ABSTRACT
Background
Congenital aniridia is a rare genetic disorder of the eye characterized by visual impairment and progressive vision loss. While prior research has focused on ocular manifestations in individuals with aniridia, there is a dearth of research on impacts on cognition and mental health. The aims of this study were to describe subjective symptoms of everyday executive functioning, fatigue and sleepiness in adults with aniridia and to compare self-reported health status with that of a normative reference group.
Methods
Twenty-nine adults (aged 18–79 years) with congenital aniridia were included in this online survey, of whom 52% were females. Participants completed self-report measures of executive functioning (The Behavior Rating Inventory of Executive Function–Adult Version), sleepiness, fatigue, and health status (EQ-5D-5L).
Results
Participants reported relatively few problems in everyday executive functioning, with only 14% experiencing impaired executive functioning. Scores on the five EQ-5D-5L domains (mobility, self-care, usual activities, pain, and anxiety/depression) did not differ from those of the normative reference group. The frequencies of excessive daytime sleepiness and severe fatigue were 17% and 38%, respectively. Ocular pain was experienced by 62% of participants.
Conclusions
The findings show that cognitive problems are related to and reflect self-reported health status and extent of fatigue. Moreover, those who suffered from ocular pain reported more difficulties with executive functioning, sleepiness and fatigue. These findings are important for understanding this disorder and supporting patients.
Introduction
Congenital aniridia is a rare genetic disorder of the eye (OMIM # 106210) in which individuals are born with impaired vision that reduces further during life in most cases (Landsend et al., Citation2018; Orphanet, Accessed February Citation23, Citation2023). Ocular manifestations in aniridia include corneal disease, glaucoma, cataracts and underdevelopment of the retina and optic nerve, which can vary in severity (Kit et al., Citation2021; Landsend et al., Citation2021; Netland et al., Citation2011). In most cases, congenital aniridia is caused by pathogenic variants of the PAX6 gene located on chromosome 11p13 (Prosser & van Heyningen, Citation1998). The reported prevalence of aniridia in Norway and Sweden is 1:72,000 (Eden et al., Citation2008).
PAX6 is specifically involved in eye and early neural development (Grant et al., Citation2021; Ochi et al., Citation2022; Yogarajah et al., Citation2016). Prior research on aniridia has largely focused on ocular complications. However, a systematic literature review suggested that pathogenic variants of PAX6 may also negatively affect brain health (Grant et al., Citation2021). Therefore, more research is needed to better understand the neurological and cognitive implications of pathogenic variants of PAX6 (Grant et al., Citation2021).
Central nervous system abnormalities have been identified in the anterior and posterior commissure, pineal gland, corpus callosum, and anterior cingulate cortex of individuals with aniridia (Berntsson et al., Citation2020; Ellison-Wright et al., Citation2004; Grant et al., Citation2017; Hanish et al., Citation2016; Yogarajah et al., Citation2016). In addition, adults with PAX6-associated aniridia exhibited an age-related decrease in cortical thickness in the inferior parietal lobe, prefrontal areas, and premotor areas in both hemispheres compared to the control group (Yogarajah et al., Citation2016). Damage to the prefrontal lobe is known to lead to personality and behavioral changes as well as deficits in executive functioning, i.e. abstract reasoning, mental flexibility, and behavioral inhibition (Rabinowitz & Levin, Citation2014). Executive functioning is defined as a set of interrelated cognitive processes required for complex goal-directed activity (Henri-Bhargava et al., Citation2018).
Functional neural abnormalities, such as reduced olfaction, deficits in working memory, and altered auditory processing, have been described in individuals with aniridia (Bamiou et al., Citation2007; Sisodiya et al., Citation2001; Thompson et al., Citation2004; Yogarajah et al., Citation2016). Previous research has shown that individuals with aniridia may display worse performance on IQ tests and higher rates of psychiatric disorders than controls (Ellison-Wright et al., Citation2004; Heyman et al., Citation1999; Malandrini et al., Citation2001). In contrast, other studies have not found cognitive dysfunction in adults with aniridia after comparing their performance with that of healthy controls on neuropsychological assessment (Han et al., Citation2013; Thompson et al., Citation2004). Several studies have reported sleeping disorders and low melatonin levels associated with PAX6-associated aniridia (Hanish et al., Citation2016; Hanish & Han, Citation2018). In chronic disorders, nondisease-specific problems such as motivational and concentration problems, pain, sleep disturbances and reduced activity seem to explain fatigue much better than the diagnosis in itself (Menting et al., Citation2018). There is evidence that mental health and quality of life are negatively impacted across a range of ophthalmic disease populations (Chaumet-Riffaud et al., Citation2017; Klauke et al., Citation2023; Langelaan et al., Citation2007; Senra et al., Citation2022).
