Abstract
Abrin toxin (AT) consisting of an A chain and a B chain is a potential agent for bioterrorism and an effective vaccine against AT poisoning is urgently required. In this study, AT B chain (ATB) was successfully expressed in the Escherichia coli (E. coli) and assessed the protection capacity against AT intoxication. The recombinant ATB (rATB) subunit induces a good immune response after 4 immunizations. All BALB/c mice immunized intraperitoneally (i.p.) with the purified rATB protein survived after challenged with 5 × LD50 of AT. Transfusion of sera from immunized mice provided passive protection in naive mice. Furthermore, histological findings showed that immunization with rATB decreased the severity of toxin-related tissue damage. This work indicates that the rATB protein may be a promising vaccine candidate against human exposure to AT.
Abbreviations:
- AT, abrin toxin
- ATB, abrin toxin B chain
- RT, ricin toxin
- RTB, ricin toxin B chain
- E. coli, Escherichia coli
- LD50, 50% lethal dose
- i.p, intraperitoneal or intraperitoneally
- PBS, phosphate-buffered saline solution
- SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
- ELISA, enzyme-linked immunosorbent assay
- pAb, polyclonal antibody
Introduction
Abrin is a toxic protein obtained from the seeds of the tropical plant Abrus precatorius, which consists of 2 subunits, an enzymatic abrin A chain (ATA) and a lectin-active abrin B chain (ATB), linked by a disulfide bond.Citation1 ATB has galactose-binding activity, while ATA has N-glycosidase activity. AT shows significant similarities to ricin toxin (RT) at the sequence level as well as the structural level, but it is 75 times more toxic than the latter (the estimated LD50 of AT in mice is 0.04 μg/kg of body weight, and the LD50 of RT is 3 μg/kg).Citation2 In addition, AT can be extracted using a relatively simple and cheap procedure. These characteristics make AT a choice for a suitable assassination weapon or a biological warfare agent .3
Toxin poisoning usually can be prevented by vaccination with toxoid,Citation4,5 or by passive administration with antibodies in experimental animals.Citation2,6 Both procedures are effective in the prophylaxis or therapy of toxin poisoning in mice. Currently, only vaccines based on a toxoid of AT ( attenuated with formalin as reported by Griffiths Citation5) and A chain subunit ( ATA mutant Citation1 and truncated ATACitation7) produced in our lab, were effective in neutralizing intoxication by AT. This toxoid was effective in preventing death by intoxication. However this toxoid may still have residual toxicity or could revert to a holotoxin, and accordingly it is considered too high a risk for use as a vaccine antigen. As for ATA vaccines, no any data concerning tissue damage by histology were reported, although they can elicit a good immune response and ultimately protect animals from exposure to lethal doses of AT. Therefore, it is urgent and important to develop a safe and effective vaccine against AT poisoning.
Ongoing efforts to develop an effective vaccine for toxins including AT and RT, have focused almost exclusively on A chain, Citation1,8-16 despite the long-standing evidence for the existence B chain specific antibodies that are capable of fully neutralizing toxins.Citation17-21 For example, in 1992, Lemley and colleagues demonstrated that anti-RTB antibodies can be as effective as anti-RTA antibodies.Citation17 In 2006, Mantis and colleagues described that IgA antibodies against RTA and RTB can protect mucosal epithelial cells from RT intoxication. Citation19 And several lines of evidence also suggest that B chain may be useful as a toxin subunit vaccine.Citation22 Firstly, B chain is non-toxic without A chain. Secondly, B chain of toxins themselves has adjuvant activity owing to its specific binding to the cell surfaces of M-cells. Thirdly, other toxins belonged to the A–B family such as Cholera and Shiga toxins have been demonstrated to induce a good protective effect with their B subunit-based vaccines.
Therefore, with the long-term objective of developing an ATB vaccine as countermeasure against AT intoxication, the capacity of recombinant ATB to elicit immunity was defined in this study. It is the first report that rATB is used to develop an effective vaccine candidate against AT exposure threat.
