Abstract
Tolerance controls the magnitude of inflammation, and balance between beneficial and harmful effects of inflammation is crucial for organ function and survival. Inadequate tolerance leads to various inflammatory diseases. Antigen specific tolerance is ideal for inflammation control as alternative anti-inflammatory interventions are non-specific and consequently increase the risk of infection and tumorigenesis. With inherent antigen specificity, tolerogenic vaccines are potentially ideal for control of inflammation. Although the concept of tolerogenic vaccines is still in its infancy, tolerogenic mucosal vaccines and specific immuno-therapies have long been proven effective in pioneering examples. Now a body of evidence supporting the concept of tolerogenic vaccines has also accumulated. Here we comment on recent successes of the tolerogenic vaccine concept, present new evidence with a type 1 diabetes vaccine as an example and draw conclusions on the advantages and potential for inflammatory disease control at the bedside.
Introduction
Tolerance controls the magnitude of inflammation, and balance between beneficial and harmful effects of inflammation is crucial for an inflamed organ's function and an individual's survival.Citation1,2 Inadequate tolerance leads to various inflammatory diseases, including those with proven autoimmune basis such as type 1 diabetes (T1D),Citation3,4 allergic diseases including asthma,Citation4-6 inflammatory cardiac disease such as atherosclerosisCitation7 and newly identified examples in neuro-degenerative Alzheimer DiseaseCitation8-10 and cancer.Citation11-13 Thus, the need to cure or control chronic and pathogenic inflammation can be urgent if not desperate, and vaccines to induce tolerogenic responses are the top priority for this task ().
Figure 1. Tolerogenic vaccines are proposed to induce antigen specific control of pathogenic inflammation. (A) Balance between inflammation and tolerance guarantees immune homeostasis and individual survival. (B) Pathogenic inflammation leads to diseases such as autoimmunity, allergy, etc. (C) Non-specific intervention impairs protective immune responses and increases risks of infection or tumorigenesis. (D) Tolerogenic vaccine, as antigen specific intervention, may restore balance and provide a cure.
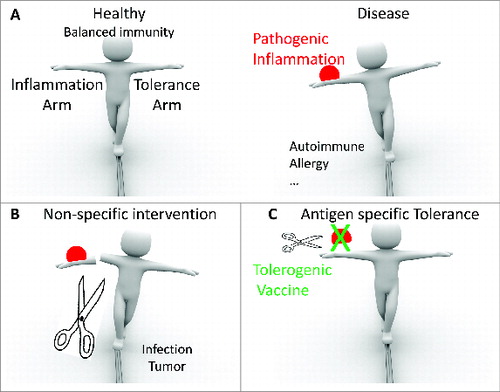
Antigen specific tolerance is ideal for inflammation control.Citation14 Classical interventions suppress inflammation through depletion (mono-clonal antibodyCitation15-17) of key parts of the immune system, or universally suppress the whole immune system (cyclophosphamideCitation18 or steroid immuno-suppressantsCitation19). With these interventions, pathogenic inflammation is surely in some degree brought under control but at the cost of impaired or retarded normal immune responses, a situation which increases the risks of infection and tumorigenesis (). Therefore, the development of specific interventions that target only the pathogenic antigen(s) and suppress only the problem-causing immune responses could be an ideal strategy that circumvents the risks while efficiently delivering safer tolerance.
Indeed, with their inherent antigen specificity, tolerogenic vaccines have long been reasoned to be an ideal means of controlling inflammation (). Since the first discovery of the live smallpox vaccine, vaccination approaches have evolved to contain a spectrum of immunogens ranging from live or dead whole pathogens, viral vectors, subunit proteins, to DNA or RNA molecules encoding antigens.Citation20-22 However, most vaccines are intended to enhance host immune responses rather than to suppress them. Only recently has the use of vaccines that induce tolerogenic immunity to suppress host immune responses gained research momentum following the discovery of Treg cells and their suppressive functions. Although the tolerogenic vaccine concept is still in its infancy, mucosal tolerance induced by vaccinationCitation23 and by specific immunotherapy (SIT) for allergy desensitizationCitation24 were long ago proven effective in successful pioneering studies.
Now a body of evidence is accumulating under the concept of tolerogenic vaccination. Here we provide a perspective and new data regarding a tolerogenic vaccine against autoimmune diabetes. In view of this constraint of content, readers interested in mucosal induced tolerance or allergen-specific immuno-tolerance (SIT) should read reviews by L Mayer,Citation25 O Pabst,Citation26 Valenta,Citation24 Mark LarchéCitation27 and Ciprandi.Citation28
Taming inflammation
Reflecting its importance, inflammation is normally meticulously controlled in body. However, genetic changes, environmental pollutants, infections, aging or unidentified factors could alter such tight control and lead to disease. Therefore, interventions targeting the armory of disease-causing inflammatory cells and molecules have been proposed and tested. As listed in , non-specific interventions that are routinely used include depletion of cell populations by cyclophosphamide,Citation18 depletion of cell subset by antibodies(anti-CD3 mAb for T cell depletionCitation29 or anti-CD20 mAb for B cell depletionCitation30,31]), depletion of inflammatory cytokines by antibody,Citation32,33 universal tolerance induction by immunosuppressant drugs (dexamethasone, FK506, rapamycin or cyclosporin A),Citation34-36 entrapment of all leukocyte into lymph nodes away from inflammatory tissues (FTY720),Citation37,38 etc. As already pointed out above, these approaches run into the risk of increasing the chance of infection and tumorigenesis(). A better approach to avoid these problems would be to use the induction of antigen specific tolerance against the bad or unregulated inflammatory reactions. Such an approach has recently undergone rapid development.
