ABSTRACT
Objectives
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex and debilitating chronic illness for which there are no well-accepted treatments, aside from some preliminary trials and symptom management strategies, such as low-dose naltrexone and NICE guidelines. A clinician administered a synergistic off-label treatment (SOT) involving spironolactone, colchicine, low-dose naltrexone, and multivitamins to ME/CFS patients, resulting in reported symptom improvement. This study sought to investigate neuroimaging characteristics linked with the treatment.
Methods
A group of treatment-naive patients (N = 8, control) was selected to age – and sex-match eight patients who had received the treatment (SOT). Diffusion-weighted imaging data and clinical measures from the two groups were compared.
Results
Compared to the control group, the SOT group showed reduced depression (Bonferroni corrected P value (PBonf) = .04) and mental deficit symptoms (PBonf = .02). Furthermore, the SOT group at post-treatment showed significantly reduced clinical scores (PBonf < .001) than at the pre-treatment baseline. We also observed higher fractional anisotropy and lower diffusivity measures in various white-matter tracts of the SOT group compared to the control group (Family-wise-error-corrected P value (PFWE) ≤ .05).
Conclusion
These findings provide a first line of evidence for the potential effectiveness of SOT treatment in ME/CFS patients, as indicated by microstructural changes in the brain associated with improved clinical symptoms. Formal randomized controlled trials are required to determine the efficacy of SOT components in treating ME/CFS.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex multisystemic debilitating chronic illness associated with unexplained fatigue, unrefreshing sleep, autonomic dysfunction, and cognitive problems. However, the pathophysiology of ME/CFS is not yet fully understood, and currently, no effective treatments are available.
Changes in immunoglobulin levels, cytokine profiles and B – and T – cell phenotype, as well as declined cytotoxicity of natural killer cells, are commonly reported features of immune dysregulation in ME/CFS [Citation1]. Studies in ME/CFS showed altered levels of inflammatory cytokines in both the plasma and cerebrospinal fluid, which correlated with the severity of symptoms [Citation2–4]. Furthermore, distinct cytokine patterns characterize various stages of ME/CFS. Early stages feature increased IL-1α and IL-8 alongside decreased IL-6, while later stages show elevated IL-1α and IL-6 with reduced IL-8, with a promising 75–88% accuracy in ME/CFS classification [Citation4]. Pro-inflammatory cytokines such as TNFα and IL-6 have been implicated in contributing to an increase in blood–brain barrier (BBB) permeability [Citation5], which may subsequently lead to heightened microglial activity as part of both direct and immune-mediated damage [Citation6]. A positron emission tomography study has shown widespread activation of the brain’s innate immune system, specifically activated microglia or astrocytes, in ME/CFS patients, which correlates with the severity of neuropsychological symptoms in these individuals [Citation7]. Also, some nutrient deficiencies (vitamin C, vitamin B complex, sodium, magnesium, zinc, folic acid, L-carnitine, L-tryptophan, essential fatty acids, and coenzyme Q10) appear to be important in the severity and exacerbation of CFS symptoms [Citation1].
A clinician (JDC) started a treatment protocol for patients with ME/CFS by prescribing Spironolactone, Colchicine, low dose naltrexone, and multivitamins to alleviate the ME/CFS symptoms [Citation8,Citation9]. Spironolactone, a mineralocorticoid receptor antagonist, has anti-inflammatory and immunomodulatory properties that might help inhibit chronic systemic inflammation [Citation10,Citation11]. Furthermore, colchicine, a treatment for gout and familial Mediterranean fever, is known to interfere with various inflammatory pathways [Citation12]. Given their anti-inflammatory properties, spironolactone and colchicine might resolve some underlying inflammatory pathologies in ME/CFS. Preliminary evidence also exists for the efficacy of low dose naltrexone in ME/CFS [Citation13]. Additionally, given the potential relationship between deficiencies in certain nutrients and the severity and worsening of ME/CFS symptoms [Citation1], it could be beneficial to provide the patients with multivitamins to reduce the severity of ME/CFS symptoms. Notably low dose naltrexone (LDN), as an opioid receptor antagonist, has been suggested to treat other diseases such as fibromyalgia, multiple sclerosis and regional pain syndromes, due to its anti-inflammatory properties [Citation14].
In the current study, we investigated the effectiveness of this treatment protocol (SOT) in ME/CFS, using diffusion weighted imaging (DWI), to explore its potential underlying mechanism for treating ME/CFS.
