Abstract
As the most potent antigen-presenting cells, dendritic cells (DCs) are pivotal players in regulating immune responses. DC-based technologies have generated a series of typical and promising therapeutic options, especially after the first DC-based cancer vaccine was approved by US. Food and Drug Administration (US. FDA). In this context, this paper employs patents and citation networks to conduct a fundamental analysis in order to show overall landscape of DC-based technologies. The results in this research can be used as references for decision-making in developing efficacious DC therapeutic products.
Introduction
Dendritic cells (DCs) are a crucial component of the immune system through their high capacity to take up, process and present antigens to T cells, by which DCs control an array of responses. Ralph Steinman first described DCs in 1973, and for this discovery and further research on DCs, he received the Nobel Prize in Physiology or Medicine in 2011.
Owing to DCs’ high efficiency at generating immune responses against antigens such as viruses, other pathogens and endogenous tumors, DC-based immunotherapy represents a new and promising approach.Citation1 The first DC-based cancer vaccine, sipuleucel-T, was approved by the US. Food and Drug Administration (US. FDA) for treating metastatic prostate cancer,Citation2 and has inspired clinical research and development (R&D). Clinical trials of DC-related pharmaceutical products are being conducted in different phases despite various difficulties and challenges.Citation3-5
For successful research and development (R&D), potential market profit of products should be guaranteed through wise intellectual property management of relevant technologies. Patents as an important element of R&D outcomes set up hurdles for technology advances by claiming exclusivity but also imply value and shed light on development possibilities.Citation6 However, no studies have examined the technological indications of overall patent activity. To navigate the hurdles and identify opportunities, we analyzed the DC patent landscape based on multiple dimensions of patent information to examine main trends in DC-based technology development.
Patent data has long been recognized as a fruitful source of well-organized and up-to-date technology information about innovation. Recently, investigations of patenting activities specified the current status, discovered the technological position, determined technical landmarks and forecast technology development tendency in a sector, technology domain or company, which had proved effective in previous studies.Citation7-12
Our study aims to identify patent activities relevant to DCs, to capture a profile of technologies related to DCs and further provide researchers, policy-makers and investors with data for making sound decisions. Facilitating R&D of DC-related products and therapeutic tactics is important. Therefore, we searched the US. Patent and Trademark Office (US. PTO) database for granted patents that contained “dendritic cell” in patent title, summary or claims. This search strategy reduced noise and revealed the patents that are most related to DCs. To make this research more manageable, we retrieved relevant granted patents by 2013. Thus, information on 395 patents was collected.
Dendritic cell patenting activity
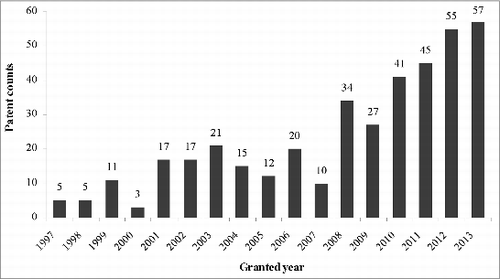
As knowledge on DC immunobiology has accumulated over the past few decades, antigen-presenting roles of DCs are critical in immune response process. The scale of patent activity indicates the research and development in a particular technology field.Citation13 The number of DC-related patents issued over time is depicted in .
The earliest relevant patents were issued in 1997, and the period generally corresponds to the initial trials of DC vaccines launched for disease therapy.Citation14 The total number of patents has increased with much more active growth in the last several years. The only 2-consecutive-year decline was detected in 2004-2005. This low may be due to the disappointing records of effectiveness observed in clinical trials of DC therapeutic vaccines.Citation14 Slight disease responses made this new immunotherapy controversial, which also led to the rejection of sipuleucel-T in 2007 by the US. FDA. This affected patenting activity. However, DC-based research received a notable boost and refreshed impetus after sipuleucel-T was approved as the first DC-based cancer vaccine in 2010.