Table 1. Non-specific interventions of inflammation
Induction of antigen specific tolerance by vaccination
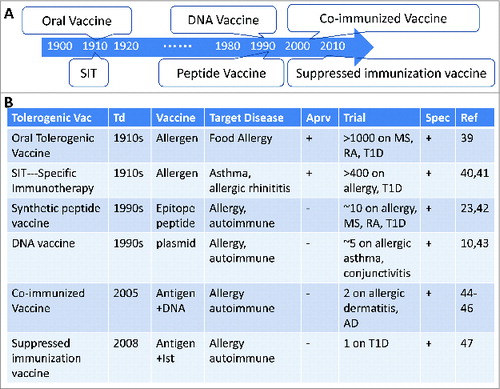
In contrast to a vaccine augmenting immune responses against a pathogen, a tolerogenic vaccine is aimed at inducing tolerogenic responses to suppress or impair immunity against antigens, which in most cases are auto-antigens or allergens.Citation39 Besides the mucosal tolerance and SIT, more attractive approaches to achieve tolerogenic responses and induce antigen specific suppression have emerged in recent yearsCitation14,27,40-44 ().
Figure 2. A history of tolerogenic vaccines as specific interventions for inflammatory diseases. (A) Timeline of vaccine discovery. (B) Current state of tolerogenic vaccines. SIT: specific immunotherapy. TolerogenicVac: tolerogenic vaccine. Td: Time of discovery. Trial: clinical trials from USA, Europe and China. Ist: Immunosuppressant. Aprv: Approved by USA FDA or European Union. Spec: antigen specificity. RA: rheumatoid arthritis. MS: multiple sclerosis. T1D: type 1 diabetes. AD: Alzheimer Disease.
Among these novel approaches, we have discovered 2 vaccine technologies to induce antigen specific tolerogenic responses. One of which is “co-immunized vaccine” (protein antigen plus its cognate DNA),Citation45-47 the other one is “suppressed immunization vaccine”(protein antigen plus immunosuppressant)Citation48(). The co-immunized vaccine was discovered by accident when we compared a prime-boost protocol of DNA-protein with a formulation of mixed DNA and protein vaccines for the ease of application. To our surprise, such co-immunized DNA and protein regimens induced impaired cellular immune responses.Citation45 This co-immunized vaccine can be applied to different antigens as long as the DNA encoded antigen is the same as the co-delivered protein. We observed that the co-immunized vaccine could induce CD4+CD25-Foxp3+ Treg cells in animals, including mice, cats, dogs and monkeys, and the suppression of activation of T effector cells was specific for the same antigen used in the co-immunized vaccine. This suppression could translate into tolerogenic responses in vivo and into effective protection/treatment of animals with inflammatory diseases including autoimmune diseases, (diabetes,Citation48,49 auto-immune ovary diseaseCitation50), asthma,Citation51 allergic dermatitisCitation52) and Alzheimer disease.Citation47 Taking autoimmune diabetes for example:1) co-immunized vaccine (insulin plus DNA encoding proinsulin) prevented type 1 diabetes (T1D) progress from hyperglycemia in NOD mice with a 93.8% efficacy for 36Citation49 and 52 weeks (unpublished data). Controlled inflammation in pancreas and suppressed T cell response in spleen were observed in the co-immunized vaccine group, consistent with the proposed mechanism; 2) “suppressed immunization vaccine” of antigen + immunosuppressant (insulin dominant epitope B:9–23 plus dexamethasone, Dex) prevented T1D in NOD mice and protected 10/12 mice from hyperglycemia for 35 weeks (83.3% efficacy). Tolerance establishment was verified under auto-antigen re-challenge.Citation48 We report here further studies undertaken to clarify the mechanisms involved.