Aniridia may impose multiple challenges regarding daily functioning, cognition and general health. To our knowledge, no studies have evaluated self-reported cognitive complaints, health status or ocular pain in the aniridia population. Prior research has indicated that health-related quality of life is worse among low vision populations than among the general population (Crews et al., Citation2016; Langelaan et al., Citation2007). It is also clinically meaningful to explore cognitive symptoms in individuals with aniridia, as such symptoms may reflect underlying brain dysfunction. The aims of this study were to describe subjective symptoms (everyday executive dysfunction, sleepiness and fatigue) in individuals with aniridia and to compare the self-reported health status of adults with aniridia with that of a normative reference group (Garratt et al., Citation2022) and with that of individuals with other eye diseases (Senra et al., Citation2022). Because aniridia is an eye disorder and individuals may experience ocular pain, the final aim was to determine whether experiencing ocular pain was associated with more severe symptoms of everyday executive dysfunction, fatigue and sleepiness.
Materials and methods
Participants and procedure
Participants with aniridia were recruited from the Norwegian Association of Aniridia mailing list and from the user register at the Centre for Rare Disorders, Oslo University Hospital, Norway. The inclusion criteria were as follows: aged 16 years or older, had a genetically and/or clinically verified congenital aniridia diagnosis, and were able to read and write in Norwegian. Individuals with traumatic or post-surgical aniridia were excluded. An e-mail invitation and two reminders were sent to 49 individuals (aged 16–84 years) from May to August 2022. The invitation included a link to an online survey, containing informed consent forms and questionnaires. For signing the consent, login with a Norwegian Bank-ID was used for safe identification. As a part of the consent, participants authorized researchers to retrieve information from their medical records about previous genetic tests. If genetic analyses had not been performed or a disease-causing variant had not been detected, the participant was offered genetic testing to identify disease-causing variants related to congenital aniridia. A secure online data collection platform approved by Oslo University Hospital, Norway was used to administer the survey. Twenty-nine adults with aniridia participated, yielding a response rate of 59%.
Instruments
The online survey collected demographic information and contained study-specific questions about health, prior and current mental and/or medical diagnoses, and prior and current health care follow-ups. Participants were asked about their age at diagnosis as well as to provide genetic test results. The research group formulated questions on participant background. Prior to inclusion, two adults with aniridia completed the forms (consent form, background-related questions) and questionnaires.
The Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A) is a standardized measure that assesses everyday executive dysfunction and contains 75 items on specific problems in daily life that are rated from 1 (never) to 3 (often) (Roth & Gioia, Citation2005). The BRIEF-A was developed for individuals 18–90 years of age. Scores are reported on two indices (Behavior Regulation Index, Metacognitive Index) and a composite index (Global Executive Composite) and nine subscales (inhibit, shift, emotional control, self-monitor, initiate, working memory, plan/organize, task monitor, and organization of materials). The BRIEF-A scoring program calculates age-specific norms, expressed as T scores (mean: 50, standard deviation: 10), with higher scores indicating greater executive dysfunction in daily activities. A T score ≥ 65 is used as the clinical cut-off in each domain to indicate impaired executive functioning.
The Epworth Sleepiness Scale (EES) includes eight questions referring to soporific situations (Johns, Citation1991). Responses are provided on a scale from 0 (would never doze) to 3 (high chance of dozing), with excessive daytime sleepiness defined as a score ≥ 10.