Methods
Participants provided written informed consent in accordance with the Human Research Committee of the University of the Sunshine Coast (A211664). The treatment and control groups originate from different sources. ME/CFS patients in the treatment group were administered a synergistic off-label treatment (SOT) protocol for more than 6 months by Dr. do Campo, as part of his routine practice following a comprehensive explanation of potential risks. The research team (AZM, PDF, RK, VC, and ZYS) subsequently approached these patients for inclusion in this pilot study (through advertisement materials), following the establishment of collaboration and receipt of ethics approval. Interested participants from these patients submitted expression of interest (EOI) forms, with no involvement from Dr. do Campo in the recruitment process. A total of 10 ME/CFS patients expressed their interest in our study, as being treated with SOT protocol for their symptoms for more than 6 months. The treatment protocol consisted of 25 mg/day of spironolactone and 0.5 mg/day of colchicine in combination with low dose naltrexone (LDN, 1.5–4.5 mg/day) and multivitamins (B12, B1, B6, C, D, folic acid, magnesium, and Omega3) (for a complete list of full medication in suppl. Table 1). Two selected participants were excluded as one was 68 years old, and another had to stop taking Spironolactone as they developed postural orthostatic tachycardia syndrome (POTS). We ended up with 8 ME/CFS patients treated (SOT; Age: 52 ± 9). Dr. do Campo had excluded, for possible enrollment in this study, patients with postural orthostatic tachycardia syndrome (POTS) as spironolactone is a potassium-sparing diuretic that can lead to a decreased blood volume, potentially exacerbating POTS symptoms, such as tachycardia (rapid heart rate) and light-headedness when standing [Citation15].
To match numbers in the SOT treatment group, the control group consisted of eight age-gender matched treatment-naïve ME/CFS patients (control; age: 52 ± 8), who were randomly selected from our ongoing study which aims to develop neuroimaging biomarkers for enhanced ME/CFS diagnosis [Citation15], to match the gender and age demographics of those in the SOT group[Citation16].
All participants were screened for eligibility according to inclusion/exclusion criteria described in our protocol paper [Citation16]. ME/CFS diagnosis was based on Canadian Consensus Criteria (CCC) [Citation17,Citation18]. Dr. do Campo initially diagnosed all SOT patients based on CCC criteria. Their diagnosis was reconfirmed when they independently participated in our study as they answered a CCC-based questionnaire and through clinical interviews with clinicians (RK and PD). Participants were asked to stop all medications influencing the brain for at least 7 days before their MRI scan, excluding the SOT regime in the group taking it.
Data collections
Upon inclusion, participants were asked to complete self-reported questionnaires and undergo an MRI scan at our site. Participants filled out online questionnaires to aid in understanding ME/CFS symptoms, including the 36-item Short-Form (SF-36) Health Survey and Hospital Anxiety and Depression Scale (HADS). Furthermore, a clinical assessment questionnaire based on the CCC criteria (CCC-JDC score; Supplementary Material) was collected from participants within the SOT group at baseline (pre-treatment) and prior to the MRI scan of the current study to assess the effectiveness of treatment on ME/CFS symptoms.
MRI data acquisition and pre-processing
MRI data was acquired using a 3 T MRI scanner with a 64-channel head coil (Skyra, Siemens). Participants completed a Fatigue State Questionnaire [Citation19] before and after the MRI scan. MRI data, including T1-weighted structural MRI and DWI, were collected as described in the study protocol [Citation16]. The DWI pre-processing was performed as described in as described in a previous study [Citation20].
In brief, the DWI data were denoised (MRtrix-dwidenoise [Citation21]), corrected for susceptibility distortion (FSL-Topup [Citation22]), corrected for eddy current (FSL-eddy_openmp [Citation23]), corrected for field bias (MRtrix-dwibiascorrect [Citation24]), and skull stripped (MRtrix-dwi2mask). To estimate the DTI metrices, we used the MRtrix-dwi2tensor to compute the corresponding tensor components, then used MRtrix-tesnor2metric to generate the eigenvector maps used to estimate the fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity (AD), and radial diffusivity (RD). Tract-based spatial statistics was used for the voxel-based analysis of the DTI data [Citation25]. To normalize the data, the FA maps of individuals were normalized to the FMRIB58 template-space using ANTs [Citation26], then the transformation and warp files were used to normalize the rest of the maps (i.e. AD, RD, and ADC) to the FMRIB58 template. The group mean FA images were generated and then skeletonized to identify the centers of all WM tracts with a threshold FA value of 0.2.
