ABSTRACT
Background
Post-exertional malaise (PEM) is a defining characteristic of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) but there is insufficient research dissecting the nature of PEM from the patients’ perspective.
Methods
A PEM questionnaire administered to 150 ME/CFS patients. It included open-ended questions about triggers, experiences, recovery, and prevention. Responses were re-coded into concise, representative topics. Chi-Square tests of independence were then used to test for differences and relationships between duration of ME/CFS illness (<4 years and >10 years), PEM onset and duration, and gender with PEM trigger, experience, recovery, and prevention.
Results
Physical exertion was the most common trigger of PEM. The onset of PEM occurred within minutes after physical exertion compared to within hours after cognitive exertion (<0.05). ME/CFS patients sick for <4 years reported stress as a trigger significantly more often than those sick for >10 years (<0.001). ME/CFS patients sick for <4 years experienced more orthostatic symptoms during PEM than those sick for >10 years. ME/CFS patients sick for >10 years reported using medications to recover from PEM significantly more that those sick for <4 years (<0.01). Pacing and avoiding specific triggers were common approaches to prevent PEM.
Conclusions
There are differences in PEM triggers, experiences and recovery based on duration of illness. Asking about PEM is important for diagnosis and to understand how to manage PEM. Given that PEM occurs more quickly after physical versus cognitive exertion, these results should instigate research on the relationship of upright posture, hypoperfusion and PEM.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating disease with significant unmet medical needs that affects as many as 2.5 million people in the U.S. and causes enormous burden for patients, their caregivers, the healthcare system, and society. The disease is characterized by impaired function accompanied by severe fatigue, unrefreshing sleep, cognitive impairment, and orthostatic intolerance, all of which are worsened by physical and cognitive exertion causing post-exertional malaise (PEM) [Citation1]. ME/CFS is generally considered to be a post-viral or post-infection syndrome with immune, metabolic, and neurologic sequelae [Citation2]. Because fatigue is a common symptom of many different medical and psychiatric conditions, the rate of misdiagnosis of ME/CFS can be as high as 40–50% of cases [Citation3]. Furthermore, numerous case definitions and the lack of objective diagnostic biomarkers result in many people not getting a diagnosis [Citation4]. At least one-quarter of ME/CFS patients are house- or bedbound at some point in their lives [Citation5]. The economic impact of ME/CFS is $17 to $24 billion annually for direct costs and $9.1 billion from lost household and labor force productivity [Citation6,Citation7].
Post-exertional malaise (PEM) is the cardinal and distinguishing feature of ME/CFS. As the phrase suggests, PEM is an increase in severity of symptoms (e.g. fatigue, weakness, orthostatic intolerance) and signs (e.g. heart rate variation, temperature dysregulation) that occurs following physical and cognitive exertion. Patients report that PEM can be triggered by the most mundane of daily activities including sitting upright at the dining table, standing to make a salad, taking a shower, driving a car, grocery shopping and cleaning the house. Cognitive exertion that triggers PEM can occur by listening to a lecture, socializing, having a conversation or reading. Upright posture (defined as feet on the floor) that occurs during physical and cognitive activities (e.g. walking, sitting at a desk) may be sufficient exertion to trigger PEM. Our clinical observations of hundreds of ME/CFS patients indicate that less time in an upright posture with feet on the floor over a 24-h period is associated with severe illness symptoms and disability. Patients with <5 h of upright activity (with feet on the floor) were more likely to be home or bedbound and unemployed compared to patients with ≥5 h of upright activity [Citation8].
We were interested in understanding ME/CFS patients’ perspective of PEM in order to identify point-of-care methods to assess PEM for diagnosis. ME/CFS patients participating in an ongoing longitudinal study responded to an online PEM questionnaire during the first year. The PEM questionnaire used open-ended text responses to capture patients’ personal stories about PEM triggers, experiences, recovery, and prevention. Our objective was to review the responses, recode them to categorical topics and determine if there were differences in PEM by duration of illness.