The Chalder Fatigue Scale (CFQ) is a measure of fatigue that contains 11 items (Chalder et al., Citation1993), with responses reported on a scale from 0 (better than usual) to 3 (much worse than usual). It yields a total score (0–33) as well as scores on physical (range: 0–21) and mental fatigue (range: 0–12). The questionnaire allows bimodal scoring for fatigue (scores 0–1 = 0 and scores 2–3 = 1), with a cut-off score ≥ 4 (bimodal score of the 11 items) considered to meet the criteria for severe fatigue.
The EuroQoL five dimensions with five severity levels (EQ-5D-5L) is a generic health status instrument that consists of five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (Nord, Citation1991). Each domain has five levels ranging from ‘no problems’ to ‘extreme problems/unable to’ (scores 1 - 5). The EQ-5D-5L index values were based on the UK value set as used in Norway (Garratt et al., Citation2022). In this study, descriptive levels of each dimension were dichotomized to ‘no problems’ (level one) or ‘any problems’ (levels two to five) and compared to Norwegian normative data (Garratt et al., Citation2022). Permission to use the EQ-5D-5L (Norwegian language) was obtained from the EuroQoL Group.
Ocular pain was assessed as the presence or absence of pain (binary: yes/no), with responses provided for daily, weekly, monthly, or less than monthly periods. Ocular pain intensity was rated using a visual analogue scale from 0 (no pain) to 10 (worst imaginable pain).
Ethics statement
All protocols and methods were approved by the Norwegian Regional Committee for Medical Research Ethics in southeastern Norway (number 172031) and the Institutional Data Protection Officer (20/25374). Informed consent was obtained from all participants.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 28 (IBM Corporation, Chicago).
The normality of data distributions were examined with the Shapiro‒Wilk test. Descriptive statistics for continuous variables are summarized with the means (M) and standard deviations (SD) for normally distributed data and with the medians and interquartile ranges (IQRs) for nonnormally distributed data. Categorical data are presented as frequencies. Student’s t test or the Mann‒Whitney U test was conducted to assess differences between sexes and between ocular pain groups. Participants who reported ocular pain were compared to participants without ocular pain. Bivariate Pearson correlation analyses were conducted to examine the relationships between all scales. Statistical significance was determined at p < 0.05.
Results
Sample characteristics
Demographic and health information is presented in . Among the 29 participants, the median (IQR) age was 37 (32) years, the age range was 18–79 years, 48% were male, 48% had > 12 years of education, 52% were single and 38% were employed or students at the time of the study. Most of the participants (59%) had family members with aniridia. Only three individuals had a driving licence and were able to drive. Regarding eye health, most individuals (72%) had undergone eye surgeries, 62% had glaucoma and 52% had keratopathy. At the time of the study, 62% reported that they had sleeping problems.
Table 1. Participants’ demographics and self-reported health problems (N = 29).
Scores on the BRIEF-A scales and indices are summarized in . Scores on four of the BRIEF-A scales violated the assumption of normal distribution (Shapiro‒Wilk test of normality: p < 0.05); therefore, the mean (SD) and median (IQR) values are presented for all subscales. Four individuals (14%) reported impaired executive functioning (T score ≥ 65) as indicated by scores on the Global Executive Composite index. One-fifth of the participants had scores over 65 on the Task Monitor Scale.
Table 2. Scores on the Behavior Rating Inventory of Executive Function-Adult Version subscales and indices.
As shown in , significant sex differences were found only in the Global Executive Composite score (p < 0.05). Seventeen percent of participants reported excessive daytime sleepiness (ESS score ≥ 10), 38% met the criteria for fatigue (CFQ score ≥ 4) and 62% reported ocular pain. The median (IQR) and mean (SD) EQ-5D-5L index scores were 0.86 (0.19) and 0.85 (0.15), respectively.
Table 3. Scores on symptoms scales and frequency of symptoms by sexes.
Within the group of participants with ocular pain (n = 18), 53% experienced daily to weekly pain, with a mean (SD) score of ocular pain of 3.8 (1.8) on the visual analogue scale. Participants with ocular pain scored significantly worse on the Metacognition Index and Global Executive Composite (p < 0.05) than participants without ocular pain (). No significant differences were found in daytime sleepiness (p = 0.07) or fatigue (p = 0.05) between the ocular pain group and the group without ocular pain. Comparison of scores on the EQ-5D-5L did not reveal significant differences in scores between the two groups (Mann‒Whitney U test: U = 78.0; z = −0.96; p = 0.338).