Result
Construction of the rATB
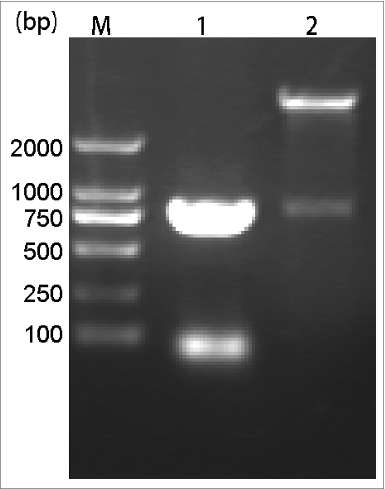
According to E. coli codon usage, a DNA fragment of the natural ATB amino acid sequence was synthesized with optimal codons usage. This rATB was constructed into pQE-80L vector by restriction enzymes BamH I and Hind III. PCR and restriction analysis showed that the construction of expression vector was correct and an insert of 804 bp was detected ().
Expression and purification of rATB
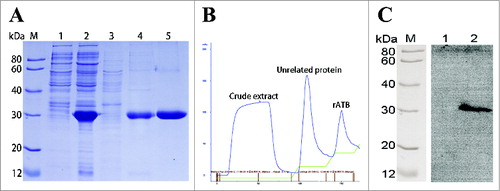
The recombinant vector pQE80L-ATB containing rATB gene was successfully transformed into E. coli M15 strain, and the rATB protein with a 6 × His-tag (30-kDa) was expressed in insoluble form () after the induction with IPTG. Ni2+-chelating affinity chromatography resin column was applied to purify the target protein. Elution buffer containing 500 mM imidazole was used to elute the rATB (). Then, the purified protein was tested using SDS-PAGE and up to 97% purity was acquired according to Total Lab 2.01 software after renaturation to PBS ().
Figure 2. Purification and Western blotting analysis of rATB protein. (A). SDS-PAGE analysis and purification of the rATB. Lanes 1 and 2, total cellular lysate of M15/pET80L-ATB induced without IPTG and with IPTG, respectively. Lanes 3 and 4, cell supernatants and cell debris after centrifugation at 12,000 rpm for 30 min. Lane 5, rATB eluted with elution buffer containing 500 mM imidazole. (B). Affinity chromatographic profile. (C). Western blotting analysis. Lane 1, pQE-80L vector-transformed cell lysates recognized by the rabbit pAb against AT. Lane 2, purified rATB recognized by the rabbit pAb against AT.
Immunoblotting
The immunoreactivity of the recombinant protein was assessed by Western blotting. As shown in , lane 2 presented that the rATB could be recognized by the rabbit pAb against AT, while lane 1 displayed pQE-80L vector-transformed cell lysates as negative control. Result indicated that the rATB was specific and correct.
Vaccination and AT challenge
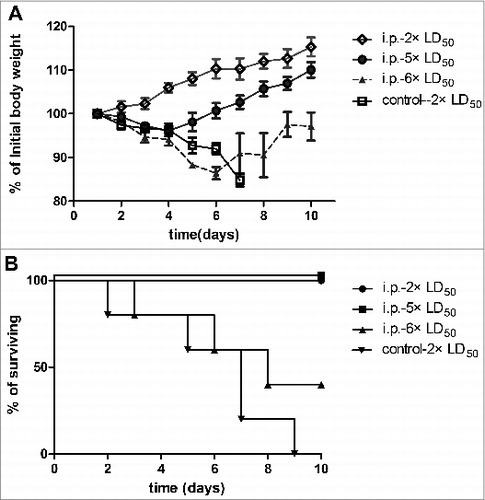
AT challenged results are shown in . All immunized mice survived a challenge with 2 or 5 × LD50 of AT. A slight weight loss occurred in the first 2–3 d and within the following days, mice regained weight and exceeded of the initial weight. No significant difference in body weight was observed for mice, which survived a challenge with 2 × LD50 or 5 × LD50 of AT (p = 0 .291). Following a challenge with 6 × LD50 of AT, the survival rate dropped to 60%.
Figure 3. Vaccination with the rATB followed by different doses of AT administered by i.p. injection. (A). Average weights (as percentage of initial weight). (B). Survival curves. n = 4 groups of 5 mice = 20.
In contrast, all LD50 toxin doses resulted in 100% mortality within 1–2 d in mice given PBS (only data of 2 × LD50 shown).