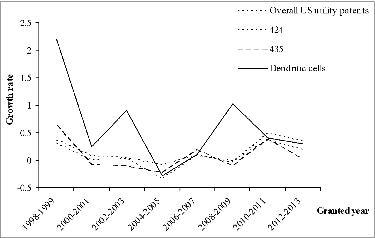
Different technology topics have unique development characteristics. Compared to the stem cell patenting profile,Citation15 the technology relative to DCs differs. To identify the specific growth trajectory of DC-based patents, patenting activity of relevant technology areas and overall US utility patents are presented as reference. In this research, US. Patent Classification (USPC) of 424 [drug, bio-affecting and body treating compositions] and 435 [chemistry: molecular biology and microbiology] are involved, which are determined by the leading USPC attached to DC relevant patents. shows the growth rate in the number of patents in the same time frame but in 2-year cohorts for more stable results. The growth rates of patents in 424 and 435 are the same, which hinges on the overall US utility patents. More obvious fluctuation is observed in the growth rate of DC-related patents, indicating special activity over time. After the dramatic drop and then increase observed from 2004-2005 to 2008-2009, the growth rate of patents related to DCs appears to follow 3 other technology areas, i.e., USPC 424, USPC 435, and overall US utility inventions.
Patenting strategy distribution
Patent information analysis is important for identifying potential issues and specific productive research areas to accelerate DC-targeted research. Fortunately, this information is disclosed clearly when patents are granted, which provides insight into patenting strategies.
The interest in R&D activity related to DCs can be illuminated in terms of the distribution of patenting strategies over time. Since patenting strategies cover upstream and downstream products,Citation12 we manually organized and cataloged the patents into upstream technologies (DCs obtainment and/or enrichment, antigen-targeted assays), downstream inventions (DC-targeted immune modulating formulation, immune response assessment) and other limbic products. The subsequent studies discussed in this paper also use this classification system.
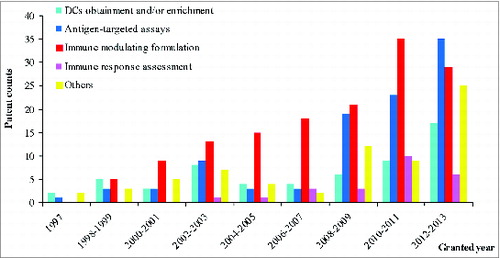
In the typical definition of DC vaccines, antigen-targeted research, including various antigen manipulations and transport system optimization, and immune modulating formulation are the vital topics in inducing efficient immunologic response via DCs.Citation16 This is consistent with the structure of patenting strategies displayed in . These two research categories also provide main contributions to the patenting activity performance shown in .
Patent counts related to formulations that modulate immune responses have grown steadily for most of the development period, while antigen-targeted research differs conspicuously in patenting activity between early and the later periods (). The steady growth in immune modulating formulations depends upon balanced development involving disease-derived antigens or genetic encoding antigen loading to DCs, antibody-mediated immune modulation, virus/bacteria stimulating DCs and other immunoregulators. After the relevant patent summaries were screened, the recent sharp increase (from 2008-2009 to 2011-2012) in antigen-targeted research led to many new antigen-manipulating inventions as well as antigen transport systems, which makes the performance of antigen-targeted research so remarkable. Additionally, the momentum might continue.
Although patenting strategies manifest separate development rules, the strategies are also mutually influenced by intrinsic technical bond. For example, inventions that provide methods for obtaining and enriching the population of DCs from precursors or cell mixtures occupied a prominent role in the early years to consolidate the base technology that supports other downstream DC-related research. In addition, the number of patents that involve immune response assessment approaches has rising lately in spite of the late start.
Patent assignee diversity
The essence of a granted patent is the right to exclude others from applying the invention commercially for a limited period in exchange for disclosing the invention to encourage innovation and creation. In other words, patentees affirm their technological territories through granted patents, which reflect the patentees’ research activities, priorities and accomplishments. Patent ownership is an important part of patent information analysis. In and , the features and patenting strategy distribution of patent ownership by sector and independent assignee are identified.