Table 4. Comparison between participants with ocular pain (n = 18) and no ocular pain (n = 11).
The most frequently affected domains on the EQ-5D-5L (any problems from slight to severe) were pain (65%), anxiety/depression (43%), and usual activities (38%) (, ). In the domains of anxiety/depression, 10% of participants reported severe problems. None of the participants reported ‘extreme problems’ on any of the five domains. No significant differences were found between the aniridia sample and the Norwegian general population (Garratt et al., Citation2022) in the prevalence of the five domains of the EQ-5D-5L () or the index score. Regarding the comparison of the aniridia sample with populations with other chronic eye diseases (Senra et al., Citation2022), there were no significant differences between adults with aniridia (n = 29, M = 0.85, SD = 0.15) and adults with other eye diseases (n = 71, M = 0.82, SD = 0.20) on the EQ-5D-5L index score (t = 0.82, df = 54, p = 0.825).
Figure 1. Single dimensions by levels for the EQ-5D-5L in aniridia (N = 29).
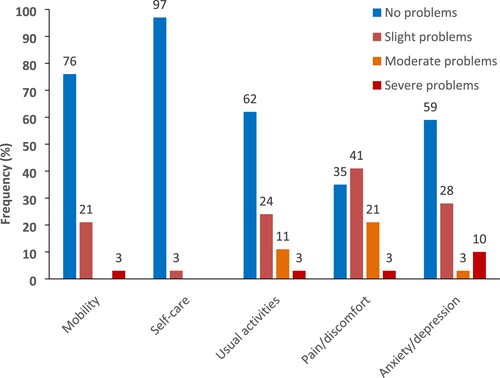
Table 5. Proportions of adults with aniridia (N = 29) reporting problems across the five domains of the EQ-5D-5L. Comparison with the normative data in Norway (N = 3120) #
As shown in , the correlation coefficients of the relationships between the Metacognition Index (BRIEF-A) and all the other scales were statistically significant (p < 0.05; r = 0.39–0.82). Scores on the CFQ mental scale were also correlated with scores on all the other scales (p < 0.05; r = 0.37–0.49). There were no significant associations between age and scores on the symptom scales.
Table 6. Correlations.
Discussion
To the best of our knowledge, this is the first study to describe the everyday executive functioning and self-reported health status of adults with aniridia. Importantly, adults with aniridia reported relatively few problems in daily executive functioning. Furthermore, this aniridia sample reported a similar frequency of health problems (EQ-5D-5L) as the general Norwegian population (Garratt et al., Citation2022). Their EQ-5D-5L index scores were also similar to those of adults with other chronic eye diseases (Langelaan et al., Citation2007; Senra et al., Citation2022). Pain/discomfort was the most frequently (65%) affected health domain of the EQ-5D-5L, and pain/discomfort appeared higher in this aniridia sample than in other populations with ophthalmic diseases (Langelaan et al., Citation2007; Macedo et al., Citation2022). The EQ-5D-5L has been widely used in quality-of-life research in populations with chronic conditions and ophthalmic diseases (Langelaan et al., Citation2007; Senra et al., Citation2022; Zhou et al., Citation2021). However, this generic measure may underestimate the impact of impaired vision or cognitive difficulties on the health status of individuals with aniridia because these domains are not included in the EQ-5D-5L.
