ABSTRACT
Background
Tuberculosis (TB) has a significant treatment burden for patients, requiring at least six months of anti-TB treatment (ATT) with multiple medicines. Ensuring good adherence to ATT is central to global TB strategies, including those in high-income, low-TB incidence (HILI) settings. For adherence interventions to be successful and deliverable, they need to address the personal and environmental factors influencing patient and provider behaviour.
Purpose
This paper describes the application of theory and research evidence to inform the design process of the IMPACT manualised intervention to support ATT adherence for adults with TB disease in the United Kingdom (UK). It also provides a full description of the resulting intervention.
Methods
We synthesised findings from our formative research (qualitative and quantitative scoping reviews and patient and carer interviews) and supplemented these with clinic observations, a literature review, and healthcare provider interviews. Findings were mapped to the guiding theoretical framework (Perceptions and Practicalities Approach) which was operationalised to design the intervention components and content. An Intervention Development Group (IDG) of relevant stakeholders were consulted to adapt the intervention to local clinical settings.
Results
The pragmatic, deliverable components and content for the IMPACT intervention included: (1) an enhanced, structured, risk assessment to systematically identify risk factors for non-adherence plus locally-adapted guidance to mitigate these; and (2) patient educational materials (an animated video and interactive patient booklet) about TB and its treatment, to communicate the need for treatment and address common concerns.
Conclusions
Using a theory– and evidence– based approach incorporating stakeholder input, we have developed a multi-component, pragmatic, manualised intervention, which addresses patients’ personal barriers to adherence within local service resources to improve adherence to ATT within UK TB services.
Introduction
Even within high-income, low tuberculosis (TB) incidence (HILI) countries, the infectious disease TB is a public health concern (Van De Berg et al., Citation2018). HILI countries are those where there are fewer than 10 new TB cases per 100,000 of the population per year, including the USA, Australia, almost all of Western Europe, and the UK. The 2022 UK Health Security Agency TB Surveillance Report indicates that the estimated number of TB cases in the UK in 2021 was 4,795 (NHS England and UK Health Security Agency, Citation2022). This makes the UK’s TB incidence rate the second highest in Western Europe. Anti-tuberculosis treatment (ATT) can cure TB infection, however as regimens include multiple medicines to be taken generally for at least six months, poor adherence can occur (Alipanah et al., Citation2018; Munro et al., Citation2007). Non-adherence, where a patient’s medication-taking behaviour deviates from the mutually-agreed recommendations of the prescriber (Horne et al., Citation2005), increases the likelihood of death and disease from TB (Waitt & Squire, Citation2011), and can lead to harder-to-treat, drug-resistant disease (Pradipta et al., Citation2018; Rockwood et al., Citation2015). Non-adherence also has public health consequences ofincreasing the risk of transmitting infection to others (Hayward et al., Citation2018). The need to develop innovative, implementable, patient-centred interventions to support adherence to anti-TB treatment (ATT) and improve patient outcomes has been highlighted by the WHO’s End TB strategy (World Health Organisation, Citation2015b) and is reflected in national plans in HILI settings, such as the UK (NHS England and UK Health Security Agency, Citation2021).
At present, the most effective and pragmatic approaches to support ATT adherence remain unclear. Directly-observed therapy (DOT) has been the recommended strategy since the 1990s (World Health Organisation, Citation1999). However, recent reviews have identified mixed results for the effectiveness of DOT compared to self-administered therapy (SAT) in improving ATT adherence (Alipanah et al., Citation2018; Pradipta et al., Citation2020). This includes evidence from low-incidence TB settings (Van De Berg et al., Citation2018). In addition, DOT is both resource-intensive, and can create a negative experience for patients (Arakelyan et al., Citation2021; Craig & Zumla, Citation2015; Hansel et al., Citation2004; Karat et al., Citation2021; Sagbakken et al., Citation2012).
More recently, WHO has emphasised the utility of digital adherence technologies (DATs), which may include video-observed therapy (VOT) (a remote version of DOT delivered via smartphones or other devices) (World Health Organisation, Citation2015a). However, DATs are largely reminder-based interventions, and as such can be rigid and inflexible by failing to consider the influence of psychosocial, structural, and health-systems factors upon patients’ adherence to ATT (Arakelyan et al., Citation2021). The importance of addressing these determinants of treatment behaviour has been highlighted in recent guidance from the International Union Against TB and Lung Disease (International Union Against Tuberculosis and Lung Disease, Citation2021). Furthermore, the UK National Institute for Health and Care Excellence (NICE) clinical guidance for TB (National Institute for Health and Care Excellence, Citation2020) has stressed the lack of robust research into this wider range of factors. Indeed, recent evidence suggests that interventions utilising person-centred approaches, where support is tailored to individual need, may be most effective in improving adherence (Pradipta et al., Citation2020).
Both DOT and VOT essentially target the practicalities of adherence. NICE guidance (National Institute for Health and Care Excellence, Citation2009) emphasises the need to address not just the practical issues impacting on patients’ opportunity and ability to adhere but also to take account of their perceptions of the illness and treatment. The latter is essential as patients’ beliefs are a common reason for non-adherence, and consideration of patients’ perceptions of illness and treatment is also necessary to support informed choice (Horne et al., Citation2013). There is a need, therefore, for evidence-based, person-centred, implementable interventions to improve adherence to ATT that address the limitations of existing support strategies such as DOT and DATs, and yet are deliverable within existing service resources. This paper describes the intervention development process and intervention used in the IMPACT study (Intervening with a Manualised Package to Achieve treatment adherence in people with TB) (Stagg et al., Citation2019), and specifically demonstrates how formative research was used to enhance the theoretical framework to inform intervention content and design.
Materials and methods
Study aim
The IMPACT study aims to develop, pilot, and evaluate a manualised package of interventions to improve adherence to ATT. We use the term manualised to refer to an intervention which is performed according to specifically-created guidelines (an intervention manual) to maximise consistency of delivery across HCPs and clinical settings. The study objectives addressed in this paper were to: develop a manualised intervention package with multiple components that could (a) identify patients most at risk of non-adherence; (b) discern salient modifiable barriers to adherence; and (c) tailor support mechanisms to meet individual needs by matching appropriate interventions to specific barriers, as recommended by NICE, that would be deliverable within standard TB care.