Figure 5. The distribution of strategies among the leading assignees related to DC patents. Private entities are marked with stars.
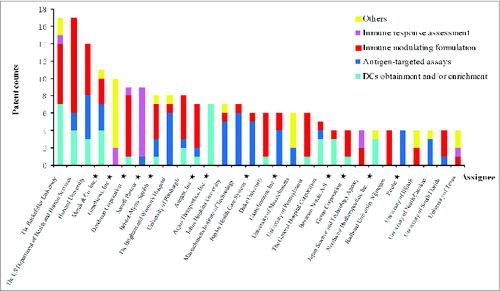
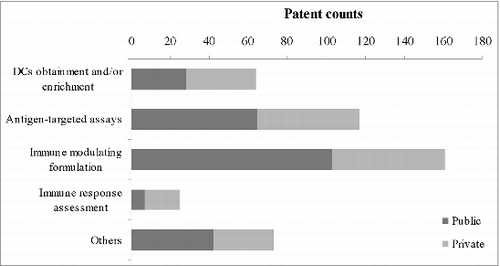
According to the grouping method used by Bergman and Graff,Citation17 all assignees are characterized into 2 sectors: (i) the private sector, which includes privately held companies, publicly traded corporations and individuals, and (ii) the public sector, which includes government agencies and academic or nonprofit research institutions. Overall, public organizations participated more than private companies. Of the DC-related patents, 56% are assigned to public sector organizations, and 44% to private sector entities. Since DCs are still a young technology domain, exploratory R&D seems to be generally supported by public funds.
In the comparison of patenting strategy distribution (), the proportion of the public sector is greater than that of the private sector when referring to patents related to antigen-focused products and DC-targeted immune modulation. Private sector entities express more interest in protocols for obtaining or enriching DCs and downstream immune response assessment methods, which are more accessible in practice than basic research.
The analysis of patent ownership from assignees showed research interest, leadership position and competitive relationship of certain innovators. A total of 195 organizations and individuals hold patents related to DCs (395 patents in total). Until the end of 2013, the 2 institutions possessing the most patents (Rockefeller University and the US. Department of Health and Human Services) accounted for only tiny fractions of the total relevant patents (4% each). All of these illustrate the fragmented situation of assignees related to DC patents. No monopoly has formed in this research field.
Of the private organizations, most are small biopharmaceutical companies with a specific focus on DCs or disease immunotherapy. At the same time, some large pharmaceutical companies such as Merck, Sanofi Pasteur, Amgen and Roche are also prominent participants (). These pharmaceutical companies do not put much effort into DCs but more likely build significant positions by merging or acquiring other biotech companies for technical or business reasons, such as BioVex (acquired by Amgen in 2011), Schering-Plough (acquired by Merck in 2009), VaxDesign (acquired by Sanofi Pasteur in 2010), GlycArt Biotechnology (acquired by Roche in 2005) and so on.
Another important point is that most leading assignees have decentralized patenting strategies, while few focus on only one patenting strategy (). In other words, leading assignees prone to create a patent portfolio, within which inventions across different patenting strategies are all interlocking. Organizations with complex patenting strategies covering upstream and downstream paths have more opportunities to get projects launched, due to less required complementary technologies and increasing difficulty in inventing around.
Patenting citation network
Patent citations are determined when patents take other early inventions as references. This pattern of information reflects technology connections among inventions, which offers insight into DC-related technology development. Patent citation network constructed based on this relationship is useful for providing a holistic and dynamic perspective on a specific technical area.Citation9,11,18,19 Analyzing these networks, similar to a navigation map of a specific technical area, helps figure out crucial participants and visually marks the intellectual boundary, which aids in making sound decisions for moving forward.