The scores on the BRIEF-A were well within normal limits in this study, suggesting that adults with aniridia exhibit good cognitive functioning in everyday life. However, a subgroup of participants (14%) reported impairment in various areas of executive functioning (Global Executive Composite), and 21% reported problems with their ability to assess their own performance during a task (Task Monitor). Self-reported cognitive complaints are not necessarily related to cognitive dysfunctions. Individuals may markedly underreport their daily difficulties compared to objective results on neuropsychological tests, or they may overreport their competency compared to the reports of family members. While prior studies on aniridia patients have described deficits observed on neuropsychological tests (Heyman et al., Citation1999; Yogarajah et al., Citation2016) and developmental delay (Grant et al., Citation2017; Grant et al., Citation2021; Netland et al., Citation2011), complete neuropsychological testing can be difficult for individuals with aniridia due to their vision loss. In this regard, neuropsychological tests may not fully evaluate cognitive dysfunction in aniridia. Although scores on the BRIEF-A do not reflect actual cognitive functioning, this instrument may be considered a complimentary measure regarding cognitive functioning in ophthalmic disease populations with vision loss and visual impairment.
Scores on the EQ-5D-5L and the BRIEF-A indices were significantly correlated in the present study. There were also strong correlations between the BRIEF-A indices and mental fatigue (CFQ scores), indicating that cognitive problems may often occur along with fatigue, as reported in other chronic disease populations (Menting et al., Citation2018). This is unexpected because the BRIEF-A does not include items assessing general health. However, there is an emotional component in the BRIEF-A that might influence these correlations. In that respect, our findings may suggest that problems with everyday executive functioning are related to and reflect an individual’s general health, including the extent of fatigue. Another study identified associations between BRIEF-A scores and emotional distress in neurological and neuropsychiatric patient groups (Løvstad et al., Citation2016).
Sixty-two percent of our participants experienced ocular pain. These individuals reported significantly more problems with executive functioning than those without ocular pain. They also tended to experience greater daytime sleepiness and fatigue than those without ocular pain. The majority of participants (72%) had undergone eye surgery, 62% reported having glaucoma, and 52% reported that keratopathy already affected vision or was about to do so. Ocular pain might be associated with the severity of patients’ eye disease (e.g. dry eyes, glaucoma, keratopathy) and the number of treatments they have received. The combination of all these factors could influence cognitive functioning in daily life. However, because of the small sample size and multiple comparisons, statistical analyses were not performed to compare subgroups according to eye surgeries or eye diseases with regard to ocular pain.
In our study, 62% of participants reported sleeping problems, 38% reported severe fatigue (CFQ score ≥ 4) and 17% reported excessive daytime sleepiness (EES scores ≥ 10). The instruments employed in this study did not capture insomnia, sleep onset latency or sleep disruption. The present findings only provide insight into sleep-related problems experienced by adults with aniridia. Another study on four family members with congenital aniridia found that all were diagnosed with sleep disorders and pineal gland hypoplasia, and one person was diagnosed with narcolepsy (Berntsson et al., Citation2020). Furthermore, a smaller size of the pineal gland (where melatonin is produced), lower melatonin secretion and greater sleep disturbance have been reported in children with aniridia compared to healthy controls (Hanish et al., Citation2016). A systematic literature review found that fatigue resulting from visual impairment was not associated with the severity of visual loss (Schakel et al., Citation2019). Mental fatigue was associated with executive functioning, quality of life, and daytime sleepiness in our study, all factors related to fatigue in other chronic disorders (Menting et al., Citation2018).
There are limitations of this study, including the following: its small sample size, the possibility of false-positives (given the number of comparisons conducted), and the low response rate (of 59%). Another limitation is that assessments were performed remotely via laptop, tablet and smartphone, and not in person. This might have affected the results and excluded individuals not using, or not being able to use computer programs. This is a descriptive study with a small sample size; therefore, no statistical causal conclusions or relationships can be drawn.
Implications for clinicians
Difficulties encountered by adults with aniridia during daily life are of utmost clinical importance to guide further assessment and health care. Although few participants reported impairments in daily executive functioning, the findings may suggest that such problems are related to and reflect an individual’s general health and extent of fatigue. Short screening tools that target problems in daily activities may be important, such as the EQ-5D-5L, visual analogue scales (of pain, fatigue) or simple questions regarding the frequency of ocular pain. Topics such as cognitive problems, sleep disturbances and fatigue should be explored in both children and adults, as such difficulties may affect academic achievement (in children) and work achievements (in adults). More research with a larger sample size is needed to confirm our findings and to elucidate how sleep and vision-related fatigue as well as ocular pain affect children, adolescents and adults with aniridia in daily life.