The aim of the study intervention aligned with one of the four pillars of WHO’s End TB strategy – ‘Integrated, patient-centred care and prevention’ (World Health Organisation, Citation2015b). Within this, the strategy states that in order to achieve its goal of ending the TB epidemic, there is a need to expand ‘the scope and reach of interventions for TB care and prevention, with a focus on high-impact, integrated and patient-centred approaches’. This study therefore aimed to address the adherence problem and ultimately improve patient outcomes by developing a patient-centred intervention.
Intervention context
Until the COVID pandemic, TB in the UK has been steadily declining over the past decade, although not at a rate that will meet the WHO’s End TB Strategy notification target of a 90% reduction by 2035 (Public Health England, Citation2020). TB incidence is concentrated within major cities of the UK, with London (the site of the pilot study) accounting for a third of all TB cases (Public Health England, Citation2020). In 2019, approximately 14% of all people who developed TB were identified as having a social risk factor (such as substance misuse, homelessness, or imprisonment), highlighting the complex psychosocial and structural barriers that patients may face when accessing and completing treatment (National Institute for Health and Care Excellence, Citation2009).
In high-burden areas in the UK, outpatient treatment is delivered by multi-disciplinary TB services, including senior doctors, nurses, and social care and DOT outreach workers. These services are designed to support the complex social and medical needs of TB patients through a case management approach to comprehensively follow-up confirmed or suspected cases (Royal College of Nursing, Citation2019). Each patient is assigned a case manager, most commonly a trained TB nurse, who is responsible for their care. At treatment initiation, case managers conduct a risk assessment to highlight specific medical needs the patient may have, such as complex TB disease, or social care needs (e.g. housing or employment needs). This informs the case manager’s judgement about whether enhanced case management (ECM) may be appropriate for the patient. ECM includes forms of treatment supervision, such as DOT or VOT, increased support (e.g. home visits), and medication-organising aids (e.g. dosette boxes).
Standard ATT regimens for drug sensitive disease are currently six months in length, and use multiple drugs, although treatment may last up to two years for patients with drug-resistant or complex disease. As part of standard TB care, all patients are reviewed by their case manager at treatment initiation, week two, and then usually monthly from months one to six (see Supplementary Material 1 for an overview of standard care).
Theoretical framework
Intervention development was guided by the theoretical framework the Perceptions and Practicalities Approach (PaPA) (Horne et al., Citation2019). The PaPA is designed to aid the development of interventions to support informed treatment decisions and optimise adherence, as recognised within NICE guidance (National Institute for Health and Care Excellence, Citation2009). The PaPA is based on a ‘no-blame’ approach to non-adherence, whereby patients are supported to make informed decisions about taking medicines which are not based on misperceptions of the treatment or illness. The PaPA has underpinned the design of interventions delivered to a range of chronic illness groups (Chapman et al., Citation2020; Clifford et al., Citation2006; Odeh et al., Citation2019). To date, the PaPA has not been applied to the design of adherence support for ATT.
The PaPA suggests that adherence is best understood in terms of the interaction between an individual and a specific treatment. The PaPa provides a simple framework for addressing this interaction, which acknowledges that while adherence is influenced by a range of external (e.g. health-systems) and internal factors (e.g. symptom experience), these ultimately influence adherence through the extent to which they affect the patients’ motivation and ability to take their treatment (Horne et al., Citation2019).
Adherence support therefore needs to address the perceptions and practicalities which influence an individual’s motivation and ability to start and continue with treatment (Horne et al., Citation2019). Perceptual barriers include the beliefs that an individual has about their treatment (Horne & Weinman, Citation1999) and illness (Leventhal et al., Citation1980). Two key barriers are beliefs about how necessary a treatment is for a given illness (necessity beliefs), versus any concerns about taking this (concern beliefs), known as the Necessity-Concerns Framework (NCF) (Horne et al., Citation2013). Therefore, in addition to addressing the practicalities of taking treatment by making this as easy as possible for the patient, adherence support should also promote a ‘common-sense fit’ between the problem (i.e. the illness) and the solution (i.e. the treatment) through increasing the perceived necessity for treatment and decreasing any concerns about taking it. The PaPA therefore specifies three basic components that should be addressed in effective adherence support: (1) necessity beliefs: explain why treatment is necessary to maintain or protect health, (2) concerns: elicit and address patient concerns about treatment, and (3) practicalities: address practical factors to make treatment as easy as possible for the patient.
An additional key element of the PaPA is the desirability to tailor adherence support to meet individual patient needs. However, the ability to fully achieve this is limited by the resources available within the given health-system. We therefore utilised formative research to enhance the utility of the PaPA applied to adherence to ATT in the UK, which could be implemented using the resources available within each UK TB clinic.
Formative research
Literature reviews
We synthesised current knowledge on (a) determinants of adherence to ATT, and (b) interventions to support adherence to ATT, with a particular emphasis on highlighting the social and cultural barriers to operationalise the intervention framework (see ). Specifically, the TB adherence literature was reviewed to answer the following questions:
What are the personal, socio-cultural, and health-systems-related, factors affecting individuals’ ability to adhere to ATT?
What interventions have been developed to address the multiple levels (personal, socio-cultural, health-systems, and structural) at which barriers may operate?
What is the evidence for the successful impact of interventions on adherence to ATT?
Figure 1. Conceptual map of formative research and intervention development.Note: HCP = healthcare provider, LMIC = low or middle-income countries, PaPA = Perceptions and Practicalities Approach, IDG = Intervention Development Group.
