of CWN-1/Wnt (green) and EGL-20/Wnt (blue) are depicted based on published expression studiesCitation25,36 mig-14/Wntless is necessary to facilitate Wnt secretion and was found to have substantial impact on egl-20/Wnt gradients.Citation29,50 The last migration phase of the DTCs (phase 3) occurs once again on the A/P axis where the DTCs migrate back toward the mid-body. The polarity and consequently the direction of the migration throughout this migration phase is determined by a fine balance between different Wnts (such as cwn-1 and egl-20) or Wnts (such as lin-44) and unc-5.Citation23 Dorsal is up and anterior is left. Red arrows mark the direction of DTC movement. Black arrows represent genetic interactions (positive or negative). Gray arrows represent the route yet to be taken by the DTCs, which stop migrating as they reach the mid-body.
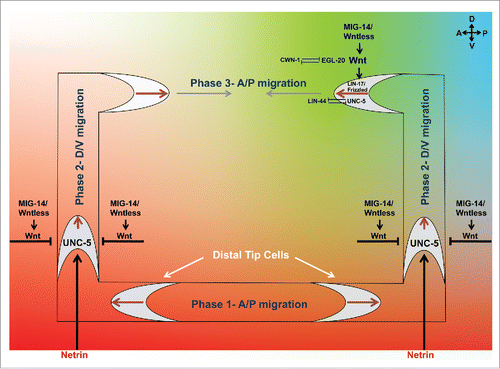
Wnts display complex interactions in regulating polarity of cells and axons in C. elegans; they widely function redundantly but often display opposing functions such that a balance of their activity levels defines explicit polarized patterns.Citation8,30 Multiple Wnts are involved in establishing DTC polarity on the A/P axis, and similarly also here, a fine balance between various Wnts determines the polarity of the DTC and consequently the direction of its migration. For example, phase 3 A/P polarity reversal defects resulting from mutations in egl-20/Wnt can be markedly rescued by impairing the function of another Wnt, cwn-1, implying that a balance between the activity of these 2 Wnts is required to elicit normal A/P polarity of the DTCs.Citation23 The Netrin receptor, UNC-5, seems to be an integral component of this Wnt signaling network; it functions redundantly with some Wnts, while opposing the function of others, to maintain a fine balance of activities required for proper A/P polarity, more specifically, a balance between UNC-5 and LIN-44/Wnt activities promotes normal DTC phase 3 A/P polarity.Citation23 The balance in activities of Wnts and UNC-5 determines whether anterior or posterior polarities are established (and hence the direction taken on the A/P axis), whether the cell reorients to the D/V axis, and likely also whether the cell halts. UNC-5 was further found to be a target for negative regulation by the Wnt frizzled receptor MOM-5. This regulation is mediated by a small GTPase signaling cascade involving ced-12/Elmo, ced-10/Rac and mig-2/RhoG.Citation31
Redundant functions of Wnt and Netrin signaling pathways were also observed in axon guidance. Impairing both Netrin and Wnt signaling components simultaneously causes synergistic axon guidance defects of the CAN neuron, which extends bipolar axons along the A/P axis, or the mechanosensory neurons, which extend axons along the A/P and D/V axes.Citation23 This indicates a functional redundancy between Wnt and Netrin signaling components in orchestrating axon guidance. Other examples of functional redundancy in mechanisms regulating axon guidance were recently demonstrated in commissural axons of the mouse spinal cord.Citation32 Thus, redundant regulatory mechanisms seem to be prevalent in axon guidance and likely provide a safety measure to ensure accurate navigation and proper connectivity. Interestingly, in addition to uncovering redundant functions for Wnt and Netrin signaling in axon guidance, the analysis of the mechanosensory neurons also revealed a novel function of the UNC-5 receptor. For example, although unc-5 or egl-20/Wnt mutants rarely displayed defects in D/V guidance toward ventral sources of Netrin (attraction response) the unc-5 egl-20 double mutants displayed a synergistic interaction causing high penetrance of failures of the AVM and PVM axons to migrate toward the ventral source of UNC-6/Netrin.Citation23 With the exception of the HSN,Citation33 UNC-5 is not known to be involved in attraction toward ventral sources of its ligand UNC-6/Netrin, but rather to elicit migration away from these sources.Citation15,26,34,35 These results demonstrate that UNC-5 has a role in establishing D/V polarity that extends beyond its conventional role in mediating Netrin induced axon repulsion,Citation15,26,34,35 and that this role is redundant with a role for EGL-20/Wnt in D/V guidance.Citation23 The dual function of UNC-5 in A/P and D/V guidance raises the untested possibility that both Wnt and Netrin signaling pathways converge on UNC-5 and that this receptor assimilates information from both cues. Hypomorphic unc-5 alleles, such as unc-5(ev644) that has impaired A/P guidance in the background of mig-14/Wntless mutants, but almost intact D/V guidance,Citation23 indicates that the functional requirements for UNC-5 in A/P signaling versus its conventional D/V instructive signaling are genetically separable.