Ethics approval
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and was approved by an Institutional Review Board/Ethics committee. See details under Methods.
Acknowledgements
We gratefully acknowledge the assistance of the following members of Aniridia Norway: Sølvi Ørstenvik, Heidi Haug, and Tove Hauge. The authors would like to thank the participants for their contribution to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bamiou, D. E., Free, S. L., Sisodiya, S. M., Chong, W. K., Musiek, F., Williamson, K. A., van Heyningen, V., Moore, A. T., Gadian, D., & Luxon, L. M. (2007). Auditory interhemispheric transfer deficits, hearing difficulties, and brain magnetic resonance imaging abnormalities in children with congenital aniridia due to PAX6 mutations. Archives of Pediatrics & Adolescent Medicine, 161(5), 463–469. https://doi.org/10.1001/archpedi.161.5.463
- Berntsson, S. G., Kristoffersson, A., Daniilidou, M., Dahl, N., Ekstrom, C., Semnic, R., Markstrom, A., Niemela, V., Partinen, M., Hallbook, F., & Landtblom, A. M. (2020). Aniridia with PAX6 mutations and narcolepsy. Journal of Sleep Research, 29, https://doi.org/10.1111/jsr.12982
- Chalder, T., Berelowitz, G., Pawlikowska, T., Watts, L., Wessely, S., Wright, D., & Wallace, E. P. (1993). Development of a fatigue scale. Journal of Psychosomatic Research, 37(2), 147–153. https://doi.org/10.1016/0022-3999(93)90081-P
- Chaumet-Riffaud, A. E., Chaumet-Riffaud, P., Cariou, A., Devisme, C., Audo, I., Sahel, J. A., & Mohand-Said, S. (2017). Impact of retinitis pigmentosa on quality of life, mental health, and employment among young adults. American Journal of Ophthalmology, 177, 169–174. https://doi.org/10.1016/j.ajo.2017.02.016
- Crews, J. E., Chou, C. F., Zack, M. M., Zhang, X., Bullard, K. M., Morse, A. R., & Saaddine, J. B. (2016). The association of health-related quality of life with severity of visual impairment among people aged 40-64 years: Findings from the 2006-2010 behavioral risk factor surveillance system. Ophthalmic Epidemiology, 23(3), 145–153. https://doi.org/10.3109/09286586.2016.1168851
- Eden, U., Iggman, D., Riise, R., & Tornqvist, K. (2008). Epidemiology of aniridia in Sweden and Norway. Acta Ophthalmologica, 86(7), 727–729. https://doi.org/10.1111/j.1755-3768.2008.01309.x
- Ellison-Wright, Z., Heyman, I., Frampton, I., Rubia, K., Chitnis, X., Ellison-Wright, I., Williams, S. C., Suckling, J., Simmons, A., & Bullmore, E. (2004). Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. European Journal of Neuroscience, 19(6), 1505–1512. https://doi.org/10.1111/j.1460-9568.2004.03236.x
- Garratt, A. M., Hansen, T. M., Augestad, L. A., Rand, K., & Stavem, K. (2022). Norwegian population norms for the EQ-5D-5L: results from a general population survey. Quality of Life Research, 31(2), 517–526. https://doi.org/10.1007/s11136-021-02938-7
- Grant, M. K., Bobilev, A. M., Branch, A., & Lauderdale, J. D. (2021). Structural and functional consequences of PAX6 mutations in the brain: Implications for aniridia. Brain Research, 1756, 147283. https://doi.org/10.1016/j.brainres.2021.147283
- Grant, M. K., Bobilev, A. M., Pierce, J. E., DeWitte, J., & Lauderdale, J. D. (2017). Structural brain abnormalities in 12 persons with aniridia. F1000Research, 6, 255. https://doi.org/10.12688/f1000research.11063.2
- Han, J. C., Thurm, A., Golden Williams, C., Joseph, L. A., Zein, W. M., Brooks, B. P., Butman, J. A., Brady, S. M., Fuhr, S. R., Hicks, M. D., Huey, A. E., Hanish, A. E., Danley, K. M., Raygada, M. J., Rennert, O. M., Martinowich, K., Sharp, S. J., Tsao, J. W., & Swedo, S. E. (2013). Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex, 49(10), 2700–2710. https://doi.org/10.1016/j.cortex.2013.02.009
- Hanish, A. E., Butman, J. A., Thomas, F., Yao, J., & Han, J. C. (2016). Pineal hypoplasia, reduced melatonin and sleep disturbance in patients with PAX6 haploinsufficiency. Journal of Sleep Research, 25(1), 16–22. https://doi.org/10.1111/jsr.12345
- Hanish, A. E., & Han, J. C. (2018). Delayed onset of sleep in adolescents with PAX6 Haploinsufficiency. Biological Research For Nursing, 20(2), 237–243. https://doi.org/10.1177/1099800417753670
- Henri-Bhargava, A., Stuss, D. T., & Freedman, M. (2018). Clinical assessment of prefrontal lobe functions. Continuum (Minneap Minn), 24(3, behavioral neurology and psychiatry), 704-726. https://doi.org/10.1212/con.0000000000000609.