Materials and methods
Participants: This analysis included responses from 151 ME/CFS patients who had been seen at Bateman Horne Center (Salt Lake City, UT) for routine clinical care between February 2018 and September 2019 and consented to participate in a longitudinal study (described in [Citation9]). This study aimed to recruit and compare shorter duration illness to longer duration illness. While choosing <4 year as short duration illness is somewhat arbitrary, this timeframe was a clinical decision based on the findings that ME/CFS patients sick for shorter periods of time had different profiles compared to those with longer duration illness [Citation10]. Patients sick for >10 years with ME/CFS were selected to provide as great of contrast between ‘short’ and ‘long’ duration of illness as possible. The 151 ME/CFS subjects included 75 sick with ME/CFS for <4 years (<4 ME/CFS) and 76 sick for greater than 10 years (>10 ME/CFS). The age range of ME/CFS participants was 18–65 years at the time of informed consent. Enrolled ME/CFS participants were required to fulfill at least one of ME/CFS case definitions [Citation1,Citation11,Citation12].
PEM Questionnaire: The PEM Questionnaire was designed by the Bateman Horne Center so ME/CFS patients could describe PEM in their own words. It was an online survey administered to ME/CFS patients one time during the first year of the study. The PEM Questionnaire included the following six questions, and the type of response is in parentheses:
What kind of exertion triggers your PEM? (open-ended text response)
Does PEM happen … (single choice),
minutes after exertion
hours after exertion
a day or more after exertion
not at all
Please describe what happens to you when PEM occurs (open-ended text response)
How long does it take you to recover from PEM? (single choice)
at least a day
several days
at least a week
several weeks
a month or more
What do you do to recover from PEM? (open-ended text response)
What do you do to prevent PEM from occurring? (open-ended text response)
Responses were exported to Microsoft Excel for analysis. Open-ended text responses were reviewed and summarized for each PEM category (triggers, experiences, recovery, and prevention) followed by assigning a topic/thematic variable name. It should be noted that ME/CFS is a fluid disease, which often presents differently in individuals from day to day. If a subject mentioned multiple types of triggers, it was recorded as such. The assigned variables are shown in .
Table 1. Patients described their experiences with the four clinically relevant categories of PEM that were than transcribed into a medically relevant term or phrase.
Statistical analysis: The responses to the PEM questionnaire were analyzed in Microsoft Excel. Comparisons were made using Chi-Square test of independence. For all statistics, a p-value of <.05 was considered statistically significant, with the null hypothesis as all variables are independent. Initially, independence was tested for between gender, duration of illness, onset of PEM, and duration of PEM. Then, repeated measures of Chi-Square were used to detect differences with respect to duration of illness (<4 years and >10 years) and gender. The mean and standard deviation for quantity of categories per individual in each subgroup were calculated. Chi-Square tests were similarly performed on the quantity of categories for both gender and duration of illness. Lastly, chi-square tests were applied to the data based on onset of PEM for trigger categories, and duration of PEM in the experience and prevention categories.
Results
The characteristics of the ME/CFS patients are shown in . The <4 ME/CFS group reported being sick between 1 and 4 years. All subjects were either sick for <4 years or >10 years. The average age of the <4 ME/CFS patients was younger than the >10 ME/CFS patient group (<0.05). There were approximately three times more female than male patients. There were no differences in PEM onset or PEM duration between the <4 and >10 ME/CFS groups. All 151 ME/CFS patients reported PEM. Duration of illness (<4 years and >10 years), gender, PEM onset and duration were cross-compared and there were no significant findings indicating that these characteristics were independent of one another. The majority of <4 ME/CFS patients and >10 ME/CFS patients met case criteria with some differences noted between the two groups (for the <4 ME/CFS group, 99% IOM [Citation1], 97% Fukuda [Citation11], 96% CCC [Citation12]; for the >10 ME/CFS group, 100% IOM [Citation1], 99% Fukuda [Citation11], 99% CCC [Citation12]).
Table 2. Characteristics of ME/CFS patients and PEM duration and onset.
A patient could note as few or as many PEM triggers, experiences, recovery, and prevention approaches as desired in their open-ended responses. (a–d) shows the total number of responses for each PEM category. The most common type of PEM trigger was medium level physical exertion followed by medium level cognitive exertion ((a)). It is notable that 58% of patient responses indicated physical exertion (low, medium, and high; noted in (a) in shades of blue) compared to 31% of responses for the cognitive trigger (noted in (a) as shades of orange). All other PEM trigger types accounted for the remaining 11% of responses. Patients had on average 2.2 (SD ± 1.04) types of PEM triggers. Having 1 or 4 PEM triggers was significantly associated with duration of illness (p = .021, ). Interestingly, PEM resulted with one trigger for most of the >10 ME/CFS patients whereas <4 ME/CFS patients reported at least 4 types of PEM triggers.