Results and Discussion
Expanding the concept of treatment for diabetes
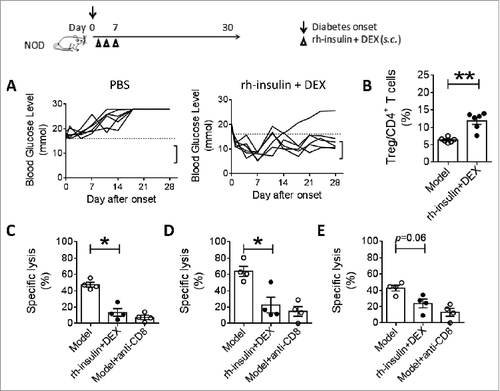
We tested if the suppressed immunization vaccine could also induce tolerogenic responses when used to treat newly diabetic animals. When NOD mice showed signs of diabetes at week 12, they were treated with the suppressed immunization vaccine (recombinant insulin and dexamethasone) at days 1, 4 and 7. Their hyperglycemia was controlled for at least 1 month at 83.3% (5/6) efficacy (). The apparent tolerogenic response was antigen-specific since irrelevant antigen suppressed immunization vaccines did not show any benefit for such amelioration (not shown). To confirm this induced tolerogenic response, Treg cells were analyzed. They were found to be up-regulated significantly in draining lymph nodes after the suppressed immunization (). The main function of Treg cells is to suppress T cell mediated inflammation and in the case of diabetes, the disease is a result of attack on islet cells by auto-reactive T cells, most of which are insulin specific cytotoxic CD8+ T cells. We therefore assessed the activities of cytotoxic CD8+ T cells reacting against islet cells (), insulin 10–18 epitope () or preproinsulin 15–24 (). The level of specific cytotoxic CD8+ T cell activity was suppressed substantially after the suppressed immunization vaccine (), although suppression of activity against preproinsulin 15–24 epitope did not reach statistical significance. This data suggested not only that induced Treg cells inhibited diabetes by suppressing auto-reactive cytotoxic CD8+ T cells but also that the epitope specificity of the target CD8+ cells influenced the degree of susceptibility to inhibition by the Treg. Overall, the degree of suppression by the induced Treg cells was similar to that seen in mice treated with anti-CD8 antibodies. The induced tolerogenic responses were antigen-specific since irrelevant antigen suppressed vaccines did not show any benefit for such amelioration.
Figure 3. Combination vaccination controlled new-onset diabetes and cytotoxic inflammation in NOD mice. New-onset diabetic NOD mice were treated with rh-insulin plus DEX (10 μg+10 μg) at days 1, 4 and 7. (A) Blood glucose was measured at intervals for 28 d Dashed line indicates the diabetes threshold (16.7 mmol/L). Square bracket indicates the normal glucose range of healthy animals (5∼12 mmol/L). (B) Percentage of regulatory T cells among draining lymph node cells. n = 6. On day 14, in vivo cytolytic assay (CTL) was performed against islet cell (C), insulin 10–18 epitope (D) or preproinsulin 15–24 (E). Unvaccinated mice were used as CTL control. Anti-CD8 mAb was i.p. injected to block CTL in unvaccinated mice as positive control. n = 4. Data represent 3 independent experiments.
With its inherent advantage of an antigen specific tolerogenic response, tolerogenic vaccination has shown proof-of-concept in these animal studies and become an attractive approach for translational research and human trials.
Translational research for tolerogenic vaccines
Before a move into human trials, there are 2 issues of concern in translating a candidate tolerogenic vaccine from lab into bedside: safety and efficacy.
In considering safety, it is evident that caution is needed when new formulations are used to develop tolerogenic vaccines since there is no history of such applications being tested in humans, For this reason, drugs such as dexamethasone, rapamycin or cyclosporin A that are already established for use in humans should be considered the first choice for administration with the antigen since their history of adverse effects is well documented.
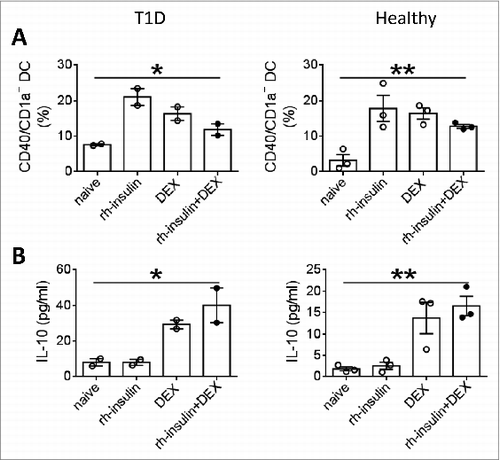
For efficacy, evidence from studies in larger animals should be obtained before test in humans. To this end, we tested the T1D tolerogenic suppressed immunization vaccines in rabbits and dogs, assessing tolerogenic responses in addition to safety. As shown in , the levels of Treg cells, IL-10 and TGF-β were increased when immunized with the suppressed immunization vaccine compared with other groups in rabbits and dogs. The tolerogenic responses are apparently dose-dependent response. When this suppressed immunization vaccine regimen was incubated with fresh isolated PBMC of T1D or healthy individuals in vitro, we observed that co-stimulation molecule CD40 was down-regulated () and IL-10 was upregulated () in CD1a+ dendritic cells compared with incubations of other regimens. The results suggest that the tolerogenic suppressed immunization vaccine could induce tolerogenic responses against auto-reactive responses in larger animals, and maybe in humans as well. In the safety evaluations, we did not observe notable signs of hypoglycemia or behavior abnormalities in the vaccinated large animals ().