In our networks, the nodes represent patents, and the edges represent citation connections from cited patents pointing to citing patents. Aware of the varying number of patents over time (), we sorted 395 patents by granted date and set 2 time points to split the patents into 3 groups with the same number. Then, 3 stages were produced: Stage 1 contained the first group of patents (132); stage 2 contained the first and second groups (264); and stage 3 covered all the patents (395). We separated the data in a successional and accumulative manner. Three citation networks were generated based on the citation relations of patents involved in the stages regardless of single nodes that have no links to a patent from the same stage (). We present the dynamic developing trace of DC-based technology over the stages and uncover technological trends in network topological analysis.
Figure 6. Technology development detected by patent citation networks. The node colors represent different patenting strategies. Patents involved in disease treatment or prevention information are circled and noted in networks.
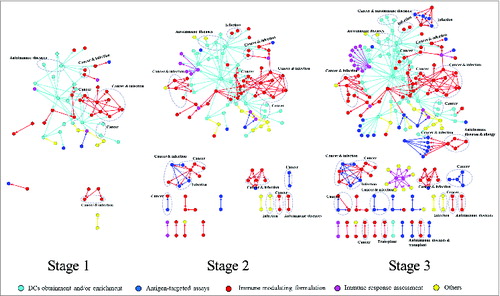
Technology development profiles. The citation network of a specific stage was limited to connections between patents of the same stage, while single nodes in the stage were removed to keep focus on the connections among the inventions. Thus, connected node ratio is introduced to reflect the coverage of internal technical references. Increasing connected node ratio over the 3 stages suggests that newly granted patents depend on earlier technologies more widely (Table 1).
As visualized in citation networks of the 3 stages, we can easily identify the expanding evolution when more relevant patents are granted (). Network framework specifics, which refer to one huge component consisting of diverse patenting strategies and other small components, are observed as early as stage 1. The extension from existing connections coupled with the emergence of new components contributes effectively to expanding the networks. This performance discloses the practice of technology development: forming new research interests or improving established topics.
Citation relationship frequently expresses meaningful facts in conjunction with other information.Citation9,18 Large components consist of more than one patenting strategy, distinguished by different colors. In addition, based on the citation networks, we introduce 2 ownership indicators for the tech-inputting and tech-outputting properties in DC research, Ro and Ri (see the definitions in Methods). In the networks, out-degree represents the citations of a patent, and in-degree represents the number of citations, which measures technology outputting and inputting, respectively. Since Ro is larger than 1, public sector is the major tech-outputting player, which owns basic inventions that are cited by other inventions (Table 1). Private sector is the major tech-inputting player, which develops relevant products. The gradually decreasing Ro and Ri both signify the weaker influence of public institutions in the citation networks of 3 stages.
Most-cited dendritic cell patents in networks. Citation frequency is a meaningful indicator of the influence of a technological innovation and the pervasiveness of technology information.Citation20,21 In addition, patent citation networks can reveal key patents described as network hubs, which are characterized by high citation frequency.Citation19 Thus, the most-cited patents (with the highest out-degree) are summarized in Table 2 to identify noteworthy technologies toward subsequent innovations in the 3 networks ().
Of the most-cited patents, half are held by public organizations and half by private entities. The public organizations concentrate in the earlier stage while private organizations predominate in the later stages. This confirms the influence of private sector throughout the stages. It is important to note that none of these patents are held by traditional large pharmaceutical companies.
Some patents appear as hubs in the 3 stages meanwhile. Surprisingly, 3 hubs issued as immune response assessments arise in stage 3, meaning that research efforts are partly shifting to downstream research.
Dendritic cell patents related to disease. For initiating and coordinating both innate and adaptive immune responses, DCs have shown pathogenic roles in many disease processes. Together with accessibility in immune system, manipulating DCs is appropriate as a therapeutic approach.Citation22 Patent system protects the clinical potential of important DC research through proper patent claims that ensure assignees’ advantages in advancing technology. Thus, we provide primary disease treatment or prevention information of patents in networks to perceive the status and development activity of DC inventions in therapeutic applications ().