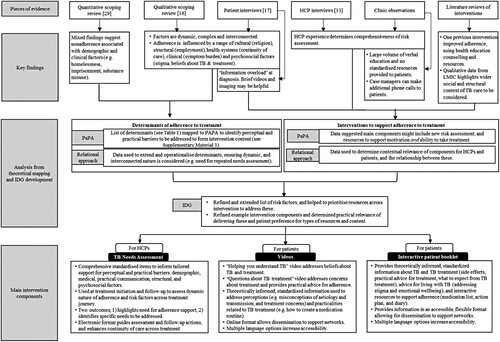
Questions 1 and 2 were answered by synthesising findings from two existing pieces of research we conducted as part of formative research: (1) a quantitative scoping review synthesising data on determinants of non-adherence to ATT in HILI settings (Jones et al., Citation2021) [question 1], and (2) a qualitative scoping review of the experiences of patients taking ATT in HILI settings (Arakelyan et al., Citation2021), and of interventions to improve adherence [questions 1 and 2]. Their methods and results are published elsewhere. For the current study, we additionally conducted a review of studies examining the effectiveness of interventions to improve adherence to ATT in HILI settings [question 3].
The reviews focused on evidence from settings similar to the UK (HILI) as determinants in these contexts likely differ from those in low-incidence settings (Centers for Disease Control and Prevention, Citation2019; Public Health England, Citation2019; Zumla et al., Citation2013). However, the qualitative interventions review found no evidence from HILI countries; and so relevant evidence from low- or middle- income settings was analysed. Ethical approval was not required for the literature reviews.
Qualitative interviews
As part of formative research, we conducted in-depth interviews with patients (n = 18)and caregivers (n = 4) to understand the experiences of adults receiving TB care across the UK. The methods and findings of this work are published elsewhere (Karat et al., Citation2021). For the intervention development process, we synthesised these results with additional interviews conducted with healthcare providers (HCPs) (n = 27) to understand the experiences of delivering TB care across the UK. Interviews were conducted at three, geographically diverse sites with differing TB populations and care settings: Edinburgh, London and Southampton (Karat et al., Citation2021). The semi-structured interviews with HCPs (nurses, doctors, outreach workers, and administrators) focused on perceived patient experiences of TB and ATT, service delivery, and systems-level barriers and enablers in the diagnostic and treatment pathway (IMPACT Study Group, Citation2020). Ethical approval was obtained for all interviews (as described in Karat et al., Citation2021).
Clinic observations
To better understand standard TB care, clinic observations were conducted by a researcher in the Intervention Development Group (IDG; see below) at study sites. Field notes collected information on the delivery of the risk assessment, methods used to assess adherence and assign support, and the education and information provided to patients. Data were collected through observing patient appointments, and discussing procedures with case managers, social care and outreach workers. Observations were conducted in parallel to the development of intervention content, to ensure that the feasibility of delivering emerging components in standard care was considered throughout the development phase. Ethical approval was gained for observations from both patients and healthcare providers as part of the main IMPACT pilot study (reviewed by Research Ethics Committee 17/0726; IRAS231542).
Intervention development group
An intervention development group (IDG) were consulted to guide development and ensure the relevancy of intervention components and content to HCPs and patients. Members of the IDG were recruited by approaching TB patient groups, TB non-governmental organisations, and researchers with experience of working in related areas who had developed adherence interventions. The IDG included patient advisors with previous experience of ATT, members of the multi-disciplinary IMPACT research team, with expertise in respiratory medicine, infectious disease, epidemiology, behavioural medicine, clinical trials, and medical anthropology, plus additional relevant stakeholders, such as HCPs and a UK TB Charity (TB Alert).
Results
Formative research findings
In synthesising findings across the literature reviews, we identified a variety of demographic, clinical, psychosocial, structural and health-systems determinants of adherence to ATT (Arakelyan et al., Citation2021; Jones et al., Citation2021), which were further extended by patient and carer interview data (Karat et al., Citation2021). The range of risk factors was much broader than those listed on the standard care risk assessment form used in National Health Service (NHS) TB practice. Importantly, the qualitative research (scoping review and interviews) also put forward a relational approach to adherence, highlighting the complexity of the interlinked and dynamic factors influencing patients’ experience of being on treatment for TB.
Existing data on interventions to support adherence to ATT in HILI settings were minimal. Two previous RCTs of adherence interventions were identified in the quantitative literature (MacIntyre et al., Citation2003; Morisky et al., Citation1990), of which only one was found to improve adherence compared to usual care (Morisky et al., Citation1990). This intervention provided health education counselling and incentives to patients initiating ATT in Los Angeles in 1990. The qualitative review found 46 articles reporting on intervention studies, all from low- and middle-income countries. In these contexts, more successful interventions improved relationships and strengthened communication between HCPs and patients, reduced feelings of isolation, and were developed and implemented with buy-in from administering HCPs. Less successful interventions were those which increased costs and travel for patients, and increased or did not account for issues around stigma.
Formative research also suggested potential intervention components. In interviews, patients described experiencing ‘information overload’ at treatment initiation; this was supported by clinic observations reporting the large volume of verbal information delivered at that time. Patients expressed that brief videos about TB and ATT may be helpful resources. They also found that seeing imaging of their TB disease was helpful and motivated treatment adherence by providing reassurance about treatment efficacy.
HCP interviews revealed that experienced case managers delivered a comprehensive risk assessment, by checking for risk factors beyond the items listed on the standard form. These included patient attitudes and motivation (perceived or stated) towards treatment, their existing social support, their understanding of TB, and perceived need for treatment (IMPACT Study Group, Citation2020). Clinic observations supported this, and additionally found that adherence support was assigned based on the case managers’ judgement of the patient’s social and clinical needs. This suggested that case managers utilise a ‘mindlines’ over ‘guidelines’ approach (Gabbay & le May, Citation2004), whereby the strength and validity of the risk assessment and adherence support provided is based on experience and judgement, rather than the tool itself.
Formative research analysis and operationalisation of theoretical framework
provides a conceptual map of the intervention development process, demonstrating how formative research findings were used to contextualise the theoretical framework and inform intervention content and components. The list of determinants extracted from the formative research is provided in Supplementary Material 2. Targets for intervention content and components were identified by mapping each determinant to the PaPA framework to identify the modifiable, perceptual and practical barriers resulting from each risk factor (see Supplementary Material 3). The researchers’ interpretation of these barriers was informed by the qualitative data (e.g. patient and carer interviews describing treatment experiences) and the expertise of the research team. The relational approach to adherence identified in the qualitative formative research helped to operationalise the PaPA framework and ensure the contextual relevance of intervention content to the UK TB setting.