Table 2. Safety assessments
Figure 4. Combination vaccination induced immune suppression in rabbit and dog. Rabbits and dogs were vaccinated at day 1, 4 and 7. At day 10, Tregs were analyzed in draining LNs of rabbits (A) or dogs (C). After in vitro restimulation with rh-insulin for 24 h, IL-10/TGF-β mRNA was analyzed in LN cells of rabbits (B) or dogs (D). n = 4. Data represent 3 independent experiments.
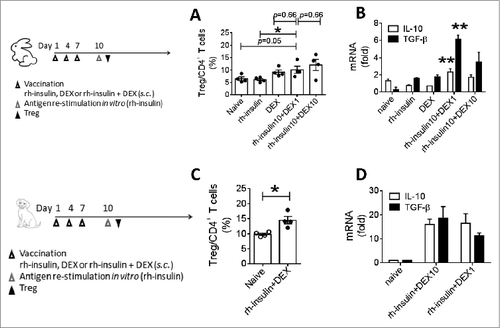
Figure 5. Suppressed immunization vaccine controlled human dendritic cell maturation. PBMC from 2 T1D patients (A, C) and 3 healthy donors (B, D) were cultured with rh-insulin, DEX or the combination for 3 d. Dendritic cell maturation (A, B) and IL-10 secretion in culture were analyzed (C, D).
Taking the results together, tolerogenic vaccination appears to be a promising strategy for treating T1D and should be considered for moving to clinical tests if other requirements are met.
Mechanisms behind induction of antigen specific tolerance
Proper interpretation of immune regulation, particularly after the discovery of regulatory T cell (Treg), requires an understanding of tolerance mechanisms.Citation53 In simple terms, Treg is a subset of CD4+ T cells that is characterized by expression of transcription factor Foxp3, and this subset helps to keep inflammation under control in healthy individuals. Deficiency of Treg or Foxp3 leads to inflammatory reactions and multi-organ autoimmune diseases. Elegant reviews on Treg's origin, development and function have been published elsewhere.Citation54-56
The use of Treg cells as a tool to treat inflammation and autoimmune diseases has been proposed and well received in the scientific community. There are several approaches as induction of tolerogenic responses and their applications listed in and . Two of them, the co-immunized vaccineCitation57 and suppressed immunization vaccine,Citation48 were discovered in our labs. Both of these approaches are aimed at inducing antigen specific Treg cells to control inflammatory reactions and autoimmune diseases. We also discovered that the co-immunized vaccine regimens entered DC cells via a caveolae-dependent route that generated negative signaling, to shut down the IRANK-NF-kB pathway and CD40 expression, but turned on the expression of IL-10 and TGF-b. In this way, the DC became CD40lowIL-10+ regulatory dendritic cells (DCreg) that could in turn cause naïve T cells to differentiate into inducible regulatory T cells (iTreg).Citation58 Since this differentiation into iTreg required MHC-II-TCR signaling, this production of iTreg cells required antigen specific recognition and accordingly resulted in suppression of cognate antigen specific T cell activation and inflammation.
Table 3. Mechanism of induction of Tregs by tolerogenic vaccines
Figure 6. Treg as a mechanism for tolerogenic vaccines. Specific tolerance targeting pathogenic inflammation (A) can be induced by tolerogenic vaccine through various mechanisms including Treg (B), and in turn ameliorates disease progression (C).
In another hand, the understanding of exact mechanism of tolerance induction by the suppressed immunization vaccine (e.g. protein+Dex) is improved. Dexamethasone preferentially induces apoptosis of dendritic cells (cDCs and pDCs),Citation59 effector CD4+ T cells (Teff)Citation60 and B-2 cells;Citation61 whereas the CD11cloCD40loCD86hiMHCIIlo macrophages, CD4+Foxp3+ Treg cells, and IL-10+ B-1 cells are relatively protected.Citation59-61 As a result, Ag immunization in the presence of Dexamethasone preferentially expands Ag-specific Treg cells and B-1 cells while blocking the recall responses of Teff and B-2 cells, which leads to tolerance.Citation59,61
Taking these new findings together, iTreg induction by vaccination is an attractive and feasible approach toward human applications although its detailed mechanisms remain to be further investigated.
We note that besides the CD4+ Treg cell, immuno-tolerance can be induced by other types of cells including CD8 Treg,Citation62,63 regulatory B cells (Bregs)Citation64-66 and IL-10 producing B cells (B10 cells).Citation67 Since these cells can also participate in tolerance induction in inflammatory contexts, they too might potentially be drawn into application in tolerogenic vaccines against autoimmune related diseases.
Concluding remarks
In conclusion, the tolerogenic vaccine provides a feasible easy means to induce specific tolerogenic responses to reduce inflammation and has a great potential for clinical application against major inflammatory diseases, such as allergy, asthma, autoimmune diseases, atherosclerosis and Alzheimer disease. Accordingly, we propose here that efforts should be devoted to development of these novel vaccine strategies, expansion of the spectrum of diseases targeted and translation from bench to bedside. To achieve this the underlying mechanisms will require further exploration.