DC patents in networks display therapeutic concerns in various diseases that are also hotspots among academics, such as cancer, infection, autoimmune disease and transplants.Citation4,22-25 Patents are organized as sub-components by considering similar disease treatment claims and network positions. Over the stages, disease-relevant sub-components increased impressively with the same manner as the network expansion. These applications have been claimed carefully within patenting strategies of antigen-focused research and immune modulating formulations. Inventions involved in cancer and infection, mostly to stimulate immune responses, show the dominant role for clinical DC applications in managing diseases in the 3 stages, which predicts that future disease-related attention should be kept on cancer and infection.
Discussion
The greatest advantage of patent data is that they represent a direct outcome of invention process. As a fundamental work, this study compiled and analyzed the DC patent landscape, providing a valuable resource for researchers, policy-makers and investors to accelerate DC-based immunotherapy research and longer-term commercial production. The landscape exhibits patenting activity, technical structure, owners and the interrelationship of patent advances and development in DC-based techniques, and thus indicates not only the exclusive hurdles but also implies opportunities for developing optimal vaccine formulations.
A reasonable scale of patents has been granted within this field. They show an active performance over whole period, especially since 2007. This can be seen in the number of patents as well as the 2 time points that generated 3 stages (early 2007 and middle 2010). If patenting activity follows this trend in the future, there will soon be a significant mass that will direct more potential products to market.
Based on a study of the complex technical content, the most intense patenting activities in antigen-targeted research and immune modulating formulation have recently made major contributions to the active patent performance. Due to high interdependence of DC vaccine system, weak downstream innovations may cause bottlenecks,Citation17 which should attract much attention from researchers.
Though low response rate frustrated the initial enthusiasm in DC-based clinical immunotherapy at some level, substantial research offered vaccine tactics to revise or optimize current protocols in order to improve the efficacy.Citation5,26,27 Furthermore, clinical trials have yielded encouraging clinical outcomes against various cancers.Citation4 The problem in correlations between immune responses and matching clinical outcomes should be addressed specifically. More than that, scientists might have faced the lack of standardization to administer the immune response induced by DCs, including criteria from generating DCs to assessing clinical efficacy.Citation3,4,16,28
The ownership of DC patents is quite fragmented in terms of a large number of organizations involved. This fragmentation might lead to a costly and intensive coordination process to develop complex new technologies.Citation17 However, mixed patenting strategies exist in one organization, which implies fewer requirements for technical complements but more crucial support from the commercialization process covering clinics, capital and marketing, because of the high engagement of typical biotechnology companies. However, despite the smaller percentage of private entities, conclusive evidence from citation network analysis confirmed the growing role and their outstanding achievements in the DC development process. All of this can inspire policy-makers to help with efficient integration of resources from relevant sectors.
Citation networks provide a dynamic angle for examining the development of DC patents. New inventions based on existing technologies are likely to emerge, which might expand and mature the technical cluster. The therapeutic potential of DCs is the main motive of researchers. Of the disease cluster in the networks, cancer is the major concern; meanwhile, the tolerogenic properties of DCs against autoimmune disorders or transplantation are being increasingly investigated.Citation22,24
In sum, our study presents a landscape for understanding technology development related to DCs, which examines outside of laboratory experiments and shifts the focus to practical application patterns. Analyzing patents and citations is also useful and provides convenient tools for depicting other areas of biotechnology. However, the study has limitations. An inevitable limitation is the time lag between the technology advance and the issuance of related patents caused by the long period of patent drafting and examination. In addition, not all new technologies have been submitted for patent approval or met the patent criteria. Thus, our data may not be complete, but this does not diminish their representativeness. On the other hand, although DC-based technology is closely associated with research articles, we do not intend to consider the link between patent and research articles in this research, which might be part of our future work.
Methods
Data retrieving. In order to investigate the landscape of DC patents, it is the first step to obtain rational data sample. Thus, we searched the US. PTO database for retrieving patents granted by the end of 2013 that contained “dendritic cell” in patent titles, summaries or claims. The titles, summaries and USPC codes were comprehensively reviewed to exclude completely unrelated patents. Finally, 395 patents were collected as research sample.