Figure 1. Distribution of categories out of total responses. Multiple categories were listed per individual. (a) represents trigger responses, where physical triggers make up 58.2% of all trigger responses. (b) shows variance within the experience responses, with no single category having more than 21% (fatigue). Recovery responses are shown in (c), and revealed that rest, sleep, and limited stimulation compose 56.9% of these responses. (d) is for prevention, and shows that pacing, avoidance, and physical awareness compose over two-thirds of prevention responses (78.2%).
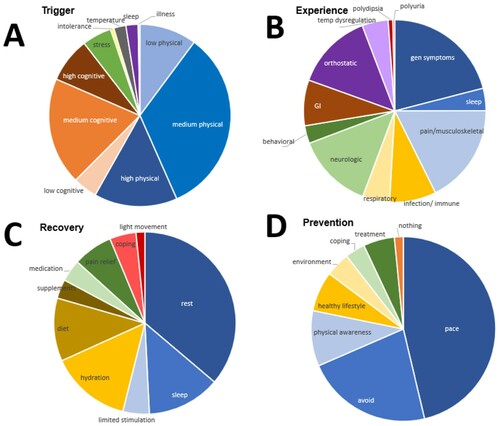
Figure 2. Quantity of trigger per individual with respect to duration of illness. The indication of having one and four trigger was found to be statistically significant between <4 ME/CFS and >10 ME/CFS. There is a notable trend of <4 ME/CFS listing more triggers compared to >10 ME/CFS.
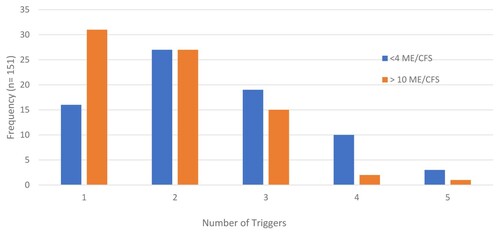
Patients experience a variety of different symptoms during PEM ((b)). Fatigue (e.g. weakness, fatigue, heavy limbs, flu-like symptoms, feeling ill) comprised 21% of total responses and accounted for the most common PEM experience. Pain/musculoskeletal symptoms (17.7%) and orthostatic intolerance symptoms (13.7%) were the next most common PEM experiences with the remaining experiences ranging from 13.6% (neurologic) to 0.33% (polyuria). The average number of symptoms experienced during PEM was 4.0 (SD ± 1.62). There was no significant difference in the symptoms experienced during PEM by either gender or duration of illness. The number of symptoms experienced during PEM was similar for both the <4 ME/CFS patients (3.84 SD ± 1.55) and the >10 ME/CFS (4.17 SD ± 1.68) patients.
Patients indicated that rest (36.2%) was most often used for recovering from PEM followed by hydration (14.3%) and sleep (13.0%) ((c)). Rest, sleep, and limited stimulation make up over half of the total responses for PEM recovery (56.9%). The average number of ways patients used to recover from PEM was 2.5 (SD ± 1.43). There were no significant differences in how patients recovered from PEM by either gender or duration of illness.
(d) shows that pacing (46.3%), avoidance (22.2%), and physical awareness (9.7%) make up the majority of responses for PEM prevention. The remaining prevention strategies accounted for less than 20% of the remaining categories. The average number of prevention strategies used by patients was 1.7 (SD ± 0.87). There were no significant differences based on gender, duration of illness, quantity of strategies, or PEM duration regarding prevention strategies for PEM.
The frequency of PEM triggers, experiences, recovery, and prevention for the total patient cohort versus total responses was analyzed (). 71.5% of patients reported medium level physical exertion as their PEM trigger. Also, the majority of patients (84%) reported fatigue (e.g. fatigue, weakness, ‘heavy limbs’, flu-like symptoms, feeling ill) during PEM. Rest was the predominant PEM recovery strategy reported by 92% of patients. Finally, 79% of patients use pacing to prevent PEM. Generally, PEM triggers, experiences, recovery, and prevention were similar for <4 ME/CFS and >10 ME/CFS patients. The exception was stress as a PEM trigger for the <4 ME/CFS patients (p = .0286) and medications for PEM recovery for >10 ME/CFS patients (p = .0009). Low level physical exertion triggered PEM within minutes (p = .0508), whereas high level cognitive exertion triggered PEM within hours (p = .0305). Males reported using supplements more often than females to recover from PEM (p = .0206).