In parallel, researchers considered potential components to deliver intervention content. The formative research highlighted limitations in the delivery of the risk assessment and assignment of adherence support. It also identified a need for additional patient resources to help manage/avoid ‘information overload’. With these potential components in mind, draft content was developed based on the perceptual and practical barriers identified in the theoretical mapping of determinants.
Intervention development group
Formative research findings and analysis were presented to the IDG throughout development. The process was iterative, as formative research findings were continuously incorporated when they became available. The IDG helped to prioritise the most pertinent determinants to be targeted by the intervention, and highlighted additional risk factors to be considered. The IDG agreed that standardising and expanding the risk assessment could improve the identification of risk factors across all HCPs. The IDG also agreed that repeated needs assessments were necessary to account for the fluctuating social and structural issues that patients may face during their several-month treatment for TB. The IDG discussed ideas for feasible and scalable interventions to address the specific social and structural risk factors highlighted in the formative research (e.g. homelessness, substance abuse, mental health needs). Given the varied capacity of TB services and resources available, it was suggested that interventions should be site-dependent (e.g. referral to dedicated social care teams or signposting to relevant external services when these are not available). Additionally, the IDG suggested creating an electronic risk assessment to maximise usability and continuity of care.
The IDG agreed that flexible, standardised patient resources at treatment initiation would support patient motivation and ability to take treatment. Prior work from study researchers supported our formative research, by demonstrating the utility of visual representations of disease and treatment efficacy (including animations) in modifying illness and treatment beliefs and improving adherence (Jones et al., Citation2016; Jones et al., Citation2017; Jones et al., Citation2019). The IDG agreed that brief videos could be an effective patient resource. The researchers also suggested providing a patient booklet, as a supplementary resource to address the full range of perceptual and practical barriers identified in the formative research.
In a final meeting, the IDG agreed upon the design of a draft intervention structure and components (see ). Sub-groups of the IDG, including researchers, HCPs, and patient advisors, met regularly to finalise intervention content, and circulated this to the wider IDG before production. Co-development with HCPs ensured that components would be complementary to standard care procedures delivered by case managers. Members of the IDG also attended meetings with third party production companies to provide feedback on initial iterations of the booklet and videos and to finalise materials.
Intervention components
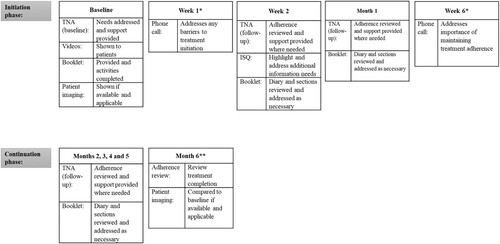
The logic model () demonstrates the anticipated mechanisms of action of intervention components on the primary outcome of treatment adherence. The resulting intervention is a manualised approach to adherence support in TB, delivered by case managers as part of standard care. summarises the three main intervention components, which are delivered at common touchpoints in the treatment pathway (see ) and supplemented by two additional phone calls at weeks one and six. The reporting of intervention components follows the TIDieR checklist for intervention description and replication (Hoffmann et al., Citation2014).
Figure 2. Logic model for the IMPACT intervention. Note: TNA = TB needs assessment. HCP = Health care professional. Dotted lines demonstrate where anticipated effects are repeated across mechanisms.
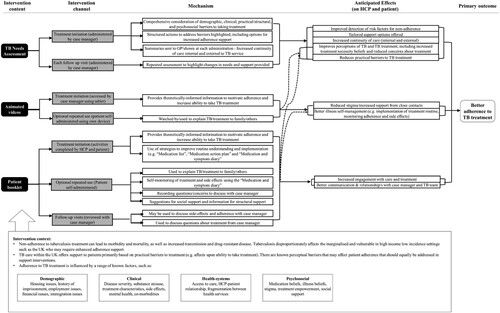
Figure 3. IMPACT intervention component delivery across patient treatment pathway.Note: TNA = TB Needs Assessment, ISQ = Information and Support Questionnaire. *Point of touch occurs outside of standard care treatment pathway. **Month 6 is treatment completion for standard treatment. If treatment continues monthly, the TNA follow-up is completed at each session.
Tb needs assessment
The TB Needs Assessment (TNA) was developed as an enhanced, structured risk assessment to be administered by case managers at treatment-start and each subsequent appointment. The TNA is web-based (run in the Research Electronic Data Capture [REDCap] database [Harris et al., Citation2009, Citation2019]) and completed using a digital tablet. The TNA identifies a comprehensive list of risk factors and provides case managers with standardised solutions to address patients’ specific needs using locally available resources (e.g. referrals or signposting to external services such as drug and alcohol services, housing or social care services, and/or addressing perceptual barriers using brief conversations). Therefore, the TNA is designed to respond to individualised barriers that may be present for patients at different times across their treatment trajectory. The research team felt this was important to ensure the adaptability of the intervention to support the diverse and variable structural, social, psychological, and treatment-related factors that can affect adherence to ATT (Karat et al., Citation2021). To identify local service resources to support the cost of the intervention, the research team consulted with nursing leads at each service to identify provisions that were already present. Therefore, each service had an individualised version of the TNA which provided links to the resources that were available locally. The TNA also highlights the need to assign additional adherence support, which includes elements of ECM used in standard care (DOT, VOT, organisational aids such as dosette boxes, or increased support), and additionally reminders that could be set on the electronic medication boxes used to measure adherence during the intervention pilot trial (Stagg et al., Citation2019).