Material and Methods
Animals and samples
Foxp3-eGFP mice of Balb/c background, NOD mice, New Zealand White female rabbits and female Beagles dogs were purchased from VitalRiver (Beijing, China) and maintained in Specific-Pathogen-Free conditions. Peripheral blood was collected from healthy donors with informed consent. All animal experiments were reviewed and permitted by the Experimental Animal Ethics Committee of Shanghai Medical College. All experiments using human PBMC were reviewed and permitted by the Clinical Experiment Ethics Committee of Shanghai Medical College.
Vaccination, re-challenge, re-stimulation and culture
The rh-insulin was purchased from Eli Lily(Indianapolis, Indiana, USA) in 400IU/10 ml packages, and dose conversion was calculated as 1IU = 35 μg as instructed. Dexamethasone was purchased from Sigma Aldrich and dissolved first in 0.2 MNaOH then diluted in PBS to final concentration of 10 mM and the pH adjusted to 7.2–7.4. Suppressed immunization vaccines were prepared at doses suitable for the different animals: mouse, 10 μg insulin + 10 μg DEX; rabbit 10 μg insulin + 10 μg DEX or 10 μg insulin + 1 μg DEX; dog, 0.15IU/kg insulin + 10 μg/kg DEX or 0.15IU/kg insulin + 1 μg/kg DEX. At days 1,4 and 7, animals were s.c. injected with single or suppressed immunization vaccine in 100 μl (mouse and rabbit) or 200 μl (dog) PBS.
Vaccinated mice were given a challenge dose of rh-insulin, B9–23 epitope or ovalbumin(OVA) at day 10 and after 24 h, the draining lymph nodes(Inguinal LNs) were collected to test for Treg responses. Vaccinated rabbits or dogs were sacrificed then draining lymph nodes were collected for in vitro re-stimulation. Single cell suspensions were cultured with rh-insulin 10 μg/ml for 24 h, then total RNA was extracted by Trizol and reverse transcribed into cDNA with oligo-dT as primer in 42°C for 1 h. PCR was performed to test expression of IL-10 and TGF-β. For human PBMC stimulation, PMBCs from patients or healthy donors were first induced into dendrtic cells with GMCSF and IL-4, then rh-insulin and/or DEX were added into cultures at a final concentration of 10 μg/ml each for 3 d CD40 expression on CD1a+ cells was then analyzed by FACS, and IL-10 secretion into the supernatant was analyzed by FlexSet(BD Biosciences, San Jose, CA,USA). All cell culture was performed with DMEM with 5% FBS, in 5% CO2 incubator at 37°C.
Type 1 diabetes model
Young female NOD mice were monitored for hyperglycemia every 2 d Mice with 2 consecutive elevations of blood glucose level (>16.7 mmol/L) were defined as new-onset diabetes (Day 0) and then treated with vaccine at days 1, 4 and 7. Blood glucose was monitored until day 28.
Flow cytometry
To detect murine Treg, single cell suspensions were prepared then stained with anti-CD4-APC andanti-CD25-PE. Foxp3 was labeled with endogenous GFP or was intracellular-stained with anti-Foxp3-FITC mAb. For rabbit or dog, surface staining of CD4-FITC(clone KEN-4 or clone YKIX302.9) was followed by intracellular staining of Foxp3-APC(clone FJK-16s) with Treg staining kit (eBioscience, San Diego, CA, USA). For PBMC maturation markers, 72 h after in vitro re-stimulation, cells were washed with PBS and stained with CD1a-FITC, CD40-PE/CD80-PE/CD83-PE/CD86-PE. All antibodies were checked for species specificity and ordered from eBioscience. Data were collected with BD Calibur(BD Biosciences) and analyzed with Flowjo (Treestar, USA).
In vivo CTL
New-onset diabetic NOD mice were treated with suppressed immunization vaccine at days 1, 4 and 7. At day 14, target cells were prepared either with islet cells from naive NOD mice, or with naive NOD splenocytes incubated with 500 μg/ml peptide epitope (insulin B10–18 epitope or preproinsulin 15–24 epitopeCitation68,69). Control cells were prepared with naive NOD splenocytes without epitope. Target cells and control cells were labeled with eFluor670 (10 μM for target cells, 2 μM for control cells), mixed at 1:1 ratio, then i.v. transferred (2 × 106 totalcells/mouse) into vaccinated diabetic mice. After 12 h, spleens of recipient mice were collected to assess specific cytolysis. Percentage of specific cytolysis was calculated as (Control cell-Target cell)/Control cell×100%. Purified anti-CD8 mAb (100 μg) was i.p. injected at days11, 12 and 13 into new-onset diabetic NOD mice as a depletion control for CTL suppression.
Evaluation of hypoglycemic symptom
Each NOD mouse was closely observed for hypoglycemic signs (vomit, seizure, shock or death) and behavior abnormalities (depression or dysphoria) for 4 h after vaccination and then once a day between injections. The incidence of these signs was recorded as either positive or negative. Overdose of rh-insulin (500 μg per rabbit or 0.3IU/kg for dog) was used as hypoglycemia positive control, and animals experiencing hypoglycemic shock were injected i.v. with saline containing 10 g glucose to rescue.