Table 3. Frequency of PEM triggers, experiences, recovery and prevention.
Discussion
This study provided the unique opportunity to compare PEM in patients sick with ME/CFS for <4 years to ME/CFS patients sick for >10 years to determine if PEM triggers, experiences, recovery and prevention vary with duration of illness. Furthermore, allowing patients to describe their PEM triggers, experiences, recovery, and prevention in their own words provided us the opportunity to identify common experiences of PEM. This study enrolled one of the largest, ‘short duration’ illness ME/CFS patient cohorts sick for less than 4 years to compare to ‘long duration’ ME/CFS patients sick for greater than 10 years.
All patients were clinically evaluated and fulfilled the 1994 International CFS case definition criteria [Citation11], the Canadian case definition criteria [Citation12], or the IOM clinical diagnostic criteria [Citation1]. Each definition includes a PEM criterion and 100% (151) of ME/CFS patients acknowledged the presence of PEM during their clinical evaluation and in response to the PEM questionnaire. This is consistent with 100% patients reporting that PEM occurs within minutes to days following exertion on the PEM questionnaire (). Most of the ME/CFS patients in this study reported that PEM occurred within minutes to hours following exertion and lasted for several days to a week. Chu et al. [Citation13] also reported that most patients reported PEM onset immediately or within 24 h of physical or cognitive exertion with similar PEM duration.
PEM is pathognomonic of ME/CFS, yet there are no PEM-specific standardized questionnaires or validated methods to fully assess it. NINDS Common Data Elements for ME/CFS recommends use of a Core PEM Assessment case report form (CRF) that uses 5 PEM questions extracted from the DePaul Symptom Questionnaire [Citation14,Citation15]. This CRF asks about the frequency and severity of post-exertional symptoms but does not elicit information about triggers, recovery, and prevention, all of which can provide clues to PEM and how best to help patients manage it. The PEM questionnaire used in this study originated from extensive clinical experience with hundreds of ME/CFS patients and the need for the clinician and patient to understand these PEM domains. This helped us understand that it was important to elicit information about not only PEM symptoms and experience, but also what triggers PEM and what strategies patients use to recover and prevent PEM.
PEM triggers and potential explanations
Low, medium, and high physical activity was the most common type of PEM trigger (58.2%). The common theme among these different levels of physical activity is time spent in an upright posture with feet on the floor. We have long used hours of upright activity (HUA) to assess physical impairment in our ME/CFS clinical patients and incorporated asking about HUA into our clinical research protocols. We recently reported that ME/CFS patients with less than <5 HUA over a 24-h period had more orthostatic intolerance related symptoms than patients with ≥5 HUA [Citation9]. Patients with <5 HUA were more severely ill and less likely to be employed compared to ME/CFS patients with ≥5 HUA. Patients reporting severe or very severe PEM are more likely to be unemployed and on disability compared to ME/CFS patients with mild or moderate PEM [Citation16]. Furthermore, in ME/CFS patients undergoing an orthostatic challenge called the 10-minute NASA Lean Test, a heart rate (HR) increase of 1 beat per minute (bpm) resulted in a decrease of 10 minutes of upright activity and HUA decreased by 1.4 hours in ME/CFS patients with POTS (defined as a HR increase of 30 bpm upon standing) [Citation9].
All these results implicate orthostatic intolerance and hypoperfusion of the brain as a possible contributor to PEM in many ME/CFS patients. This would help explain why even a low level of physical exertion can trigger PEM in minutes, although medium physical exertion was the most common type of PEM trigger. High, medium, and low levels of cognitive exertion accounted for 31.4% of what triggered PEM. It is difficult to disentangle the effect of upright posture and coincident brain hypoperfusion that occurs in ME/CFS patients. Many cognitive activities occur while in upright posture (e.g. seated with feet on the floor). Upright postures will increase splanchnic pooling, decrease cerebral blood flow, and impair neurocognition in ME/CFS patients [Citation17]. Neuroinflammation is also likely to contribute to the inability of ME/CFS patients to sustain cognitive activities without triggering PEM. Activated microglial cells are characteristic of ME/CFS neuroinflammation [Citation18]. There are numerous animal models demonstrating how activated microglial cells injure the brain following cerebral hypoperfusion and cause neurocognitive deficits [Citation19]. Chronic brain hypoperfusion coupled with neuroinflammation are plausible causal pathways of PEM.