Antibody titers
Serum titers of anti-rATB antibody following each immunization and the challenge with AT are given in . ELISA results showed that the titers of the immunized mice increased following booster immunizations with the highest titer (1:106) reached after the last vaccination. Meanwhile, a strong secondary response was triggered in the mice when challenged with toxin. In contrast, the antibody titer in serum of mice given PBS was less than 1:10 (P < 0.05).
Table 1. The anti-rATB IgG antibody titers of sera after each immunization and challenge trial
Efficacy of passive protection
The rATB neutralization assay and efficacy of passive protection were presented in . All mice that received immune sera survived a challenge with 2 × LD50 or 5 × LD50 AT, and only showed a temporary loss of body weight during days 2 to 4. There was no significant difference between these 2 groups (p = 0 .193). Only two out of 5 mice that received immune sera survived a challenge with 6 × LD50 of toxin. In contrast, none of the mice in the control groups survived (only data of 2 × LD50 shown). These results indicated that the anti-rATB antibody can completely neutralize 5 × LD50 of AT.
Histological findings
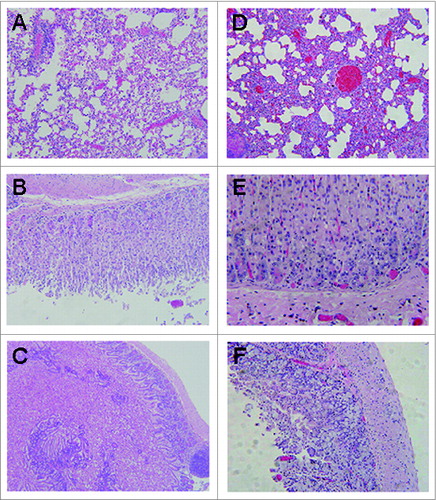
Tissues selected from 3 fully vaccinated mice that survived a challenge with 5 × LD50 of AT were used to determine the degree of tissue damage. Tissues selected from 3 dead control group mice were used as control. The results are shown in . Considerable differences were observed between the mice treated with the rATB and PBS in the lungs (, D). The former displayed mild inflammatory cells and pulmonary alveoli fibrosis, while the latter presented severe inflammatory infiltrates and alveoli extension. No detectable changes were identified in the stomach of the mice from the vaccinated group (), whereas scattered apoptotic cells were identified in the control mice (). As for the intestine, only slightly shortened villi and few necrotic epithelial cells were found in intestine tissues of vaccinated mice (), whereas apoptotic and necrotic cells was common in intestinal tissues from mice of the control group (). It has been reported that AT can cause severe inflammatory infiltrates and necrotic cells in the tissues. Therefore, compared with the control group, the severity of the major tissue damage ranged from marked to mild in the vaccinated mice.
Figure 5. Histopathologic examination in BALB/c mice post-challenge with AT (HE stain, 100×). (A). mice vaccinated with rATB showing mild inflammatory cells and alveoli fibrosis in the lung. (B). mice vaccinated with rATB showing normal stomach. (C). mice vaccinated with rATB showing villi slightly shortened and rare necrotic epithelial cells in the intestine. (D). mice vaccinated with PBS showing severe inflammatory infiltrates and alveoli extension in the lung. (E). mice vaccinated with PBS showing numerous apoptotic cells in the stomach. (F). mice vaccinated with PBS showing numerous apoptotic and necrotic cells in the intestine.
Discussion
AT is a plant-derived toxin of extraordinary toxicity, with an estimated lethal oral dose of AT in humans is 0.1–1μg/kg. The feature with extreme toxicity and easy acquirement makes AT a potential agent for biological warfare and bioterrorism.Citation1 Therefore, it is necessary to develop a viable, safe and stable vaccine candidate against human exposure to AT.