Patient videos
Two brief (four-minute), animated videos were created to increase patient’s motivation and ability to take their ATT: ‘Helping you understand TB’ and ‘Questions about TB’. The structure of the videos was based on the PaPA and the NCF, and the content of the videos informed by the qualitative formative research and relational approach. Specifically, the first video addressed step 1 of the PaPA, by targeting perceptions of TB, beliefs about the necessity of ATT, and providing a common-sense rationale for taking treatment. A visual representation of the mechanism of action of treatment demonstrated how adherence relates to the control of TB infection and disease. The second video addressed steps 2 and 3 of the PaPA by addressing common concerns about ATT (e.g. side effects, pill burden) and providing practical advice to make treatment as easy as possible to take (e.g. habit formation and anchoring).
The videos were shown to patients on a digital tablet during their baseline session. Patients were also provided with web links to share and re-watch the videos. Translated videos were available in Bengali, Hindi, and Romanian, to match the languages most commonly spoken by individuals treated for TB in the pilot study sites.
Interactive patient booklet
An interactive patient booklet, ‘TB and Me’, was designed to guide patients through their treatment. The core content and activities in the booklet were designed to address the three steps of the PaPA. Additional information also addressed the key social and structural issues highlighted in our formative research. Information topics included: what to expect when taking ATT, emotional wellbeing, addressing stigma, and side effects. Contact information for the TB service, and local social services was also provided. The booklet contained interactive resources to help patients understand and implement a treatment routine, such as creating a personalised medication list and a ‘medication action plan’ (detailing when and how patients will take their treatment). Case managers completed these resources with patients during their initial session. Patients were also encouraged to use a ‘medication and symptom diary’ to record adherence and side effects, which case managers could review at subsequent follow-up. Translated versions of the booklet were also available in, Bengali, Hindi, and Romanian.
Minor intervention components
We included standardised, structured, phone calls as part of the intervention at weeks one and six of treatment. This was done to standardise the additional phone contact that some case managers reported having with patients. The purpose of the calls was to review patient progress with treatment and address any issues. As formative research suggested that imaging can motivate and reassure patients, case managers were also encouraged to share any available and appropriate imaging with patients (e.g. chest radiographs) at treatment initiation and completion. Finally, to tailor information provision to patient needs, at week two, patients complete a short checklist to identify any booklet topics they would like to review with their case manager.
Intervention training
A training manual provided to case managers included information on intervention development and theory, intervention components, and guides on how to incorporate these into standard care. Training was provided by two IDG researchers (AJ and TC), including an initial 90-minute presentation and question and answer session at each intervention site, and one-hour individual sessions with each case manager. Case managers were also given checklists to follow intervention steps.
Discussion
We report the development and description of an intervention to improve adherence to ATT in the UK (Horne et al., Citation2019). The resulting intervention is a manualised, multi-component, complex intervention delivered within the standard TB care programme by case managers. The main intervention components are an enhanced needs assessment (with advice on how to manage any identified needs), an interactive patient booklet, and animated videos.
Overall, the theoretical and empirical basis for our intervention determined that barriers to treatment adherence in TB are both structural and perceptual (i.e. beliefs about illness and treatment) in nature. We identified clear modifiable barriers which can be amended through intervention to help patients understand the necessity for, and reduce concerns related to, their treatment. The intervention therefore provides an integrated, patient-centred approach by offering a comprehensive assessment of these barriers and risk factors for non-adherence, and then provides tailored support to address these within the context of standard TB care and the resource available within a specific TB service.
As highlighted in our literature review of interventions in HILI settings, very few other interventions to improve adherence to ATT have been developed that do not utilise directly-observed therapy. Of the studies we identified, only one was found to be effective – and this was based on changing patient’s beliefs about illness and treatment (Morisky et al., Citation1990) which underpin all three of our intervention components. Our intervention therefore provides, to our knowledge, the first example of a comprehensive, multi-component behavioural intervention to improve adherence to ATT developed for HILI TB settings.
The use of appropriate theory and evidence is integral to the development of complex health interventions; and we followed the process recommended by the UK Medical Research Council (Craig et al., Citation2008). We were motivated to report our work as the detail needed to understand the active components of an intervention are seldom found in the literature (Horne et al., Citation2005), yet seem key to an appreciation of the content, development, and implementation of an intervention. The mechanisms through which we believe these intervention components will influence patient adherence is demonstrated in our study logic model. Our approach is in line with the recommendation of the Template for intervention description and replication (TIDieR) checklist (Hoffmann et al., Citation2014).
The IMPACT intervention is guided by a theoretical framework of adherence recommended within UK NICE guidance as a method to implement research that relates to modifiable causes of non-adherence to treatment – the PaPA framework. The approach enables the tailoring of support to the needs of the individual patient, recognising the specific perceptions (e.g. thoughts and feeling about the illness and treatment) and practicalities (e.g. capability and resources) influencing their motivation and ability to start and continue with treatment for TB. This report demonstrates how formative research can be used to operationalise this framework for intervention development. The value of the PaPA framework lies in it being centred on an understanding of patients’ perspectives of both illness and treatment. In doing so, the framework does not conceptualise non-adherence as the ‘patients’ problem’, but rather emphasises the need to optimise healthcare practice and policy to enable and support adherence to appropriately-prescribed medicines. It enables a patient-centred approach to adherence; and provides the key ‘ingredients’ for adherence support that formative research can then use to contextualise the intervention for a given population and setting (Horne et al., Citation2019). The resulting adherence support therefore acknowledges and addresses the factors influencing patient adherence, within a scalable and applicable, individual-level intervention. The example process we report may be relevant to the development of adherence interventions for other conditions with long-term or chronic treatments, such as HIV or asthma. Through its consideration of the context-specific perceptual and practical barriers, it also may be helpful when the PaPA framework is applied to other intervention approaches in different TB settings.
Considerations of ‘how’ and ‘by whom’ an intervention will be delivered in practice must take place early in the development process (Medical Research Council, Citation2006), if it is to successfully bridge the frequent gap between demonstrations of intervention efficacy and implementation into services (Glasgow et al., Citation2003). Through co-production with a multi-disciplinary IDG, our development process ensured that intervention components would be complementary to UK TB services. From the patient perspective, this aligned the accessibility and relevance of intervention content to patient needs. For the HCP, this ensured the components were deliverable by TB case managers and applicable to current care practices. Furthermore, although the IMPACT intervention will be piloted in London, the intervention was designed to be relevant to other UK services and HILI settings. The IDG included researchers and patient representatives from across the UK. The formative research reviewed literature across similar HILI settings and captured perspectives across three UK sites (Southampton, London, and Edinburgh), with differing levels of TB incidence and models of care. Including stakeholders throughout the design process therefore helps to reduce implementation barriers for incorporating the intervention into standard care, assuming efficacy and acceptability are demonstrated.