- Heyman, I., Frampton, I., van Heyningen, V., Hanson, I., Teague, P., Taylor, A., & Simonoff, E. (1999). Psychiatric disorder and cognitive function in a family with an inherited novel mutation of the developmental control gene PAX6. Psychiatric Genetics, 9(2), 85–90. https://doi.org/10.1097/00041444-199906000-00006
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14(6), 540–545. https://doi.org/10.1093/sleep/14.6.540
- Kit, V., Cunha, D. L., Hagag, A. M., & Moosajee, M. (2021). Longitudinal genotype-phenotype analysis in 86 patients with PAX6-related aniridia. JCI Insight, 6(14), https://doi.org/10.1172/jci.insight.148406
- Klauke, S., Sondocie, C., & Fine, I. (2023). The impact of low vision on social function: The potential importance of lost visual social cues. Journal of Optometry, 16(1), 3–11. https://doi.org/10.1016/j.optom.2022.03.003
- Landsend, E. C. S., Lagali, N., & Utheim, T. P. (2021). Congenital aniridia - A comprehensive review of clinical features and therapeutic approaches. Survey of Ophthalmology, 66(6), 1031–1050. https://doi.org/10.1016/j.survophthal.2021.02.011
- Landsend, E. S., Utheim, O. A., Pedersen, H. R., Lagali, N., Baraas, R. C., & Utheim, T. P. (2018). The genetics of congenital aniridia-a guide for the ophthalmologist. Survey of Ophthalmology, 63(1), 105–113. https://doi.org/10.1016/j.survophthal.2017.09.004
- Langelaan, M., de Boer, M. R., van Nispen, R. M., Wouters, B., Moll, A. C., & van Rens, G. H. (2007). Impact of visual impairment on quality of life: A comparison with quality of life in the general population and with other chronic conditions. Ophthalmic Epidemiology, 14(3), 119–126. https://doi.org/10.1080/09286580601139212
- Løvstad, M., Sigurdardottir, S., Andersson, S., Grane, V. A., Moberget, T., Stubberud, J., & Solbakk, A. K. (2016). Behavior rating inventory of executive function adult version in patients with neurological and neuropsychiatric conditions: Symptom levels and relationship to emotional distress. Journal of the International Neuropsychological Society, 22(6), 682–694. https://doi.org/10.1017/S135561771600031X
- Macedo, A. F., Hellström, A., Massof, R., Tuvesson, H., Rask, M., Ramos, P. L., Safipour, J., Marteinsdottir, I., Nilsson, E., Fagerström, C., & Årestedt, K. (2022). Predictors of problems reported on the EQ-5D-3L dimensions among people with impaired vision in northern Portugal. Health and Quality of Life Outcomes, 20(1), 132. https://doi.org/10.1186/s12955-022-02043-4
- Malandrini, A., Mari, F., Palmeri, S., Gambelli, S., Berti, G., Bruttini, M., Bardelli, A. M., Williamson, K., van Heyningen, V., & Renieri, A. (2001). PAX6 mutation in a family with aniridia, congenital ptosis, and mental retardation. Clinical Genetics, 60(2), 151–154. https://doi.org/10.1034/j.1399-0004.2001.600210.x
- Menting, J., Tack, C. J., Bleijenberg, G., Donders, R., Droogleever Fortuyn, H. A., Fransen, J., Goedendorp, M. M., Kalkman, J. S., Strik-Albers, R., van Alfen, N., van der Werf, S. P., Voermans, N. C., van Engelen, B. G., & Knoop, H. (2018). Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychology, 37(6), 530–543. https://doi.org/10.1037/hea0000598