To date, vaccine researches against toxins of A-B family, including AT and RT, have focused on toxoid and A chain subunit, especially the latter. For instance, deglycosylated RTA (dgRTA),Citation8,11 RTA 1–33/44–198,Citation9,12 Rivax,Citation12,14-16,23 ATA mutant Citation1 and truncated ATA Citation7 are all based on A chain. As for AT vaccine, ATA mutant could provide the complete protection against challenge at a dose 10 times higher than the LD50 of AT. And truncated ATA provides a better protection, which can elicit 100% protective efficacy against i.p. challenge of 40 × LD50 of AT. But the toxicity of 2 vaccines indicated that they had a slight toxicity and there were no reports about histopathological evaluation. Compared with A chain subunit, conventional toxoid preparation via protein denaturation has significant shortfalls that can lead to inadequate vaccine potency and poor quality control.Citation4,5,24
ATB is a galactose-specific lectin and can bind to cell surface glycosylated receptors and promote the entry of the ATA into the cytosol, which means that ATB is essential for the toxicity of AT. Meanwhile, previous studies also have demonstrated that B chain of toxins can exhibit both antigenic and adjuvant activity.Citation20,22,25,26 Therefore, it is seemly feasible to use ATB as a target for vaccine candidate. However, few reports have examined the use of B subunit as a vaccine candidate in humans in spite of some long-standing evidence indicating that the anti-B-subunit specific antibodies have the capacity of neutralizing corresponding toxin.Citation6,20,26
In this study, we developed a B subunit-based vaccine of AT and assessed the immune response and the protective efficacy elicited by the recombinant protein without adjuvant in mice model. Firstly, the full-length gene encoding ATB was optimized and designed (). Although the rATB was expressed in E. in inclusion bodies, there is no data to prove that the galactose-binding sites affect rATB to elicit a protective immune response. After expression, purification and renaturation, the target protein was demonstrated to react with the rabbit pAb against AT by Western blot analysis (). Secondly, the rATB was applied to immunize 25μg per mouse via i.p. without adjuvant. Results indicated that the rATB is sufficient to protect the vaccinated mice when challenged with 5 × LD50 of the native toxin after 4 immunizations at 2 weeks interval (). Compared with the immunized groups, all the mice were dead in the control group. Thirdly, passive protection mediated by anti-rATB antibodies in immune sera was confirmed (). All mice survived a challenge with up to 5 × LD50 of AT, and protection broke down at 6 × LD50. This indicates that anti-rATB antibodies can provide protection for naive individuals exposed to AT. Finally, histopathological evaluation was done to determine the severity of toxin-related tissue damage in the survived mice after challenged with 5 × LD50 of AT (). It has been reported that native abrin can cause rapid irreversible lung damage leading to death when inhaled and severe gastroenteritis leading to dehydration, shock, and death when ingested. Our data from the control group confirmed these reports and the severity of the major tissue damage ranged from marked to mild when the mice were immunized the rATB protein. These results illustrated that the rATB could elicit a protective immunity in the vaccinated mice when challenged with 5 × LD50 of the native toxin.
Compared with ATA mutant and truncated ATA, which elicit protection (10 or 40 × LD50 of AT respectively), protective potency by the rATB was low (only 5 × LD50). However, both 2 A chain subunits have some drawbacks, such as residual toxicity and additional adjuvant. Although immunostimulatory adjuvants have been applied to increase the potency of other antigens, there is a risk of reactogenicity and other adverse effects. Our data have indicated that adjuvants (such as aluminum salt and Freund's adjuvant) did not significantly boost specific antibody response for immunizations (data not shown). In this study, no adjuvant was used in order to best highlight the B chain of AT platform as a safe vaccine. Therefore, ATB protein is non-toxic and can trigger a good immune response and protective efficacy without adjuvant, which means it is safer to be used in both animals and humans.
In conclusion, we have successfully produced a vaccine candidate based on B subunit of AT with good immunogenicity and immunoreactivity. The present data showed that it induces specific neutralizing antibodies against the native toxin at a dose 5 times higher than the LD50 of AT. And the recombinant protein can provide protection in the vaccinated mice when challenged with the same dose of toxin according to the performance of the survival curves and histopathological assessment. Altogether, it is the first report on developing an ATB-based vaccine as countermeasure against AT threat and rATB should be investigated deeply as a promising vaccine candidate.
Materials and Methods
Construction of the ATB expression plasmid
An optimum design of 804bp gene encoding ATB amino acid sequence (GeneBank accession no. 1908235A) was performed by replacing rare codons with synonymous codons of high-frequency, according to the inclination for codon usage in Escherichia coli (E. coli). After synthesis (Invitrogen, Shanghai, China), the full-length gene was cloned into prokaryotic expression plasmid pQE-80L through BamH I and Hind Ш restriction sites, and a new plasmid pQE80L-ATB was obtained. The recombinant vector pQE80L-ATB was analyzed by PCR and restriction digest after it was transformed into E. coli M15 (Qiagen, Germany).