Stress was a significant trigger of PEM for the <4 ME/CFS patients. Dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis, the central regulator of the stress response, has been a consistent finding in ME/CFS. At the biological level, in addition to the sympathetic nervous system predominance, chronic dysregulation of the HPA axis leads to abnormal immune responses, glucocorticoid sensitivity, and low cortisol levels [Citation20]. At the clinical level, patients are overwhelmed with an ME/CFS diagnosis and fearful about life with a chronic illness. While it may seem unusual to consider 4 years as ‘short duration,’ it takes 5 years, on average, to obtain a diagnosis of ME/CFS [Citation1]. Many of the <4 ME/CFS patients had not yet, or only recently, been diagnosed with ME/CFS before participating in this research and had been living with disabling symptoms and uncertainty, potentially amplifying the effects of stress on an already ‘stressed’ HPA axis. Furthermore, the <4 ME/CFS patients reported having more activities that trigger PEM perhaps indicating their unfamiliarity with their disease and the lack of treatments. ME/CFS patients, over time, and especially those that receive good comprehensive care, learn how to cope with chronic illness and better manage their symptoms.
Four or more symptoms of PEM, including fatigue, pain/musculoskeletal, orthostatic, and neurologic symptoms, were experienced by more than half of all of our ME/CFS patients. This PEM symptom constellation is similar to what was reported by patients participating in the Genetic Expression and Immune System Dynamics Study [Citation13]. The <4 ME/CFS patients reported experiencing slightly more orthostatic symptoms than the >10 ME/CFS group but the difference was not significant. Orthostatic intolerance (OI) is common in ME/CFS patients and is included in the diagnostic criteria recommended by the National Academy of Medicine [Citation1]. OI is a readily treated and manageable symptom in ME/CFS patients. It is likely that the <4 ME/CFS patients reported experiencing orthostatic symptoms because many had been recently diagnosed at Bateman Horne Center and when OI was identified, treatment had recently ensued.
PEM management strategies
Resting by limiting physical and cognitive activities and elevating feet was used by >90% of all patients to recover from PEM. Here again orthostatic intolerance is implicated as a cause of PEM. Resting restores physical and cognitive energy and elevating feet or becoming recumbent helps cerebral blood flow. It is interesting to note that the >10 ME/CFS group used medications to recover from PEM. This is likely due to the fact that their ME/CFS symptoms have been managed for longer and they are more familiar with what works for their disease. In the redesign of the PEM questionnaire, it will be important to ascertain the type of medications that are used to aid PEM recovery. The <4 ME/CFS group reported experiencing more OI symptoms during PEM and were significantly more likely to use hydration to recover from PEM. Oral and intravenous hydration are used to increase blood volume in patients who experience orthostatic intolerance [Citation21].
Pacing was the most common strategy used to prevent PEM following by avoiding the physical and cognitive activities the individual knows will trigger PEM. The term ‘pacing’ describes proactive, preventive behavioral changes, such as engaging in several short periods of mild to medium activity separated by periods of rest and recovery throughout the day. In the absence of scientific direction regarding pathophysiology and treatments, patients with ME/CFS have shown us the most effective existing treatment approach: activity management to prevent PEM. This follows decades of standard medical advice from the highest sources advising patients to stop fearing exercise and accusing them of not wanting to get well for a variety of emotional reasons.
Limitations
There are limitations to this study. The designation of <4 years and >10 years as short versus long duration of illness is arbitrary. However, it is based on the case definition recommendations to stratify and subgroup based on duration of illness [Citation11] as well as the finding of distinct immune profiles in short duration (<3 years) illness ME/CFS patients [Citation10]. This analysis only included responses from ME/CFS patients to the PEM questionnaire and not responses from healthy controls. Therefore, we do not know if the PEM experiences, triggers, recovery, and prevention topics identified for ME/CFS patients would be the same for healthy people or people with other diseases.