Statistical analysis
One-way analysis of variance was performed using SPSS (2022) to compare the symptoms and clinical scores between the SOT and control groups with Bonferroni correction (PBonf ≤ .05). To compare diffusion measures (i.e. FA, MD, RD, and ADC) between SOT and control groups, we ran voxel-based analyses using ‘FSL-randomise’ with 5000 permutations and family-wise error correction (PFWE ≤ .05) for multiple comparisons. In addition, general linear modeling was performed to test correlations between the changes in the clinical scores and between DTI measures. Sex, age, and BMI were used as nuisance covariates in all analyses.
Results
Demographics and symptoms
Demographics of the study groups are shown in . Compared to the control group, the SOT group showed reduced HADS depression (PBonf = 0.04) and increased SF-36 – Mental Health (PBonf = 0.01; ), with no significant differences in the Fatigue State Questionnaire levels between before and after MRI scans in any of the groups. Patients in the SOT group also showed lower clinical scores at the post-treatment (mean ± SD = 10.86 ± 4.49; PBonf < .001) compared to baseline scores (20.25 ± 2.92), based on CCC-JDC (Supplementary Material).
Table 1. Demographics and assessment results.
DWI results
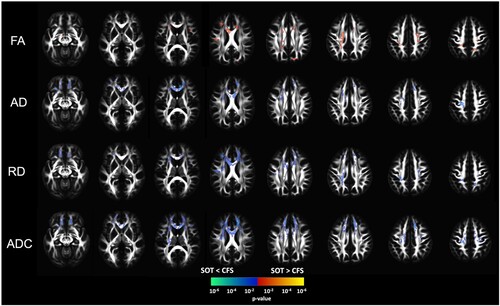
Our analysis of the DTI measures revealed widespread significant changes of higher FA and lower diffusivity measures (AD, ADC, and RD) in the SOT group as compared to the control group (PFWE ≤ 0.05). Compared to the control group, the SOT group showed higher FA values in the body of corpus callosum, bilateral anterior corona radiata, superior longitudinal fasciculus, and superior frontal-occipital fasciculus, and left posterior corona radiata, internal capsule, cerebral peduncle, and cortico-spinal tract (). The same white-matter tracts showed lower RD and ADC. Furthermore, compared to the control group, the SOT group showed lower AD, with some degree of overlap with white-matter tracts that showed increased FA, including genu and body of corpus callosum, cingulum, anterior corona radiata, superior corona radiata, and external capsule (). No significant correlations were observed between the change in clinical and between-groups DTI measures (PFWE > .05).
Figure 1. Voxel-based analysis of statistical group differences (two-sample t-test) in the different DTI measures between the ME/CFS patients taking spironolactone (SOT) and those who are not taking spironolactone (CFS). Results show that, compared to the control group, the SOT group had higher fractional anisotropy (FA) and lower diffusivity measures (AD, RD, ADC). All results were corrected for multiple comparisons using family wise error correction with a threshold of p ≤ .05.
Discussion
This report investigated the potential effectiveness of synergetic oral treatment for ME/CFS. The SOT group reported symptom improvement and exhibited reduced depression, mental burden, and clinical CCC-JDC scores as compared to the control group. Furthermore, the SOT group exhibited higher FA and lower diffusivity measures than the control group, suggesting the treatment may have a mitigating effect on the extent of microstructural changes associated with ME/CFS.
However, it is important to interpret these findings cautiously due to limitations inherent to case reports. The small sample size may result in false positive or negative findings. Additionally, multiple medications, including spironolactone, colchicine, LDN, and multivitamins, make it difficult to pinpoint the primary cause of difference. Furthermore, the absence of a sham control group may introduce placebo effects. Finally, the recruitment of patients from different resources might be another limitation. To address these limitations, formal randomized clinical trials including sham groups are necessary for a more comprehensive investigation of the treatments’ effectiveness in ME/CFS.
Our findings revealed significant improvements in ME/CFS symptoms in the treatment group, as shown by the HADS-depression and SF-36 Mental health compared to the control group, and improved CCC scores within the SPL group comparing pre – and post – treatment. In one study spironolactone was found to improve pain, mood, and quality of life in women with fibromyalgia within 4–6 weeks of treatment and persisted over 12 months [Citation27], but was not significantly evident in a later clinical trial [Citation28]. Another double-blinded study showed that spironolactone reduced negative mood, depression, and somatic symptoms in women with premenstrual syndrome when compared to a placebo group [Citation29]. This suggests that spironolactone may hold promise in alleviating the symptoms associated with ME/CFS, with future comprehensive research required to evaluate the treatments’ effectiveness more fully.
