Abstract
To evaluate antibody response induced by trivalent inactivated influenza vaccine (TIV) against circulating influenza A (H3N2) strains in healthy adults during the 2010/11 and 2011/12 seasons, a hemagglutination-inhibition (HI) assay was utilized to calculate geometric mean antibody titer (GMT), seroprotection rate (post vaccination HI titers of ≥1 :40), and seroresponse rate (4-fold increase in antibody level). In the 2010/11 season, GMT increased 1.8- to 2.0-fold following the first dose of TIV against 3 circulating strains and 2.2-fold following the second compared to before vaccination. The seroresponse rate ranged from 22% to 26% following the first dose of TIV and from 31% to 33% following the second (n = 54 ). The seroprotection rate increased from a range of 6% to 13% to a range of 26% to 33% following the first dose of TIV and to a range of 37% to 42% following the second (n = 54 ). In the 2011/12 season, GMT increased 1.4-fold against A/Osaka/110/2011 and 1.8-fold against A/Osaka/5/2012. For A/Osaka/110/2011, the seroresponse rate was 29%, and the seroprotection rate increased from 26% to 55% following vaccination (n = 31 ). For A/Osaka/5/2012, the seroresponse rate was 26%, and the seroprotection rate increased from 68% to 84% following vaccination (n = 31 ). HI assays with reference antisera demonstrated that the strains in the 2011/12 season were antigenically distinct from vaccine strain (A/Victoria/210/2009). In conclusion, the vaccination increased the seroprotection rate against circulating H3N2 strains in the 2010/11 and 2011/12 seasons. Vaccination of TIV might have potential to induce reactive antibodies against antigenically distinct circulating H3N2 viruses.
Introduction
Influenza virus causes epidemics of respiratory infection. Influenza vaccination is the primary method for preventing severe influenza-related complications.Citation1,2 Annual influenza vaccination is recommended for all persons aged ≥6 months by the Advisory Committee on Immunization Practices (ACIP),Citation3 and recommended for elderly people over 65 y in Japan. Trivalent inactivated influenza vaccine (TIV) is the most common vaccine and induces antibodies specific to the surface glycoprotein of influenza virus. The efficacy of the influenza vaccine partly depends on antigenic match between the vaccine strains and circulating epidemic strains.Citation4-6 Antigenic drift is the accumulation of mutations in the surface glycoprotein, which enable the virus to evade pre-existing immunity induced by vaccination or prior infection.Citation6,7 The antigenicity of circulating influenza viruses is therefore monitored via annual surveillance and vaccine strains are updated. The efficacy of seasonal influenza vaccines has been evaluated in many reports. However, antibody titers were generally measured by a hemagglutination-inhibition (HI) assay using vaccine strains, not circulating influenza strains.Citation8-12 Moreover, although a number of studies have evaluated the effect of previous vaccination on the vaccine-induced antibody response, they also generally utilized the HI antibody titer against vaccine strains.Citation13-18 The impact of repeated vaccination against circulating influenza strains is therefore unclear.
Vaccine compositions in the northern hemisphere influenza season of 2011/12 were unchanged from those of 2010/11. Influenza virus surveillance in Japan (http://www.nih.go.jp/niid/en/iasr-inf-e.html) showed that the H1N1pdm09 virus was dominant in the 2010/11 season, with the H3N2 virus detected in the latter half of that season, subsequently rising to dominance in the 2011/12 season. By analyzing the immune responses against circulating influenza viruses in these 2 influenza seasons, the immunogenicity of the influenza vaccine can be examined.
Here, to evaluate the effect of TIV and the impact of vaccination for 2 consecutive years on immune responses against circulating influenza viruses, we conducted a study using sera from healthy adults <65 y old in Japan in the 2010/11 and 2011/12 influenza seasons. The TIV-induced serum HI titer against circulating influenza A (H3N2) viruses was assessed based on the fold increase of geometric mean antibody titer (GMT), seroresponse rate, and seroprotection rate. The results of this study may prove useful in understanding the effectiveness of vaccination of TIV against circulating influenza viruses.
Results
In the 2010/11 season, serum samples were collected from a total of 54 healthy adults before vaccination (S0) and after the first dose (S1), and from 52 of the 54 subjects after the second dose (S2). In the 2011/12 influenza season, a total of 31 healthy adults who received TIV in 2010/11 season received the TIV, and serum samples were collected from all subjects at S0 and S1.