Statistics
Two-tailed Mann-Whitney test(, ) or 1-way ANOVA test ( and ) was used to calculate significance of data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gonzalez-Rey E, Ganea D, Delgado M. Neuropeptides: keeping the balance between pathogen immunity and immune tolerance. Curr Opin Pharmacol 2010; 10(4):473-81; PMID:20399708; http://dx.doi.org/10.1016/j.coph.2010.03.003
- Nagler-Anderson C. Tolerance and immunity in the intestinal immune system. Crit Rev Immunol 2000; 20(2):103-20; PMID:10872893; http://dx.doi.org/10.1615/CritRevImmunol.v20.i2.20
- Tse MT. Autoimmunity: Particulate promotion of tolerance. Nat Rev Immunol 2013; 13(1):6-7; PMID:23237965; http://dx.doi.org/10.1038/nri3373
- Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010; 464(7293):1293-300; PMID:20432533; http://dx.doi.org/10.1038/nature08933
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454(7203):445-54; PMID:18650915; http://dx.doi.org/10.1038/nature07204
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010; 10(12):838-48; PMID:21060320; http://dx.doi.org/10.1038/nri2870
- Libby P. Inflammation in atherosclerosis. Nature 2002; 420(6917):868-74; PMID:12490960; http://dx.doi.org/10.1038/nature01323
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010; 362(4):329-44; PMID:20107219; http://dx.doi.org/10.1056/NEJMra0909142
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2012; 2(1):a006346; PMID:22315714; http://dx.doi.org/10.1101/cshperspect.a006346
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 2006; 12(9):1005-15; PMID:16960575
- Williams KJ, Tabas I. Atherosclerosis and inflammation. Science 2002; 297(5581):521-2; PMID:12143880; http://dx.doi.org/10.1126/science.297.5581.521
- McGeer EG, McGeer PL. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr Pharm Des 1999; 5(10):821-36; PMID:10526088
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140(6):883-99; PMID:20303878; http://dx.doi.org/10.1016/j.cell.2010.01.025
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol 2007; 7(9):665-77; PMID:17690713; http://dx.doi.org/10.1038/nri2153
- Bingham CO 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010; 62(1):64-74; PMID:20039397; http://dx.doi.org/10.1002/art.25034
- Prentice HG, Blacklock HA, Janossy G, Bradstock KF, Skeggs D, Goldstein G, Hoffbrand AV. Use of anti-T-cell monoclonal antibody OKT3 to prevent acute graft-versus-host disease in allogeneic bone-marrow transplantation for acute leukaemia. Lancet 1982; 1(8274):700-3; PMID:6122004; http://dx.doi.org/10.1016/S0140-6736(82)92619-8
- Favas C, Isenberg DA. B-cell-depletion therapy in SLE–what are the current prospects for its acceptance? Nat Rev Rheumatol 2009; 5(12):711-6; PMID:19946298; http://dx.doi.org/10.1038/nrrheum.2009.218
- Jokipii AM, Jokipii L. Suppression of cell-mediated immunity by cyclophosphamide: its independence of concomitant B cell response. Cell Immunol 1973; 9(3):477-81; PMID:4543314; http://dx.doi.org/10.1016/0008-8749(73)90063-4
- Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, Bergmeister H, Wrba F, Kurtz J, Kiss C, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood 2003; 101(7):2886-93; PMID:12433677; http://dx.doi.org/10.1182/blood-2002-10-3014
- Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol 2007; 5(7):555-63; PMID:17558423; http://dx.doi.org/10.1038/nrmicro1709
- Finco O, Rappuoli R. Designing vaccines for the twenty-first century society. Front Immunol 2014; 5:12; PMID:24478777; http://dx.doi.org/10.3389/fimmu.2014.00012
- Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol 2011; 11(12):865-72; PMID:22051890
- Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C, et al. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev 1999; 170:197-222; PMID:10566152; http://dx.doi.org/10.1111/j.1600-065X.1999.tb01339.x
- Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol 2002; 2(6):446-53; PMID:12093011
- Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol 2004; 4(6):407-19; PMID:15173830; http://dx.doi.org/10.1038/nri1370
- Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol 2012; 5(3):232-9; PMID:22318493; http://dx.doi.org/10.1038/mi.2012.4
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol 2006; 6(10):761-71; PMID:16998509; http://dx.doi.org/10.1038/nri1934
- Ciprandi G, Marseglia GL, Tosca MA. Allergen-specific immunotherapy: an update on immunological mechanisms of action. Monaldi Arch Chest Dis 2006 65(1):34-7; PMID:16700191