A further strength of our development process was the use of varied research methods to obtain evidence to inform intervention content. In practice, this meant that formative research projects were conducted in parallel, such that findings were incorporated into intervention design as they became available, rather than the results of one piece of research sequentially informing the design of the next. However, the findings across projects were often complementary, meaning new evidence could be incorporated into the emerging intervention design. Using varied research methods in parallel also enhanced the development process. For example, clinic observations enabled a more rapid understanding of the context of current TB care, which increased the clinical relevance of emerging intervention components.
The IMPACT intervention is being formally trialled in a pilot feasibility study (Stagg et al., Citation2019). This will include a cost analysis of the intervention so we can further understand the implementation potential of this within the NHS. A process evaluation will assess the implementation, context, and mechanisms of impact of the intervention. This will highlight any ‘active’ intervention components and assess the feasibility of delivering these within routine TB care. In particular, this will aim to evaluate the ability of services to deliver this type of intervention (i.e. primarily face-to-face, with high levels of contact), in the context of the COVID-19 pandemic and the resulting implications on healthcare. This will also evaluate how well the intervention can be implemented in clinical practice. It will measure outcomes such as the time taken for the intervention to be delivered. This will be important when determining its ease of use in routine care. The pilot study results will determine whether the IMPACT intervention is tested in future, large-scale trials.
If found to be effective, further work could examine the adaptability of the IMPACT intervention to related patient groups, such as those taking tuberculosis preventative therapy (TPT). The intervention content related to addressing beliefs about TB and treatment would be relevant to latent tuberculosis, given there are similarities in belief barriers between the two conditions such as stigma, side effects and understanding of disease (Colson et al., Citation2010; Kwara et al., Citation2008; Spruijt et al., Citation2020). It will also be important for future work to examine if this intervention could be translated into high TB burden countries which have their own systemic barriers to overcome.
This paper reports the theory- and evidence-based approach used to develop the IMPACT intervention, a multi-component, pragmatic, manualised intervention to improve adherence to ATT within UK TB services. This example may be helpful to researchers working in both adherence and TB. Specifically, we demonstrate how to utilise the PaPA in intervention design. We also highlight the importance of stakeholder input across the development process. Reporting on complex intervention development identifies pathways and processes that future research can follow. It also ensures the transparent reporting of intervention content, which in turn helps contextualise reported efficacy and implementation trial results.
Ethical statement
The intervention development work reported here does not report any novel work with human participants and therefore did not require ethics approval. Previous work described in this paper (but published in full elsewhere) which did include human participants was conducted in accordance with the Declaration of Helsinki and was approved by an Institutional Review Board/Ethics committee. See details under Methods.
Supplemental Material
Download ()Acknowledgements
This research was conducted as part of the Intervening with a Manualised Package to Achieve treatment adherence in people with TB (IMPACT) study, supported by a National Institute for Health Research (NIHR; London, UK) Health Technology Assessment Programme (UK grant number 16/88/06). We thank the members of our Intervention Development Group who provided their expertise and input on the development of the intervention. We would also like to acknowledge In Tune For Life and UCL Creatives, who produced the animations and interactive booklet respectively. ASKJ and RH led the intervention development process. ASKJ led the drafting of this manuscript. JW, TC, MD, ASK, KK, HRS, ATH, CNJC and MCIL were all part of the intervention development group who led the design and creation of this work and contributed to the drafting of manuscript.
Disclosure statement
KK declares funding from the National Institute for Health Research, UK related to this grant. HRS declares funding from the Medical Research Council, UK, and National Institute for Health Research, UK, related to this grant. HRS declares other funding from the Latvian Society Against Tuberculosis (funding through Otsuka and Johnson and Johnson) and UiT The Arctic University of Norway for speaker engagements in the area of adherence to anti-tuberculosis medication. ASK reports salary support from a grant from the National Institute of Health Research (UK) and non-financial support from University College London (UK) during the conduct of the study. Outside the submitted work, ASK declares personal fees from The Aurum Institute (South Africa), the South African National TB Think Tank (funded by the Bill & Melinda Gates Foundation), Vital Strategies (Singapore; work funded by Bloomberg Philanthropies), The University of Cape Town (South Africa), the Center for Health Policies and Studies (Moldova), and the Edanz Group (Japan); and non-financial support from The Africa Health Research Institute (South Africa), Vital Strategies (Singapore), Kyoto University (Japan), and the Bill & Melinda Gates Foundation (USA). All other authors report no conflicts of interest.
Additional information
Funding
References
- Alipanah, N., Jarlsberg, L., Miller, C., et al. (2018). Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLOS Medicine, 15(7), e1002595. https://doi.org/10.1371/journal.pmed.1002595
- Arakelyan, S., Karat, A. S., Jones, A. S. K., et al. (2021). Relational dynamics of treatment behavior among individuals with tuberculosis in high-income countries: A scoping review. Patient Preference and Adherence, 15, 2137–2154. https://doi.org/10.2147/PPA.S313633
- Centers for Disease Control and Prevention. (2019). Reported tuberculosis in the United States, 2018. https://www.cdc.gov/tb/statistics/reports/2018/default.htm.