The observed increased FA and reduced diffusivity measures in the SOT group suggest one or more potential treatments of the underlying alterations associated with ME/CFS pathology or the potentiation of alternative pathways that may alleviate ME/CFS symptoms. A study has shown spironolactone reduces lesion progression within white matter in hypertension patients, independent of changes in blood pressure [Citation30], suggesting the spironolactone to have a positive impact of on white matter tracts, regardless of its effect on blood pressure, as a therapeutic avenue for managing ME/CFS.
Possible biological pathways for the treatment
A conclusive understanding of the underlying mechanisms that explain the potential effectiveness of spironolactone, colchicine, and LDN, specifically in ME/CFS, remains challenging. However, considering the existing hypothesis that inflammation is a crucial underlying mechanism in ME/CFS [Citation4,Citation7,Citation31], it is plausible to consider that spironolactone, colchicine and/or LDN may each exert their benefits by interfering with the ongoing inflammation associated with chronic fatigue syndrome, thereby improving the patients’ condition.
Moreover, previous studies have indicated that the onset of ME/CFS is often associated with Epstein–Barr virus. A recent study has demonstrated that spironolactone inhibits the SM protein function and production of the Epstein–Barr virus [Citation11]; suggesting that the use of spironolactone could potentially reduce the reactivation of the Epstein–Barr virus by blocking its pathway for replication. Similarly, spironolactone has been suggested to be beneficial in the prevention and treatment of various diseases, including the novel coronavirus (SARS-Covid-19) [Citation10,Citation32], which may lead to long-covid; a health condition highly overlapping with ME/CFS symptoms. Spironolactone was also found to inhibit the microglial activity, and was suggested as a potential viable therapeutic option for multiple sclerosis [Citation33].
Moreover, colchicine is well-known for its anti-inflammatory properties [Citation12] and appears to be useful in the management of COVID-19 infection [Citation34] Colchicine was suggested to interfere with various inflammatory pathways through inflammatory activation and proinflammatory cytokine release [Citation12]. By controlling the inflammatory responses, colchicine may alter the underlying inflammatory mechanisms for ME/CFS and contribute to the improved symptoms [Citation12].
Low-dose naltrexone (LDN) has been suggested to help patients with ME/CFS, although 18.3% of patients did not show improvement [Citation13]. The identification of Transient Receptor Potential Melastatin 3 (TRPM3) as a nociceptor channel influenced by certain opioid receptors and involved in calcium-dependent Natural Killer (NK) cell functions suggests that targeting TRPM3 through LDN induction could be a potential treatment for ME/CFS symptoms [Citation35]. LDN was also shown to enhancing the endorphin effect to reduce pain and boost pleasure [Citation36], potentially improving the quality of life for ME/CFS patients. Clinical trials are currently in progress to determine the effectiveness of LDN for treating fibromyalgia, a condition that significantly overlaps with ME/CFS [Citation37]. Therefore, the use of these treatments, either separately or together, might be effective in helping ME/CFS patients.
This pioneering study provides the first evidence of potential effectiveness of one or more components of SOT in helping alleviate ME/CFS symptoms by potentially influencing mood regulation, modulating brain microstructure, and possibly modulating inflammation.
Consent for publication
No individual information was used in the preparation of any of the figures. All data used in the preparation of the manuscript were of group levels, without any individual images or personal information used in the preparation of the manuscript.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgment
We wish to acknowledge our radiographer Dr. Natali Naude for her help collecting the data used in the current manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Abdalla Z. Mohamed
Dr. Abdalla Z. Mohamed is a post-doctoral research associate at New York University Abu Dhabi and previously held a post-doctoral research fellow position at the Thompson Institute, University of the Sunshine Coast. His research leverages advanced neuroimaging techniques to identify neural biomarkers for various clinical conditions, including trauma, dementia, and chronic fatigue syndrome.
Jorge do Campo
Jorge do Campo is a fellow of the Royal Australian College of Physicians and a Senior Medical Officer at the Department of Medicine at Bundaberg Hospital, Queensland Health.