Recombinant protein expression and purification
The rATB protein was expressed and purified according to the ordinary protocol performed with some modification. The recombinant vector pQE80L-ATB transformed into the E. coli strain M15 was grown in LB containing 100 μg/ml ampicillin at 37°C. After an optical density at 600 nm reached an OD600 of 0.6∼0.8, the temperature was decreased to 30°C and 1 M IPTG was added to a final concentration of 1 mM for 5 h induction. Then bacteria were harvested by centrifugation and washed with cold phosphate-buffered saline solution (PBS). Cells were resuspended in PBS at 4°C and disrupted by sonication. The bacterial lysates were analyzed by 15 % sodium dodecyl sulfate PAGE (SDS-PAGE) for protein pattern.
After being washed 4 times with wash buffer (2% Triton X-100, 2% Tween-20, 1 M NaCl, 2 M urea), the recombinant protein formed in the inclusion bodies was dissolved in 50 ml buffer A (20 mM NaH2PO4, 500 mM NaCl, 10 mM imidazole, 8 M urea, pH 8.0), and then purified by Ni2+-charged column chromatography (Amersham). The column was eluted with buffer B containing 500 mM imidazole. Then, a series of renaturation buffer (PBS containing 4 M, 2 M, 1 M, 0.5 M, and 0 M urea) was used at 4°C for protein renaturation. The purified protein was confirmed using SDS-PAGE followed by coomassie blue staining and the BCA assay kit was applied to measure the concentration.
Immunoblotting
The recombinant protein was confirmed by Western blotting. After applied to 15% SDS-PAGE, the purified protein was electro-transferred to a nitrocellulose membrane using a transmembrane device (Amersham Biosciences, Sweden). Blocking buffer (3 % BSA in PBS, pH 7.4, with 0.5 % Tween-20) was performed to block the nonspecific binding sites overnight at 4°C. Then, a 1:1000 dilution of rabbit anti-AT polyclonal antibody (pAb) (previously prepared in our laboratory) was used at room temperature for 1h. After the membrane was washed 3 times with PBST (PBS containing 0.5 % Tween-20), a 1:50,000 dilution of HRP-conjugated goat anti-rabbit IgG (Sigma) was subsequently used at room temperature for 1 h. Finally, the membrane was colored using SuperSignal Substrate Working Solution (Thermo, America) for 5 minutes and exposed in AE-1000 cool CCD image analyzer (Beijing BGI-GBI Biotech Co., Ltd).
Vaccinations
In this study, the rATB protein was employed without other adjuvants owing to its adjuvant activity. Fifteen six-week-old female BALB/c mice (purchased from Laboratory Animal Center, The Academy of Military Medical Sciences) were randomized into 3 test groups, which were administered the recombinant protein with 25 μg per mouse in 50 mM PBS (pH 7.4) via intraperitoneal (i.p.) injection. All the mice were injected at 2-week intervals for 4 vaccinations and challenged i.p. with 2, 5, or 6 × LD50 of AT one week after the last administration. In addition, 15 mice were only vaccinated PBS via i.p. injection before being challenged i.p. with 2, 5, or 6 × LD50 of the native toxin as negative. The weight and survival of all the mice were observed for 10 d Another 10 mice immunized were used for passive protection. Animal studies were conducted in compliance with the Guide for the Care and Use of Laboratory Animals and the Association for Assessment and Accreditation of Laboratory Animal Care International.
Antibody titer measurements
Three mice were randomly selected from different treatment groups and sera were pooled from the caudal vein of every individual mouse one week after each vaccination and one week after challenge with AT. In order to measure the antibody titer, the enzyme-linked immunosorbent assay (ELISA) was used in this study. A 96-well plate was coated with the purified rATB (5 μg/mL, 100 μl/well) overnight at 4°C. PBST was applied to wash plates 3 times between all incubations. A serial dilution of sera samples at 1:10 increments (1:10–1:107) was added to each well and incubated at 37°C for 1 h after the plate was blocked PBS containing 3 % BSA. Then, the HRP-coupled goat anti-mouse IgG antibodies (1:5,000, Sigma) were used as secondary antibody. The substrate solution was added to plates and 2 M H2SO4 was subsequently applied to stop the reaction. Anti-rATB reactivity was read at 450 nm using a microplate reader (Molecular Device) and the values greater than 2.1-fold negative control were considered as positive.