- Chapman, S., Sibelli, A., St-Clair Jones, A., Forbes, A., Chater, A., & Horne, R. (2020). Personalised adherence support for maintenance treatment of inflammatory bowel disease: A tailored digital intervention to change adherence-related beliefs and barriers. Journal of Crohn's and Colitis, 14(10), 1394–1404. https://doi.org/10.1093/ecco-jcc/jjz034
- Clifford, S., Barber, N., Elliott, R., Hartley, E., & Horne, R. (2006). Patient-centred advice is effective in improving adherence to medicines. Pharmacy World and Science, 28(3), 165–170. https://doi.org/10.1007/s11096-006-9026-6
- Colson, P. W., Franks, J., Sondengam, R., Hirsch-Moverman, Y., & El-Sadr, W. (2010). Tuberculosis knowledge, attitudes, and beliefs in foreign-born and US-born patients with latent tuberculosis infection. Journal of Immigrant and Minority Health, 12(6), 859–866. doi:10.1007/s10903-010-9338-4
- Craig, G. M., & Zumla, A. (2015). The social context of tuberculosis treatment in urban risk groups in the United Kingdom: A qualitative interview study. International Journal of Infectious Diseases, 32, 105–110. https://doi.org/10.1016/j.ijid.2015.01.007
- Craig, P., Dieppe, P., Macintyre, S., Mitchie, S., Nazareth, I., & Petticrew, M. (2008). Developing and evaluating complex interventions: The new medical research council guidance. BMJ, 337(7676), 979–983. https://doi.org/10.1136/bmj.a1655
- Gabbay, J., & le May, A. (2004). Evidence based guidelines or collectively constructed “mindlines?” ethnographic study of knowledge management in primary care. BMJ, 329(7473), 1013. https://doi.org/10.1136/bmj.329.7473.1013
- Glasgow, R. E., Lichtenstein, E., & Marcus, A. C. (2003). Why don’t We See more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. American Journal of Public Health, 93(8), 1261–1267. https://doi.org/10.2105/AJPH.93.8.1261
- Hansel, N. N., Wu, A. W., Chang, B., & Diette, G. B. (2004). Quality of life in tuberculosis: Patient and provider perspectives. Quality of Life Research, 13(3), 639–652. https://doi.org/10.1023/B:QURE.0000021317.12945.f0
- Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O'Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., & Duda, S. N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. https://doi.org/10.1016/j.jbi.2019.103208
- Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
- Hayward, S., Harding, R. M., McShane, H., & Tanner, R. (2018). Factors influencing the higher incidence of tuberculosis among migrants and ethnic minorities in the UK. F1000Research, 7, 461. https://doi.org/10.12688/f1000research.14476.2
- Hoffmann, T. C., Glasziou, P. P., Boutron, I., et al. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348. https://doi.org/10.1136/bmj.g1687
- Horne, R., Chapman, S. C. E., Parham, R., Freemantle, N., Forbes, A., & Cooper, V. (2013). Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: A meta-analytic review of the necessity-concerns framework. PLoS One, 8(12), e80633. https://doi.org/10.1371/journal.pone.0080633
- Horne, R., Cooper, V., Wileman, V., & Chan, A. (2019). Supporting adherence to medicines for long-term conditions: A perceptions and practicalities approach based on an extended common-sense model. European Psychologist, 24(1), 82–96. https://doi.org/10.1027/1016-9040/a000353
- Horne, R., & Weinman, J. (1999). Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. Journal of Psychosomatic Research, 47(6), 555–567. https://doi.org/10.1016/S0022-3999(99)00057-4
- Horne, R., Weinman, J., Barber, N., Elliot, R., Morgan, M., & Cribb, A. (2005). Concordance, adherence and compliance in medicine taking: Report for the National Co-Ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO).
- IMPACT Study Group. (2020). Voices from the front line: TB patients and healthcare providers in the UK. In: World TB Day Symposium. https://www.slideshare.net/TBUCL/impact-study-voices-from-the-front-line-24-mar2020-231057302/TBUCL/impact-study-voices-from-the-front-line-24-mar2020-231057302.
- International Union Against Tuberculosis and Lung Disease. (2021). Psychological counselling and treatment adherence support for people with tuberculosis. https://theunion.org/sites/default/files/2021-04/TheUnion_TBAlert_PsychosocialCounselling_April2021_webversion.pdf.
- Jones, A. S. K., Bidad, N., Horne, R., et al. (2021). Determinants of non-adherence to anti-TB treatment in high income, low TB incidence settings: A scoping review. The International Journal of Tuberculosis and Lung Disease, 25(6), 483–490. https://doi.org/10.5588/ijtld.21.0024
- Jones, A. S. K., Ellis, C. J., Nash, M., Stanfield, B., & Broadbent, E. (2016). Using animation to improve recovery from acute coronary syndrome: A randomized trial. Annals of Behavioral Medicine, 50(1), 108–118. https://doi.org/10.1007/s12160-015-9736-x
- Jones, A. S. K., Fernandez, J., Grey, A., & Petrie, K. J. (2017). The impact of 3-D models versus animations on perceptions of osteoporosis and treatment motivation: A randomised trial. Annals of Behavioral Medicine, 51(6), 899–911. https://doi.org/10.1007/s12160-017-9913-1
- Jones, A. S. K., Kleinstäuber, M., Akroyd, A., Mittendorf, A., Bognuda, P., Merrie, A. E. H., Eva, L., Fernandez, J., & Petrie, K. J. (2019). Using animated visualization to improve postoperative mobilization: A randomized controlled trial. Health Psychology, 38(8), 748–758. https://doi.org/10.1037/hea0000761
- Karat, A. S., Jones, A. S. K., Abubakar, I., Campbell, C. N. J., Clarke, A. L., Clarke, C. S., Darvell, M., Hill, A. T., Horne, R., Kunst, H., Mandelbaum, M., Marshall, B. G., McSparron, C., Rahman, A., Stagg, H. R., White, J., Lipman, M. C. I., & Kielmann, K. (2021). “You have to change your whole life”: A qualitative study of the dynamics of treatment adherence among adults with tuberculosis in the United Kingdom. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases, 23, 100233. https://doi.org/10.1016/j.jctube.2021.100233
- Kwara, A., Herold, J. S., Machan, J. T., & Carter, E. J. (2008). Factors associated with failure To complete isoniazid treatment for latent tuberculosis infection in Rhode Island. Chest, 133(4), 862–868. https://doi.org/10.1378/chest.07-2024
- Leventhal, H., Meyer, D., & Nerenz, D. (1980). The common sense representation of illness danger. In S. Rachman (Ed.), Contributions to medical psychology. Vol. 2 (pp. 7–30). Pergamon Press.