Peter Del Fante
Dr. Peter Del Fante is a General Practitioner, Public Health Physician, and Clinical Informatician in Adelaide, with expertise in Digital Health, Preventative Medicine, Geo-Spatial Medicine, and Health Data Analytics, and a background in Physics and Neuroscience. Since 1992, he has focused on translating ME/CFS research into clinical practice, developing the ME/CFS Clinical Guidelines for SA Health in 2004, and advising the SA ME/CFS Society. His collaboration with Australian researchers aims to advance ME/CFS brain research through neuroimaging to improve diagnosis and understand the neurological aspects of the disease, potentially leading to new therapeutic interventions.
Richard Kwiatek
Dr. Richard Kwiatek is a consultant rheumatologist and clinical researcher at the Thompson Institute, University of the Sunshine Coast, Queensland, Australia. Through his research collaborations with other Australian researchers, he aim to help improve the quality of life and diagnosis of chronic fatigue syndrome and fibromyalgia through neuroimaging and clinical investigations.
Vince D. Calhoun
Professor Vince D. Calhoun is the founding director of the tri-institutional Center for Translational Research in Neuroimaging and Data Science (TReNDS) and a Georgia Research Alliance eminent scholar in brain health and image analysis where he holds appointments at Georgia State University, Georgia Institute of Technology and Emory University. Dr. Calhoun is a fellow of the Institute of Electrical and Electronic Engineers, The American Association for the Advancement of Science, The American Institute of Biomedical and Medical Engineers, The American College of Neuropsychopharmacology, The Organization for Human Brain Mapping (OHBM) and the International Society of Magnetic Resonance in Medicine.
Zack Y. Shan
Associate Professor Zack Y. Shan leads the Chronic Fatigue Syndrome (CFS) research program at the Thompson Institute, University of the Sunshine Coast. He is an Associate Professor of Neuroimaging, specialising in the study of brain disorders using MRI techniques.
References
- Bjørklund G, Dadar M, Pen JJ, et al. Chronic fatigue syndrome (CFS): suggestions for a nutritional treatment in the therapeutic approach. Biomed Pharmacother. 2019;109:1000–1007. doi:10.1016/j.biopha.2018.10.076
- Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci U S A. 2017;114:E7150–E7158.
- Fletcher MA, Zeng XR, Barnes Z, et al. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. doi:10.1186/1479-5876-7-96
- Russell L, Broderick G, Taylor R, et al. Illness progression in chronic fatigue syndrome: a shifting immune baseline. BMC Immunol. 2016;17:1–11. doi:10.1186/s12865-016-0142-3
- Farkas G, Márton J, Nagy Z, et al. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neurosci Lett. 1998;242:147–150. doi:10.1016/S0304-3940(98)00060-3
- Soilu-Hänninen M, Erälinna JP, Hukkanen V, et al. Semliki forest virus infects mouse brain endothelial cells and causes blood-brain barrier damage. J Virol. 1994;68:6291–6298. doi:10.1128/jvi.68.10.6291-6298.1994
- Nakatomi Y, Mizuno K, Ishii A, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An11C-(R)-PK11195 PET study. J Nucl Med. 2014;55:945–950. doi:10.2967/jnumed.113.131045
- do Campo J, Taylor V. Clinical improvement in patients with ME/CFS with synergistic effect of colchicine and spironolactone targeting inhibition of inflammasome activity. Internal Medicine Journal, Abstracts for the RACP Congress 2022, A Climate for Change, 12–14 May 2022, Melbourne, Victoria, Australia and Online. John Wiley & Sons, Ltd; 2022. p. 13–13.
- Mohamed AZ, do Campo J, Del Fante P, et al. Combined oral treatment ameliorates symptoms and white matter microstructural integrity in myalgic encephalomyelitis/chronic fatigue syndrome [Abstract]. Int J Rheum Dis. 2024;27(Suppl. 2):e15172.
- Cadegiani FA, Goren A, Wambier CG. Spironolactone may provide protection from SARS-CoV-2: targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS). Med Hypotheses. 2020;143:110112.
- Verma D, Thompson J, Swaminathan S, et al. Spironolactone blocks Epstein-Barr virus production by inhibiting EBV SM protein function. Proc Natl Acad Sci U S A. 2016;113:3609–3614. doi:10.1073/pnas.1523686113
- Leung YY, Yao Hui LL, Kraus VB. Colchicine – update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi:10.1016/j.semarthrit.2015.06.013
- Polo O, Pesonen P, Tuominen E. Low-dose naltrexone in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Fatigue. 2019;7:207–217.