Summary
In summary, this study compares the triggers, experiences, recovery, and prevention strategies of ME/CFS patients in early (<4 years) and late (>10 years) disease. There were differences in PEM based on duration of illness that may reflect adaptation to illness over time as well as effective treatment of some ME/CFS symptoms (e.g. unrefreshing sleep, orthostatic intolerance). Physical, cognitive, emotional, and orthostatic stress may be the main contributors to PEM. Fatigue, pain, orthostatic intolerance, and neurologic symptoms are the most common symptoms of PEM. Both prevention and recovery strategies are identified that may help guide management strategies.
Ethics approval and consent to participate
The protocol was approved by The Jackson Laboratory Institutional Review Board study number 17-JGM-13 and the study was carried out according to United States federal regulations for the protection of human subjects as codified in 45 CFR 46. Procedures were carried out with the adequate understanding and written consent of the subjects.
Acknowledgements
Bateman Horne Center serves as the Clinical Core of the Collaborative Research Center (Grant project number U54NS105539) led by Derya Unutmaz, M.D. of The Jackson Laboratory. We want to thank everyone for their participation in this research. MH, SDV and LB designed the research study, analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed are available from the corresponding author upon request.
Additional information
Funding
Notes on contributors
Megan Hartle
Megan Hartle is currently a student at Drake University pursuing a Doctorate in Pharmacy.
Lucinda Bateman
Lucinda Bateman, MD, is the Medical Director and founder of the Bateman Horne Center.
Suzanne D. Vernon
Suzanne D. Vernon, PhD, is the Research Director of the Bateman Horne Center.
References
- Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington (DC): The National Academies Press; 2015.
- Komaroff AL. Advances in understanding the pathophysiology of chronic fatigue syndrome. JAMA. 2019;322:499–500.
- Newton JL, Mabillard H, Scott A, et al. The Newcastle NHS chronic fatigue syndrome service: not all fatigue is the same. J R Coll Physicians Edinb. 2010;40(4):304–307.
- Haney E, Smith ME, McDonagh M, et al. Diagnostic methods for myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(12):834–840.
- Marshall R, Paul L, Wood L. The search for pain relief in people with chronic fatigue syndrome: a descriptive study. Physiother Theory Pract. 2011;27(5):373–383.
- Jason LA, Benton MC, Valentine L, et al. The economic impact of ME/CFS: individual and societal costs. Dyn Med. 2008;8(7):6.
- Reynolds KJ, Vernon SD, Bouchery E, et al. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc. 2004;2(1):4.
- Lee J, Wall P, Kimler C, et al. Clinically accessible tools for documenting the impact of orthostatic intolerance on symptoms and function in ME/CFS. Work. 2020;66(2):257–263.
- Lee J, Vernon SD, Jeys P, et al. Hemodynamics during the 10-minute NASA Lean Test: evidence of circulatory decompensation in a subset of ME/CFS patients. J Transl Med. 2020;18(1):314.
- Hornig M, Montoya JG, Klimas NG, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1(1):e1400121.
- Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959.
- Carruthers BM. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J Clin Pathol. 2007;60(2):117–119.
- Chu L, Valencia IJ, Garvert DW, et al. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS One. 2018;13(6):e0197811.
- NINDS Common Data Elements Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. https://www.commondataelements.ninds.nih.gov/Myalgic%20Encephalomyelitis/Chronic%20Fatigue%20Syndrome.
- Cotler J, Holtzman C, Dudun C, et al. A brief questionnaire to assess post-exertional malaise. Diagnostics (Basel). 2018;8(3):66.
- May M, Milrad SF, Perdomo DM, et al. Post-exertional malaise is associated with greater symptom burden and psychological distress in patients diagnosed with Chronic Fatigue syndrome. J Psychosom Res. 2020;129:109893.
- Stewart JM, Medow MS, Messer ZR, et al. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2012;302(5):H1185–H1194.
- Nakatomi Y, Mizuno K, Ishii A, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an ¹¹C-(R)-PK11195 PET study. J Nucl Med. 2014;55(6):945–950.
- Zhang LY, Pan J, Mamtilahun M, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics. 2020;10(1):74–90.
- Tomas C, Newton J, Watson S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neurosci. 2013;2013:784520.
- Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol. 2016;37(2):278–282.