Orchestrating A/P and D/V guidance
Wnts are graded along the A/P axis in 2 opposing gradients; some Wnts (like LIN-44 and EGL-20) are secreted from posterior sources, while others like CWN-2 and CWN-1 are secreted from more anterior sources and their graded distributions intersect to varying degrees depending on the position along the A/P axis.Citation25,36 A possible mechanism by which A/P graded Wnts can affect D/V polarity is by eliciting A/P bipolar inhibition, i.e. inhibiting the possibility of leading edge formation at both the anterior and posterior poles of the cell or growth cone. Inhibiting or excluding the polarity establishment machinery from the anterior or the posterior poles would effectively restrict it to the center of the cell (aligning with the D/V axis) where it can be employed for D/V guidance. This allows the efficient distribution of polarity determinants along the orthogonal axis and provides means for translating the output of a seemingly contradicting extracellular inputs (i.e., input from an A/P guidance cue eliciting A/P polarity vs. a D/V guidance cue eliciting D/V polarity) into a cooperative output (both signaling pathways establish polarity along a single axis). An example of apparent A/P bipolar inhibition was observed in a study of the HSN neuron, where Wnt signaling components were implicated in the exclusion of the UNC-40/DCC receptor from the anterior and posterior poles of the HSN growth cone.Citation33 It is tempting to speculate that bipolar inhibition of anterior and posterior leading edges could regulate reorientations from one axis to another in complex pathfinding processes. It is also tempting to speculate that bipolar inhibition could serve as a general mechanism for regulating cessation of cell migration along a single axis; hence, opposing gradients can potentially determine cell positioning by restricting polarity formation to the same extent from both directions, consequently resulting in a halt.
Wnts and Netrin- more to uncover
It is well established that the role of Netrin and its receptors is not limited to guiding cell and axon migrations. The Netrin pathway contributes to a wide range of biological processes, such as: organogenesis, synaptogenesis, angiogenesis, cell survival, adhesion, tissue morphogenesis, dendritic self avoidance, tumor formation and metastasis.Citation37-43 Similarly, Wnts control a variety of developmental processes including: cell fate determination, polarity establishment, spindle orientation, cell migration and axon guidance Citation36,44-46; and are hence involved in tumorigenesis and human disease.Citation47,48 The indication that Wnts and Netrin signaling components share redundant functions, which are not readily revealed except by impairing both pathways simultaneously, suggests that these 2 signaling pathways might be substantially involved in more processes and to a greater extent than currently appreciated. This is further demonstrated by a synthetic fully penetrant egg-laying defect and extensive embryonic lethality observed in the mig-14/Wntless; unc-6/Netrin double mutants,Citation23 which indicates redundant functions for Wntless and UNC-6/Netrin in orchestrating vulval function and at least one essential developmental process critical for early development in C. elegans. Therefore, during normal development as well as under some pathological conditions Wnts and Netrins may have functions that are not apparent due to their redundant output a notion that is important to consider in order to fully elucidate the underlying mechanisms governing these processes.
Given their redundant functions, are these 2 signaling pathways co-regulated? Interestingly, SFRPs known to function as Wnt regulators, contain a cysteine rich domain (CRD), which shares high degree of homology to the Frizzled family CRD, and also contain a Netrin-related motif (NTR domain).Citation49 It is an interesting possibility that the SFRPs may function in some cases to co-regulate these 2 fundamental pathways.