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005 54(6):1763-9; PMID:15919798; http://dx.doi.org/10.2337/diabetes.54.6.1763
- Lunardon L, Payne AS. Rituximab for autoimmune blistering diseases: recent studies, new insights. G Ital Dermatol Venereol 2012; 147(3):269-76; PMID:22648328
- Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune diseases. Immunobiology 2002; 206(5):519-27; PMID:12607727; http://dx.doi.org/10.1078/0171-2985-00200
- Zagury D, Gallo RC. Anti-cytokine Ab immune therapy: present status and perspectives. Drug Discov Today 2004; 9(2):72-81; PMID:15012931; http://dx.doi.org/10.1016/S1359-6446(03)02955-6
- O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol 2002; 2(1):37-45; PMID:11905836; http://dx.doi.org/10.1038/nri702
- Thomson AW, Carroll PB, McCauley J, Woo J, Abu-Elmagd K, Starzl TE, Van Thiel DH. FK 506: a novel immunosuppressant for treatment of autoimmune disease. Rationale and preliminary clinical experience at the University of Pittsburgh. Springer Semin Immunopathol 1993; 14(4):323-44; PMID:7686690; http://dx.doi.org/10.1007/BF00192307
- Thomson AW, Starzl TE. FK 506 and autoimmune disease: perspective and prospects. Autoimmunity 1992; 12(4):303-13; PMID:1382646; http://dx.doi.org/10.3109/08916939209148473
- Schwaiger T, van den Brandt C, Fitzner B, Zaatreh S, Kraatz F, Dummer A, Nizze H, Evert M, Bröker BM, Brunner-Weinzierl MC, et al. Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut 2014; 63(3):494-505; PMID:23564336; http://dx.doi.org/10.1136/gutjnl-2012-303635
- Suzuki K, Yan H, Li XK, Amemiya H, Suzuki S, Hiromitsu K. Prevention of experimentally induced autoimmune type I diabetes in rats by the new immunosuppressive reagent FTY720. Transplant Proc 1998; 30(4):1044-5; PMID:9636421; http://dx.doi.org/10.1016/S0041-1345(98)00143-2
- Papadopoulos D, Rundle J, Patel R, Marshall I, Stretton J, Eaton R, Richardson JC, Gonzalez MI, Philpott KL, Reynolds R. FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J Neurosci Res 2010; 88(2):346-59; PMID:19658199; http://dx.doi.org/10.1002/jnr.22196
- Anderson RP, Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr Opin Immunol 2013; 25(3):410-7; PMID:23478068; http://dx.doi.org/10.1016/j.coi.2013.02.004
- Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann N Y Acad Sci 1996; 778:1-27; PMID:8610963; http://dx.doi.org/10.1111/j.1749-6632.1996.tb21110.x
- Noon L CB. Prophylactic inoculation against hay fever. Lancet 1911; 177: 1572–73; http://dx.doi.org/10.1016/S0140-6736(00)78276-6.
- Vandenbark AA, Morgan E, Bartholomew R, Bourdette D, Whitham R, Carlo D, Gold D, Hashim G, Offner H. TCR peptide therapy in human autoimmune diseases. Neurochem Res 2001; 26(6):713-30; PMID:11519731; http://dx.doi.org/10.1023/A:1010951706830
- Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med 2005; 11(4 Suppl):S69-76; PMID:15812493; http://dx.doi.org/10.1038/nm1226
- Mor G, Yamshchikov G, Sedegah M, Takeno M, Wang R, Houghten RA, Hoffman S, Klinman DM. Induction of neonatal tolerance by plasmid DNA vaccination of mice. J Clin Invest 1996; 98(12):2700-5; PMID:8981914; http://dx.doi.org/10.1172/JCI119094
- Jin H, Kang Y, Zheng G, Xie Q, Xiao C, Zhang X, Yu Y, Zhu K, Zhao G, Zhang F, et al. Induction of active immune suppression by co-immunization with DNA- and protein-based vaccines. Virology 2005; 337(1):183-91; PMID:15914231; http://dx.doi.org/10.1016/j.virol.2005.03.029
- Jin J, Ding Z, Meng F, Liu Q, Ng T, Hu Y, Zhao G, Zhai B, Chu HJ, Wang B. An immunotherapeutic treatment against flea allergy dermatitis in cats by co-immunization of DNA and protein vaccines. Vaccine 2010; 28(8):1997-2004; PMID:20188255; http://dx.doi.org/10.1016/j.vaccine.2009.10.092
- Wang S, Yu Y, Geng S, Wang D, Zhang L, Xie X, Wu B, Li C, Xu H, Li X, et al. A coimmunization vaccine of Abeta42 ameliorates cognitive deficits without brain inflammation in an Alzheimer's disease model. Alzheimers Res Ther 2014; 6(3):26; PMID:24987466; http://dx.doi.org/10.1186/alzrt256
- Kang Y, Xu L, Wang B, Chen A, Zheng G. Cutting edge: Immunosuppressant as adjuvant for tolerogenic immunization. J Immunol 2008; 180(8):5172-6; PMID:18390698; http://dx.doi.org/10.4049/jimmunol.180.8.5172
- Zhang W, Jin H, Hu Y, Yu Y, Li X, Ding Z, Kang Y, Wang B. Protective response against type 1 diabetes in nonobese diabetic mice after coimmunization with insulin and DNA encoding proinsulin. Hum Gene Ther 2010; 21(2):171-8; PMID:19788384; http://dx.doi.org/10.1089/hum.2009.095