- MacIntyre, C. R., Goebel, K., Brown, G. V., Skull, S., Starr, M., & Fullinfaw, R. O. (2003). A randomised controlled clinical trial of the efficacy of family-based direct observation of anti-tuberculosis treatment in an urban, developed-country setting. International Journal of Tuberculosis and Lung Disease, 7(9), 848–854. Retrieved February 26, 2021, from http://europepmc.org/article/MED/12971668.
- Medical Research Council. (2006). Developing and evaluating complex interventions: New guidance.
- Morisky, D. E., Malotte, C. K., Choi, P., Davidson, P., Rigler, S., Sugland, B., & Langer, M. (1990). A patient education program to improve adherence rates with antituberculosis drug regimens. Health Education Quarterly, 17(3), 253–266. https://doi.org/10.1177/109019819001700303
- Munro, S. A., Lewin, S. A., Smith, H. J., Engel, M. E., Fretheim, A., & Volmink, J. (2007). Patient adherence to tuberculosis treatment: A systematic review of qualitative research. PLoS Medicine, 4(7), e238. https://doi.org/10.1371/journal.pmed.0040238
- National Institute for Health and Care Excellence. (2009). Medicines adherence: Involving patients in decisions about prescribed medicines and supporting adherence. Clinical Guideline [CG76]. Retrieved May 5, 2020, from www.nice.org.uk/guidance/cg76.
- National Institute for Health and Care Excellence. (2020). Tuberculosis NICE guideline [NG33]. Published 2016. Accessed April 3, 2020. https://www.nice.org.uk/guidance/NG33.
- NHS England and UK Health Security Agency. (2021). TB action plan for England, 2021 to 2026. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/998158/TB_Action_Plan_2021_to_2026.pdf.
- NHS England and UK Health Security Agency. (2022). Reports of cases of tuberculosis to enhanced tuberculosis surveillance Systems: UK, 2000 to 2021. https://www.gov.uk/government/statistics/reports-of-cases-of-tb-to-uk-enhanced-tuberculosis-surveillance-systems-2000-to-2021.
- Odeh, M., Scullin, C., Fleming, G., Scott, M. G., Horne, R., & McElnay, J. C. (2019). Ensuring continuity of patient care across the healthcare interface: Telephone follow-up post-hospitalization. British Journal of Clinical Pharmacology, 85(3), 616–625. https://doi.org/10.1111/bcp.13839
- Pradipta, I. S., Forsman, L. D., Bruchfeld, J., Hak, E., & Alffenaar, J. W. (2018). Risk factors of multidrug-resistant tuberculosis: A global systematic review and meta-analysis. Journal of Infection, 77(6), 469–478. https://doi.org/10.1016/j.jinf.2018.10.004
- Pradipta, I. S., Houtsma, D., van Boven, J. F. M., Alffenaar, J. W. C., & Hak, E. (2020). Interventions to improve medication adherence in tuberculosis patients: A systematic review of randomized controlled studies. NPJ Primary Care Respiratory Medicine, 30(1), 21. https://doi.org/10.1038/s41533-020-0179-x
- Public Health England. (2019). Tuberculosis in England: 2019 report.
- Public Health England. (2020). Tuberculosis in England: 2020 report. Retrieved February 15, 2021, from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/943356/TB_Annual_Report_2020.pdf.
- Rockwood, N., Abdullahi, L. H., Wilkinson, R. J., & Meintjes, G. (2015). Risk factors for acquired rifamycin and isoniazid resistance: A systematic review and meta-analysis. PLoS One, 10(9), 1–23. https://doi.org/10.1371/journal.pone.0139017
- Royal College of Nursing. (2019). A case management tool for TB prevention, care and control in the UK.
- Sagbakken, M., Bjune, G. A., & Frich, J. C. (2012). Humiliation or care? A qualitative study of patients’ and health professionals’ experiences with tuberculosis treatment in Norway. Scandinavian Journal of Caring Sciences, 26(2), 313–323. https://doi.org/10.1111/j.1471-6712.2011.00935.x
- Spruijt, I., Haile, D. T., van den Hof, S., et al. (2020). Knowledge, attitudes, beliefs, and stigma related to latent tuberculosis infection: A qualitative study among Eritreans in The Netherlands. BMC Public Health, 20(1), 1–9. https://doi.org/10.1186/s12889-019-7969-5
- Stagg, H. R., Abubakar, I., Campbell, C. N. J., et al. (2019). IMPACT study on intervening with a manualised package to achieve treatment adherence in people with tuberculosis: Protocol paper for a mixed-methods study, including a pilot randomised controlled trial. BMJ Open, 9(12), e032760. https://doi.org/10.1136/bmjopen-2019-032760
- Van De Berg, S., Jansen-Aaldring, N., de Vries, G., & van den Hof, S. (2018). Patient support for tuberculosis patients in low-incidence countries: A systematic review. PLoS One, 13(10), e0205433–24. https://doi.org/10.1371/journal.pone.0205433
- Waitt, C. J., & Squire, S. B. (2011). A systematic review of risk factors for death in adults during and after tuberculosis treatment. International Journal of Tuberculosis and Lung Disease, 15(7), 871–885. doi:10.5588/IJTLD.10.0352
- World Health Organization. (1999). Communicable diseases cluster. What is DOTS ? A guide to understanding the WHO-recommended TB control strategy known as DOTS. Retrieved May 5, 2020, from https://apps.who.int/iris/handle/10665/65979.
- World Health Organization. (2015a). Digital health for the end TB strategy: An agenda for action. https://www.who.int/tb/areas-of-work/digital-health/Digital_health_EndTBstrategy.pdf?ua=1.
- World Health Organization. (2015b). The end TB strategy.
- Zumla, A., Raviglione, M., Hafner, R., Reyn, V., & F, C. (2013). Tuberculosis. New England Journal of Medicine, 368(8), 745–755. https://doi.org/10.1056/NEJMra1200894