Neutralization assay
Sera were collected from 10 immunized mice in the vaccination study and mixed with an equal volume of different doses of AT in 50 mM PBS (2, 5, 6 × LD50). After incubated at 37°C for 30 min, the mixtures were injected i.p. into fifteen new mice (5 mice in each group) using a volume of 500 μl per mouse. Meanwhile, another 15 naive mice were challenged with the mixtures of non-immunized sera and 2, 5, or 6 × LD50 of AT as control. All the mice were observed for 10 d and assessed the ability of the anti-rATB sera to protect the mice against AT challenge.
Histopathological examination
The vaccinated mice survived after being challenged with 5 × LD50 of AT for 10 d were humanly euthanized for histopathological evaluation to test whether there was toxin-related tissue damage in the mice. And three dead PBS-vaccinated mice following challenge with 2 × LD50 of AT were randomly selected and performed as control. Major organs (lungs, stomach and intestine) from all the mice were collected and fixed by formalin and embedded in paraffin. Finally, the tissues were stained with hematoxylin and eosin (HE), coded and analyzed by a pathologist blindly.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) and ANOVA and student's t-test were used to analyze the data on body weight and antibody titer. A p-value of <0 .05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Jinwen Chen (School of Vet Medicine, University of Pennsylvania) for his critical reading of the manuscript.
References
- Han YH, Gao S, Xin WW, Kang L, Wang JL. A recombinant mutant abrin A chain expressed in Escherichia coli can be used as an effective vaccine candidate. Hum Vaccin 2011; 7:838-44; PMID:21817853; http://dx.doi.org/10.4161/hv.7.8.16258
- Surendranath K, Karande AA. A neutralizing antibody to the a chain of abrin inhibits abrin toxicity both in vitro and in vivo. Clin Vaccine Immunol 2008; 15:737-43; PMID:18353919; http://dx.doi.org/10.1128/CVI.00254-07
- Balali-Mood M, Moshiri M, Etemad L. Medical aspects of bio-terrorism. Toxicon 2013; 69:131-42; PMID:23339855; http://dx.doi.org/10.1016/j.toxicon.2013.01.005
- Kende M, Yan C, Hewetson J, Frick MA, Rill WL, Tammariello R. Oral immunization of mice with ricin toxoid vaccine encapsulated in polymeric microspheres against aerosol challenge. Vaccine 2002; 20:1681-91; PMID:11858879; http://dx.doi.org/10.1016/S0264-410X(01)00484-4
- Griffiths GD, Lindsay CD, Allenby AC, Bailey SC, Scawin JW, Rice P, Upshall DG. Protection against inhalation toxicity of ricin and abrin by immunisation. Hum Exp Toxicol 1995; 14:155-64; PMID:7779439; http://dx.doi.org/10.1177/096032719501400201
- Hu WG, Yin J, Chau D, Negrych LM, Cherwonogrodzky JW. Humanization and characterization of an anti-ricin neutralization monoclonal antibody. PLoS One 2012; 7:e45595; PMID:23049820; http://dx.doi.org/10.1371/journal.pone.0045595
- Zhang T, Kang L, Gao S, Xin W, Yang H, Wang JH, Guo M, Wang J. Truncated abrin A chain expressed in Escherichia coli: A promising vaccine candidate. Hum Vaccin Immunother 2014; 10:2648-55; PMID: 25483485; http://dx.doi.org/10.4161/hv.29645
- Vallera DA, Burns LJ, Frankel AE, Sicheneder AR, Gunther R, Gajl-Peczalska K, Pennell CA, Kersey JH. Laboratory preparation of a deglycosylated ricin toxin A chain containing immunotoxin directed against a CD7 T lineage differentiation antigen for phase I human clinical studies involving T cell malignancies. J Immunol Methods 1996; 197:69-83; PMID:8890895; http://dx.doi.org/10.1016/0022-1759(96)00127-5
- Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, Smith LA. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine 2007; 25:4149-58; PMID:17408819; http://dx.doi.org/10.1016/j.vaccine.2007.03.011
- Kende M, Tan X, Wlazlowski C, Williams R, Lindsey C, Del Giudice G. Enhancement of intranasal vaccination with recombinant chain A ricin vaccine (rRV) in mice by the mucosal adjuvants LTK63 and LTR72. Vaccine 2007; 25:3219-27; PMID:17343960; http://dx.doi.org/10.1016/j.vaccine.2007.01.036
- Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I, Barta S, Ghetie V, Vitetta E, Verma A. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol 2011; 154:471-6; PMID:21732928; http://dx.doi.org/10.1111/j.1365-2141.2011.08762.x
- Legler PM, Brey RN, Smallshaw JE, Vitetta ES, Millard CB. Structure of RiVax: a recombinant ricin vaccine. Acta Crystallogr D Biol Crystallogr 2011; 67:826-30; PMID:21904036; http://dx.doi.org/10.1107/S0907444911026771
- McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: survival, immunological response, and histopathological findings. Toxicol Sci 2012; 126:72-83; PMID:21987460; http://dx.doi.org/10.1093/toxsci/kfr274
- Vitetta ES, Smallshaw JE, Schindler J. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol 2012; 19:1697-9; PMID:22914366; http://dx.doi.org/10.1128/CVI.00381-12
- Thomas JC, O'Hara JM, Hu L, Gao FP, Joshi SB, Volkin DB, Brey RN, Fang J, Karanicolas J, Mantis NJ, et al. Effect of single-point mutations on the stability and immunogenicity of a recombinant ricin A chain subunit vaccine antigen. Hum Vaccin Immunother 2013; 9:744-52; PMID:23563512; http://dx.doi.org/10.4161/hv.22998
- O'Hara JM, Brey RN, 3rd, Mantis NJ. Comparative efficacy of two leading candidate ricin toxin a subunit vaccines in mice. Clin Vaccine Immunol 2013; 20:789-94; PMID:23515013; http://dx.doi.org/10.1128/CVI.00098-13
- Lemley PV, Wright DC. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology 1992; 76:511-3; PMID:1526657
- McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun 2006; 74:3463-70; PMID:16714577; http://dx.doi.org/10.1128/IAI.00324-06
- Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun 2006; 74:3455-62; PMID:16714576; http://dx.doi.org/10.1128/IAI.02088-05
- Yermakova A, Mantis NJ. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB). Vaccine 2011; 29:7925-35; PMID:21872634; http://dx.doi.org/10.1016/j.vaccine.2011.08.075
- Hu WG, Yin J, Chau D, Hu CC, Lillico D, Yu J, Negrych LM, Cherwonogrodzky JW. Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies. Biomed Res Int 2013; 2013:471346; PMID:23484120
- Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev 2005; 57:1424-39; PMID:15935880; http://dx.doi.org/10.1016/j.addr.2005.01.017
- Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine 2005; 23:4775-84; PMID:15961194; http://dx.doi.org/10.1016/j.vaccine.2005.04.037
- Pincus SH, Smallshaw JE, Song K, Berry J, Vitetta ES. Passive and active vaccination strategies to prevent ricin poisoning. Toxins (Basel) 2011; 3:1163-84; PMID:22069761; http://dx.doi.org/10.3390/toxins3091163
- Medina-Bolivar F, Wright R, Funk V, Sentz D, Barroso L, Wilkins TD, Petri W Jr, Cramer CL. A non-toxic lectin for antigen delivery of plant-based mucosal vaccines. Vaccine 2003; 21:997-1005; PMID:12547614; http://dx.doi.org/10.1016/S0264-410X(02)00551-0
- Liu W, Xu N, Yuan H, Li S, Liu L, Pu Z, Wan J, Wang H, Chang Y, Li R. Immunomodulatory Activity of Recombinant Ricin Toxin Binding Subunit B (RTB). Int J Mol Sci 2013; 14:12401-10; PMID:23765218; http://dx.doi.org/10.3390/ijms140612401