- Li J, Jin H, Zhang F, Du X, Zhao G, Yu Y, Wang B. Treatment of autoimmune ovarian disease by co-administration with mouse zona pellucida protein 3 and DNA vaccine through induction of adaptive regulatory T cells. J Gene Med 2008; 10(7):810-20; PMID:18452236; http://dx.doi.org/10.1002/jgm.1200
- Jin H, Xiao C, Geng S, Hu Y, She R, Yu Y, Kang Y, Wang B. Protein/DNA vaccine-induced antigen-specific Treg confer protection against asthma. Eur J Immunol 2008; 38(9):2451-63; PMID:18792401; http://dx.doi.org/10.1002/eji.200737899
- Jin H, Kang Y, Zhao L, Xiao C, Hu Y, She R, Yu Y, Du X, Zhao G, Ng T, et al. Induction of adaptive T regulatory cells that suppress the allergic response by coimmunization of DNA and protein vaccines. J Immunol 2008; 180(8):5360-72; PMID:18390718; http://dx.doi.org/10.4049/jimmunol.180.8.5360
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133(5):775-87; PMID:18510923; http://dx.doi.org/10.1016/j.cell.2008.05.009
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8(7):523-32; PMID:18566595; http://dx.doi.org/10.1038/nri2343
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003; 3(3):253-7; PMID:12658273; http://dx.doi.org/10.1038/nri1032
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30:531-64; PMID:22224781; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141623
- Kang Y, Jin H, Zheng G, Du X, Xiao C, Zhang X, Geng S, Li X, Wang J, Chen A, et al. Co-inoculation of DNA and protein vaccines induces antigen-specific T cell suppression. Biochem Biophys Res Commun 2007; 353(4):1034-9; PMID:17204242; http://dx.doi.org/10.1016/j.bbrc.2006.12.124
- Li J, Geng S, Xie X, Liu H, Zheng G, Sun X, Zhao G, Wan Y, Wu Y, Chen X, et al. Caveolin-1-mediated negative signaling plays a critical role in the induction of regulatory dendritic cells by DNA and protein coimmunization. J Immunol 2012; 189(6):2852-9; PMID:22904311; http://dx.doi.org/10.4049/jimmunol.1102828
- Zheng G, Zhong S, Geng Y, Munirathinam G, Cha I, Reardon C, Getz GS, van Rooijen N, Kang Y, Wang B, et al. Dexamethasone promotes tolerance in vivo by enriching CD11clo CD40lo tolerogenic macrophages. Eur J Immunol 2013; 43(1):219-27; PMID:23001956; http://dx.doi.org/10.1002/eji.201242468
- Chen X, Murakami T, Oppenheim JJ, Howard OM. Differential response of murine CD4+CD25+ and CD4+CD25- T cells to dexamethasone-induced cell death. Eur J Immunol 2004; 34(3):859-69; PMID:14991616; http://dx.doi.org/10.1002/eji.200324506
- Chen A, Geng Y, Ke H, Constant L, Yan Z, Pan Y, Lee P, Tan I, Williams K, George S, et al. Cutting edge: Dexamethasone potentiates the responses of both regulatory T cells and B-1 cells to antigen immunization in the ApoE(-/-) mouse model of atherosclerosis. J Immunol 2014; 193(1):35-9; PMID:24899497; http://dx.doi.org/10.4049/jimmunol.1302469
- Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, et al. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 2012; 119(24):5898-908; PMID:22538855; http://dx.doi.org/10.1182/blood-2011-12-396119
- Tsai YG, Lee CY, Lin TY, Lin CY. CD8(+) Treg cells associated with decreasing disease activity after intravenous methylprednisolone pulse therapy in lupus nephritis with heavy proteinuria. PLoS One 2014; 9(1):e81344; PMID:24475019
- Chesneau M, Michel L, Degauque N, Brouard S. Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol 2013; 4:497; PMID:24427159; http://dx.doi.org/10.3389/fimmu.2013.00497
- Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012; 491(7423):264-8; PMID:23064231; http://dx.doi.org/10.1038/nature11501
- Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol 2013; 10(2):122-32; PMID:23292280; http://dx.doi.org/10.1038/cmi.2012.60
- Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 2013; 15(Suppl 1):S1; PMID:23566714; http://dx.doi.org/10.1186/ar3907
- Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol 2007; 148(1):1-16; PMID:17349009; http://dx.doi.org/10.1111/j.1365-2249.2006.03244.x
- Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A 2005; 102(51):18425-30; PMID:16339897; http://dx.doi.org/10.1073/pnas.0508621102
- Caspi RR. Tregitopes switch on Tregs. Blood 2008; 112(8):3003-4; PMID:18840719; http://dx.doi.org/10.1182/blood-2008-08-173609
- Fissolo N, Costa C, Nurtdinov RN, Bustamante MF, Llombart V, Mansilla MJ, Espejo C, Montalban X, Comabella M. Treatment with MOG-DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up-regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis. J Neuroinflammation 2012; 9:139; PMID:22727044; http://dx.doi.org/10.1186/1742-2094-9-139