Patent information analysis. For each sampled patent, the information on granted dates, assignees, USPC, citations, and technological patenting strategies (summarized from patent titles, patent summaries, and claims) were extracted. As the USPC of DC patents were concentrated in codes 424 and 435, total patent counts of the 2 technological classes and overall US utility patents were extracted from general patent statistics reports posted by US. PTO as a reference. Patenting strategies were categorized into 5 different groups according to main technical components of DC vaccine products: DCs obtainment and/or enrichment, antigen-targeted assays, DC-targeted immune modulating formulation, immune response assessment and others. Assignee information of patents, e.g., public/private ownership, and institutional affiliation were identified by reviewing institutional official websites. The frequency of sampled patents owned by different assignees was calculated respectively.
Network visualization. The network was visualized with the software Gephi, in order to analyze DC-based technology development and identify the structural and topological characteristics of networks through embedded patent information and various indicators. Each node in the network represents a patent, and an edge between 2 nodes indicates patent citation relationship. We firstly extracted the internal citation information among the 395 patents and further constructed the networks during 3 different periods according to patent approval year. Various topological indicators were defined and calculated as follows. Connected node ratio was defined as the ratio of connected nodes to relevant patents in the stage. Average degree refers to mean number of links coming out of each node. A component is an isolated sub-network, which has no connections with others components. The number of components reflects the amount of independent technology clusters. Most-cited patents were summarized to reflect the important inventions with the wide influence. Moreover, the out-degree is the number of outgoing edges emanating from a node and the in-degree is the number of incoming edges onto a node. Ro is the ratio of the sum of the out-degree of patents owned by public assignees to the sum by private assignees. Similarly, Ri reflects the relationship between public and private sectors measured by the in-degree. That is to say, the higher value of Ro represents the more dominance of public assignees in terms of technology output, whereas the higher Ri shows the more importance of public sectors in technology input.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully thank Ms. Xiaomei Geng, Mr. Zhiwei Fang, and Ms Jingyun Ni for their advice on data processing and manuscript preparation.
Funding
This study was financially supported by the University of Macau by the project MYRG119(Y1-L3)-ICMS12-HYJ.
References
- Cintolo J, Datta J, Mathew S, Czerniecki B. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol 2012; 5:1273-99; PMID:23130928; http://dx.doi.org/10.2217/fon.12.125
- Longo D. New therapies for castration-resistant prostate cancer. N Engl J Med 2010; 363:479-81; PMID:20818868; http://dx.doi.org/10.1056/NEJMe1006300
- Tabarkiewicz J. Dendritic cells: active and passive players in therapy of human diseases. Immunotherapy 2012; 4:975-78; PMID:23148747; http://dx.doi.org/10.2217/imt.12.97
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Wang J, Liao L, Tan J. Dendritic cell-based vaccination for renal cell carcinoma: challenges in clinical trials. Immunotherapy 2012; 4:1031-42; PMID:23148755; http://dx.doi.org/10.2217/imt.12.107
- Breitzman A, Mogee M. The many applications of patent analysis. J Inf Sci 2002; 28:187-205; http://dx.doi.org/10.1177/016555150202800302