- Netland, P. A., Scott, M. L., Boyle, J. W. T., & Lauderdale, J. D. (2011). Ocular and systemic findings in a survey of aniridia subjects. Journal of American Association for Pediatric Ophthalmology and Strabismus, 15(6), 562–566. https://doi.org/10.1016/j.jaapos.2011.07.009
- Nord, E. (1991). EuroQol©: health-related quality of life measurement. Valuations of health states by the general public in Norway. Health Policy, 18(1), 25–36. https://doi.org/10.1016/0168-8510(91)90141-J
- Ochi, S., Manabe, S., Kikkawa, T., & Osumi, N. (2022). Thirty years’ history since the discovery of Pax6: From central nervous system development to neurodevelopmental disorders. International Journal of Molecular Sciences, 23(11), https://doi.org/10.3390/ijms23116115
- Orphanet. (Accessed February 23, 2023). Disease: Isolated aniridia. https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=19593.
- Prosser, J., & van Heyningen, V. (1998). PAX6 mutations reviewed. Human Mutation, 11(2), 93–108. https://doi.org/10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M
- Rabinowitz, A. R., & Levin, H. S. (2014). Cognitive sequelae of traumatic brain injury. Psychiatric Clinics of North America, 37(1), 1–11. https://doi.org/10.1016/j.psc.2013.11.004
- Roth, R. M., & Gioia, G. A. (2005). Behavior rating inventory of executive function–adult version. Psychological Assessment Resources.
- Schakel, W., Bode, C., Elsman, E. B. M., van der Aa, H. P. A., de Vries, R., van Rens, G., & van Nispen, R. M. A. (2019). The association between visual impairment and fatigue: A systematic review and meta-analysis of observational studies. Ophthalmic and Physiological Optics, 39(6), 399–413. https://doi.org/10.1111/opo.12647
- Senra, H., Hernandez-Moreno, L., Moreno, N., & Macedo, A. F. (2022). Anxiety levels moderate the association between visual acuity and health-related quality of life in chronic eye disease patients. Scientific Reports, 12(1), 2313. https://doi.org/10.1038/s41598-022-06252-1
- Sisodiya, S. M., Free, S. L., Williamson, K. A., Mitchell, T. N., Willis, C., Stevens, J. M., Kendall, B. E., Shorvon, S. D., Hanson, I. M., Moore, A. T., & van Heyningen, V. (2001). PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nature Genetics, 28(3), 214–216. https://doi.org/10.1038/90042
- Thompson, P. J., Mitchell, T. N., Free, S. L., Williamson, K. A., Hanson, I. M., van Heyningen, V., Moore, A. T., & Sisodiya, S. M. (2004). Cognitive functioning in humans with mutations of the PAX6 gene. Neurology, 62(7), 1216–1218. https://doi.org/10.1212/01.WNL.0000118298.81140.62
- Yogarajah, M., Matarin, M., Vollmar, C., Thompson, P. J., Duncan, J. S., Symms, M., Moore, A. T., Liu, J., Thom, M., van Heyningen, V., & Sisodiya, S. M. (2016). PAX6, brain structure and function in human adults: Advanced MRI in aniridia. Annals of Clinical and Translational Neurology, 3(5), 314–330. https://doi.org/10.1002/acn3.297
- Zhou, T., Guan, H., Wang, L., Zhang, Y., Rui, M., & Ma, A. (2021). Health-Related quality of life in patients with different diseases measured with the EQ-5D-5L: A systematic review. Frontiers in Public Health, 9, 675523. https://doi.org/10.3389/fpubh.2021.675523