- Kučić N, Rački V, Šverko R, et al. Immunometabolic modulatory role of naltrexone in bv-2 microglia cells. Int J Mol Sci. 2021;22(16):8429. doi:10.3390/ijms22168429
- Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83(981):478–80. doi:10.1136/pgmj.2006.055046
- Shan ZY, Mohamed AZ, Andersen T, et al. Multimodal MRI of myalgic encephalomyelitis/chronic fatigue syndrome: a cross-sectional neuroimaging study toward its neuropathophysiology and diagnosis. Front Neurol. 2022;13:954142. doi:10.3389/fneur.2022.954142
- Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chronic Fatigue Syndr. 2003;11:7–115. doi:10.1300/J092v11n01_02
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Chapter 3: Current Case Definitions and Diagnostic Criteria, Terminology, and Symptom Constructs and Clusters. 2015; Washington (DC): National Academies Press (US); Available from: https://www.ncbi.nlm.nih.gov/books/NBK284898/.
- Greenberg S, Aislinn P, Kirsten D. Development and validation of the Fatigue State Questionnaire: preliminary findings. Open Psychol J. 2016;9:50–65. doi:10.2174/1874350101609010050
- Mohamed AZ, Lagopoulos J, Nasrallah FA, et al. Self-reported fatigue was associated with increased white-matter alterations in long-term traumatic brain injury and posttraumatic stress disorder patients. Neuroscience. 2023;520:46–57.
- Cordero-Grande L, Christiaens D, Hutter J, et al. Complex diffusion-weighted image estimation via matrix recovery under general noise models. Neuroimage. 2019;200:391–404. doi:10.1016/j.neuroimage.2019.06.039
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi:10.1016/S1053-8119(03)00336-7
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi:10.1016/j.neuroimage.2015.10.019
- Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi:10.1109/TMI.2010.2046908
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi:10.1016/j.neuroimage.2006.02.024
- Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi:10.1016/j.neuroimage.2010.09.025
- Wernze H, Herdegen T. Long-term efficacy of spironolactone on pain, mood, and quality of life in women with fibromyalgia: an observational case series. Scand J Pain. 2014;5:63–71. doi:10.1016/j.sjpain.2013.12.003
- Böhm R, Westermann P, Gleim M, et al. High-dose spironolactone lacks effectiveness in treatment of fibromyalgia (RCT). Eur J Pain. 2021;25:1739–1750. doi:10.1002/ejp.1784
- Wang M, Hammarbäck S, Lindhe B, et al. Treatment of premenstrual syndrome by spironolactone: a double-blind, placebo-controlled study. Acta Obstet Gynecol Scand. 1995;74:803–808. doi:10.3109/00016349509021201
- Yuan Y, Heizhati M, Liu Y, et al. Spironolactone is associated with reduced white matter lesion progression in patients with hypertension. Eur J Intern Med. 2023;114:146–149.
- Nakatomi Y, Kuratsune H, Watanabe Y. Neuroinflammation in the brain of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Brain Nerve. 2018;70:19–25.
- Wilcox CS, Pitt B. Is spironolactone the preferred renin-angiotensin-aldosterone inhibitor for protection against COVID-19? J Cardiovasc Pharmacol. 2020;77:323–331. doi:10.1097/FJC.0000000000000960
- Samanani S, Mishra M, Silva C, et al. Screening for inhibitors of microglia to reduce neuroinflammation. CNS Neurol Disord Drug Targets. 2013;12:741–749. doi:10.2174/18715273113126660177
- Danjuma MI, Sayed R, Aboughalia M, et al. Does colchicine reduce mortality in patients with COVID-19 clinical syndrome? An umbrella review of published meta-analyses. Heliyon. 2023;9(10):e20155. doi:10.1016/j.heliyon.2023.e20155
- Cabanas H, Muraki K, Eaton-Fitch N, et al. Potential therapeutic benefit of low dose naltrexone in myalgic encephalomyelitis/chronic fatigue syndrome: role of transient receptor potential melastatin 3 ion channels in pathophysiology and treatment. Front Immunol. 2021;12:687806. doi:10.3389/fimmu.2021.687806
- Ludwig MD, Zagon IS, McLaughlin PJ. Featured article: serum [Met5]-enkephalin levels are reduced in multiple sclerosis and restored by low-dose naltrexone. Exp Biol Med. 2017;242(15):1524–1533. doi:10.1177/1535370217724791
- Bruun KD, Amris K, Vaegter HB, et al. Low-dose naltrexone for the treatment of fibromyalgia: protocol for a double-blind, randomized, placebo-controlled trial. Trials. 2021;22:804.