- Erdi P, Makovi K, Somogyva´ri Z, Strandburg K, Tobochnik J, Volf P, Zala´nyi L. Prediction of emerging technologies based on analysis of the US patent citation network. Scientometrics 2013; 95:225-42; http://dx.doi.org/10.1007/s11192-012-0796-4
- Cho T, Shih H. Patent citation network analysis of core and emerging technologies in Taiwan: 1997-2008. Scientometrics 2011; 89:795-811; http://dx.doi.org/10.1007/s11192-011-0457-z
- Xu J, Kong X, Qiu L, Geng X, Hu Y, Wang Y. Research and development of anti-Alzheimer's drugs: an analysis based on technology flows measured by patent citations. Expert Opin Ther Pat 2014; 24:791-800; PMID:24798577; http://dx.doi.org/10.1517/13543776.2014.915943
- Lee S, Kim M. Inter-technology networks to support innovation strategy: an analysis of Korea's new growth engines. Innov Manage Policy Pract 2010;12:88-104; http://dx.doi.org/10.5172/impp.12.1.88
- Lacasa I, Grupp H, Schmoch U. Tracing technological change over long periods in Germany in chemicals using patent statistics. Scientometrics 2003; 57:175-95; http://dx.doi.org/10.1023/A:1024133517484
- Clark K, Cavicchi J, Jensen K, Fitzgerald R, Bennett A, Kowalski S. Patent data mining: A tool for accelerating HIV vaccine innovation. Vaccine 2011; 29:4086-93; PMID:21496469; http://dx.doi.org/10.1016/j.vaccine.2011.03.052
- Zheng J, Zhao Z, Zhang X, Chen D, Huang M, Lei X, Zhang Z, Zhao Y, Liu R. Industry evolution and key technologies in China based on patent analysis. Scientometrics 2011; 87:175-88; http://dx.doi.org/10.1007/s11192-010-0316-3
- Ridgway D. The First 1000 Dendritic Cell Vaccinees. Cancer Invest 2003; 21:873-86; PMID:14735692; http://dx.doi.org/10.1081/CNV-120025091
- Wang S. The stem cell patent landscape as relevant to cancer vaccines. Hum Vaccin 2011; 7:1100-08; PMID:21957493; http://dx.doi.org/10.4161/hv.7.10.17022
- Figdor C, de Vries I, Lesterhuis W, Melief C. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039
- Bergman K, Graff G. The global stem cell patent landscape: implications for efficient technology transfer and commercial development. Nat Biotechnol 2007; 25:419-24; PMID:17420745; http://dx.doi.org/10.1038/nbt0407-419
- Meyer M. Patent citation analysis in a novel field of technology: An exploration of nano-science and nano-technology. Scientometrics 2001; 51:163-83; http://dx.doi.org/10.1023/A:1010572914033
- Choe H, Lee D, Seo I, Kim H. Patent citation network analysis for the domain of organic photovoltaic cells: Country, institution, and technology field. Renew Sust Energ Rev 2013; 26:492-505; http://dx.doi.org/10.1016/j.rser.2013.05.037
- Narin F. Patent bibliometrics. Scientometics 1994; 30:147-55; http://dx.doi.org/10.1007/BF02017219
- Karki M. Patent citation analysis: a policy analysis tool. World Pat Inf 1997; 19:269-72; http://dx.doi.org/10.1016/S0172-2190(97)00033-1
- Pal C, Ganguly D, Paul K, Pal S. Dendritic cells and antigen trapping technology - a revolution in vaccine/immunotherapy strategy. Indian J Exp Biol 2007; 45:491-504; PMID:17585683
- García F, Plana M, Climent N, León A, Gatell M, Gallart T. Dendritic cell based vaccines for HIV infection: the way ahead. Hum Vaccin Immunother 2013; 9 :2445-52; PMID:Can't; http://dx.doi.org/10.4161/hv.25876
- Steinman R, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; http://dx.doi.org/10.1038/nature06175
- Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol 2013; 13:566-77; PMID:23827956; http://dx.doi.org/10.1038/nri3477
- Aurisicchio L, Ciliberto G. Patented cancer vaccines: the promising leads. Expert Opin Ther Pat 2010; 20:647-60; PMID:20345331; http://dx.doi.org/10.1517/13543771003720483
- Radford K, Caminschi I. New generation of dendritic cell vaccines. Hum Vaccin Immunother 2013; 9:259-64; PMID:23291951; http://dx.doi.org/10.4161/hv.22487
- Bhargava A, Mishra D, Banerjee S, Mishra P. Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Immunotherapy 2012; 4:703-18; PMID:22853757; http://dx.doi.org/10.2217/imt.12.40