To conclude, the observation that Netrins and Wnts have shared functions in A/P and D/V guidance opens new avenues for deciphering how A/P and D/V guidance signals are integrated to establish polarity in multiple biological processes and implicate broader roles for Netrin and Wnt signaling - roles that are hidden due to prevalent functional redundancy between these cues. Furthermore, it provides a novel conceptual view by which polarity establishment along the A/P axis can also be achieved by D/V guidance cues, and vice verse. Thus, for example, D/V polarity can be potentially viewed as one of 3 possible A/P polarized states; the two obvious A/P polarized states are anterior polarity (when the polarizing machinery is limited to the anterior pole) and posterior polarity (the polarizing machinery is limited to the posterior pole) while the third state is established when the polarizing machinery is limited to the center of the cell by means of exclusion from both anterior and the posterior poles, effectively positioned along the D/V axis. Thus D/V and A/P guidance cues can function collaboratively to establish polarity on a single axis, with D/V polarity being, in part, the culminating result of bipolar inhibition of A/P polarity formation at both the anterior and posterior poles providing a mean for collaborative input integration of A/P and D/V signaling pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron (1992); 9:873-81; PMID:1329863; http://dx.doi.org/10.1016/0896-6273(92)90240-E
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell (1999); 96:795-806; PMID:10102268; http://dx.doi.org/10.1016/S0092-8674(00)80590-5
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell (1999) 96:785-94; PMID:10102267; http://dx.doi.org/10.1016/S0092-8674(00)80589-9
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell (1994) 78:425-35; PMID:8062385; http://dx.doi.org/10.1016/0092-8674(94)90421-9
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron (2001) 32:25-38; PMID:11604136; http://dx.doi.org/10.1016/S0896-6273(01)00448-2
- Silhankova M, Korswagen HC. Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr Opin Genet Dev (2007); 17:320-5; PMID:17644372; http://dx.doi.org/10.1016/j.gde.2007.05.007
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, Katz WS, Sternberg PW. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell (2004) 118:795-806; PMID:15369677; http://dx.doi.org/10.1016/j.cell.2004.09.001
- Zinovyeva AY, Yamamoto Y, Sawa H, Forrester WC. Complex network of Wnt signaling regulates neuronal migrations during Caenorhabditis elegans development. Genetics (2008); 179:1357-71; PMID:18622031; http://dx.doi.org/10.1534/genetics.108.090290
- Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell (2006); 10:379-90; PMID:16516840; http://dx.doi.org/10.1016/j.devcel.2006.01.013
- Pan C.-L, Howell JE, Clark SG, Hilliard M, Cordes S, Bargmann CI, Garriga G. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell (2006); 10:367-77; PMID:16516839; http://dx.doi.org/10.1016/j.devcel.2006.02.010
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development (2006); 133:1757-66; PMID:16571624; http://dx.doi.org/10.1242/dev.02357
- Rhiner C, Gysi S, Fröhli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development (2005); 132:4621-33; PMID:16176946; http://dx.doi.org/10.1242/dev.02042
- Merz DC, Alves G, Kawano T, Zheng H, Culotti JG. UNC-52/Perlecan affects gonadal leader cell migrations in c. elegans hermaphrodites through alterations in growth factor signaling. Dev Biol (2003); 256:174-187; http://dx.doi.org/10.1016/S0012-1606(03)00014-9
- Schwabiuk M, Coudiere L, Merz DC. SDN-1/syndecan regulates growth factor signaling in distal tip cell migrations in C. elegans. Dev Biol (2009); 334:235-42; PMID:19631636; http://dx.doi.org/10.1016/j.ydbio.2009.07.020
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron (1990); 4:61-85; PMID:2310575; http://dx.doi.org/10.1016/0896-6273(90)90444-K
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron (1996); 16:35-46; PMID:8562088; http://dx.doi.org/10.1016/S0896-6273(00)80021-5
- Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development (2011); 138:2153-69; PMID:21558366; http://dx.doi.org/10.1242/dev.044529
- Honigberg L, Kenyon C. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development (2000); 127:4655-68; PMID:11023868
- Middelkoop TC, Williams L, Yang PT, Luchtenberg J, Betist MC, Ji N, van Oudenaarden A, Kenyon C, Korswagen HC. The thrombospondin repeat containing protein MIG-21 controls a left-right asymmetric Wnt signaling response in migrating C. elegans neuroblasts. Dev Biol (2012); 361:338-48; PMID:22074987; http://dx.doi.org/10.1016/j.ydbio.2011.10.029
- Teichmann HM, Shen K. UNC-6 and UNC-40 promote dendritic growth through PAR-4 in Caenorhabditis elegans neurons. Nat Neurosci (2011); 14:165-72; PMID:21186357; http://dx.doi.org/10.1038/nn.2717
- Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci (2007); 10:161-8; PMID:17237777; http://dx.doi.org/10.1038/nn1835
- Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga GC. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci (2007); 10:169-76; PMID:17237778; http://dx.doi.org/10.1038/nn1834
- Levy-Strumpf N, Culotti JG. Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in C. elegans. PLoS Genet (2014); 10:e1004381; PMID:24901837; http://dx.doi.org/10.1371/journal.pgen.1004381
- Wong M-C, Schwarzbauer JE. Gonad morphogenesis and distal tip cell migration in the Caenorhabditis elegans hermaphrodite. Wiley Interdiscip. Rev Dev Biol (2012); 1:519-531
- Harterink M, Kim DH, Middelkoop TC, Doan TD, van Oudenaarden A, Korswagen HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development (2011); 138:2915-24; PMID:21653614; http://dx.doi.org/10.1242/dev.064733
- Su M, Merz DC, Killeen MT, Zhou Y, Zheng H, Kramer JM, Hedgecock EM, Culotti JG. Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development (2000); 127:585-94; PMID:10631179
- Nishiwaki K. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics (1999); 152:985-97; PMID:10388818
- Cabello J, Neukomm LJ, Günesdogan U, Burkart K, Charette SJ, Lochnit G, Hengartner MO, Schnabel R. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol (2010); 8:e1000297; PMID:20126385; http://dx.doi.org/10.1371/journal.pbio.1000297
- Yang P-T, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell (2008); 14:140-7
- Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell (2008); 134:646-56; PMID:18724937; http://dx.doi.org/10.1016/j.cell.2008.06.026
- Levy-Strumpf N, Krizus M, Zheng H, Brown L, Culotti JG. The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells. PLOS Genet (2015); 11:e1005446; PMID:26292279; http://dx.doi.org/10.1371/journal.pgen.1005446
- Jaworski A, Tom I, Tong RK, Gildea HK, Koch AW, Gonzalez LC, Tessier-Lavigne M. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science (2015); 350:961-965; PMID:26586761; http://dx.doi.org/10.1126/science.aad2615
- Kulkarni G, Xu Z, Mohamed AM, Li H, Tang X, Limerick G, Wadsworth WG. Experimental evidence for UNC-6 (netrin) axon guidance by stochastic fluctuations of intracellular UNC-40 (DCC) outgrowth activity. Biol Open 6 (2013); 2(12):1300-12
- Hamelin M, Zhou Y, Su MW, Scott IM, Culotti JG. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steer their axons dorsally. Nature (1993); 364:327-30; PMID:8332188; http://dx.doi.org/10.1038/364327a0
- Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell (1992); 71:289-99; PMID:1384987; http://dx.doi.org/10.1016/0092-8674(92)90357-I
- Sawa H, Korswagen HC. Wnt signaling in C. elegans. WormBook 1–30 (2013).
- Mehlen P, Furne C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol Life Sci (2005); 62:2599-616; PMID:16158190; http://dx.doi.org/10.1007/s00018-005-5191-3
- Baker KA, Moore SW, Jarjour AA, Kennedy TE. When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr Opin Neurobiol (2006); 16:529-34; PMID:16935486; http://dx.doi.org/10.1016/j.conb.2006.08.002
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol (2007); 8:296-306; PMID:17356579; http://dx.doi.org/10.1038/nrm2142
- Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature (2008); 455:669-73; PMID:18776887; http://dx.doi.org/10.1038/nature07291
- Ziel J, Sherwood D. Roles for netrin signaling outside of axon guidance: a view from the worm. Dev Dyn (2010); 239:1296-1305; PMID:20108323
- Ziel JJW, Hagedorn EEJ, Audhya A, Sherwood DDR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol (2009); 11:183-9; PMID:19098902; http://dx.doi.org/10.1038/ncb1825
- Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science (2007); 318:103-6; PMID:Can't; http://dx.doi.org/10.1126/science.1143762
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci (2005); 6:351-62; PMID:15832199; http://dx.doi.org/10.1038/nrn1665
- Fradkin LG, Garriga G, Salinas PC, Thomas JB, Yu X, Zou Y. Wnt signaling in neural circuit development. J Neurosci (2005); 25:10376-8; PMID:16280576; http://dx.doi.org/10.1523/JNEUROSCI.3429-05.2005
- Park M, Shen K. WNTs in synapse formation and neuronal circuitry. EMBO J (2012); 31:2697-704; PMID:22617419; http://dx.doi.org/10.1038/emboj.2012.145
- Herr P, Hausmann G, Basler K. WNT secretion and signalling in human disease. Trends Mol Med (2012); 18:483-93; PMID:22796206; http://dx.doi.org/10.1016/j.molmed.2012.06.008
- Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol (2012); 4(5):4:pii: a008052; PMID:22438566; http://dx.doi.org/10.1101/cshperspect.a008052.
- Bhat RA, Stauffer B, Komm BS, Bodine PVN. Structure-function analysis of secreted frizzled-related protein-1 for its Wnt antagonist function. J Cell Biochem (2007); 102:1519-28; PMID:17471511; http://dx.doi.org/10.1002/jcb.21372
- Coudreuse DYM, Roël G, Betist MC, Destrée O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science (2006); 312:921-4; PMID:16645052; http://dx.doi.org/10.1126/science.1124856