In the 2010/11 season, measurement of serum HI titer was conducted using 3 circulating H3N2 strains derived from different regions in Japan. Antigenic analysis with antisera to the vaccine strain showed that the 3 circulating strains were similar to the vaccine strain (2- to 4-fold difference compared to the homologous HI titer) (). Findings regarding the antibody responses in the 2010/11 season are summarized in and . The GMT against the vaccine strain at S1 and S2 increased 2.4- (P < 0.001) and 2.9-fold (P < 0.001) compared to S0. The seroresponse rate was 30% at S1 and 38% at S2. The seroprotection rate increased from 43% (S0) to 78% (S1) and 81% (S2) against the vaccine strain. Against the 3 circulating strains, vaccination induced a 1.8- to 2.0-fold increase (P < 0.001) at S1 and a 2.2-fold increase (P < 0.001) at S2. The seroresponse rate ranged from 22% to 26% at S1 and from 31% to 33% at S2. The seroprotection rate increased from a range of 6% to 13% (S0) to a range of 26% to 33% (S1) and then to 37% to 42% (S2).
Table 1. Antigenic analysis of influenza A (H3N2) strains in the 2010/11 season
Table 2. HI antibody responses against influenza A (H3N2) strains following vaccination in the 2010/11 season
Figure 1. Geometric mean titers (±geometric standard error of the mean) against vaccine strain and circulating strains before vaccination (S0), 4 weeks after the first dose (S1) and 4 weeks after the second dose (S2) in 2010/11 season (A), and before vaccination (S0) and 4 weeks after the dose (S1) in 2011/12 season (B).
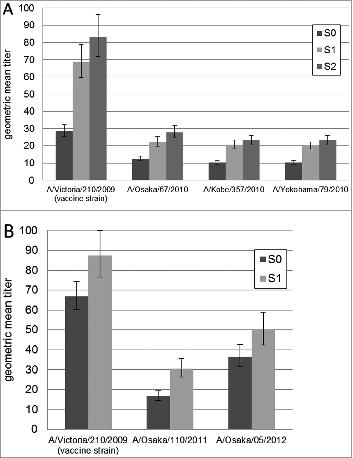
In the 2011/12 influenza season, we analyzed 2 circulating H3N2 strains in Osaka (A/Osaka/110/2011 and A/Osaka/5/2012) as the isolated regions of circulating strains did not affect the antigenic properties in the prior season. Antigenic analysis of the 2 strains with reference antisera exhibited an 8-fold difference compared to the homologous HI titer (). Results of the antibody responses in the 2011/12 season are summarized in and . Against the vaccine strain, vaccination induced a 1.3-fold increase (P < 0.001) and a seroresponse rate of 6% at S1. A total of 94% of subjects had HI titers ≥1 :40 against the vaccine strain before vaccination (S0), and the seroprotection rate was 94% following vaccination (S1). Meanwhile, GMT against A/Osaka/110/2011 increased 1.8-fold (P < 0.001), and A/Osaka/5/2012 increased 1.4-fold (P < 0.001) following vaccination (S1). For the A/Osaka/110/2011, the seroresponse rate was 29% at S1, and the seroprotection rate increased from 26% (S0) to 55% (S1). For the A/Osaka/5/2012, the seroresponse rate was 26% at S1, and the seroprotection rate increased from 68% (S0) to 84% (S1). Accordingly, the seroprotection rates against circulating strains in the 2011/12 influenza season were higher than those in the 2010/11 season.
Table 3. Antigenic analysis of influenza A (H3N2) strains in the 2011/12 season
Table 4. HI antibody responses against influenza A (H3N2) strains following vaccination in the 2011/12 season
Discussion
At present, there is a scarcity of information regarding HI antibody induced by seasonal influenza vaccines against circulating influenza viruses. Here, TIV-induced antibodies against influenza A (H3N2) strains were evaluated in the 2010/11 and 2011/12 influenza seasons. In both seasons, the seroprotection rate against the vaccine strain was 78% or more following vaccination. Although the GMT against circulating strains increased in parallel with that against the vaccine strain, the pre- and post-vaccination antibody titers were lower than those against the vaccine strain in the 2 influenza seasons. Vaccination with the same vaccine composition for 2 consecutive years showed that the seroprotection rates against circulating strains increased in the 2011/12 season. These results provide insight into the immune response induced by TIV against circulating influenza viruses.
In the present study, past vaccination history of the subjects was not corrected. However, 43% of subjects in the 2010/11 season and 94% of subjects in the 2011/12 season had a titer of ≥1 :40 against the vaccine strain before vaccination. These results indicate that a proportion of subjects have pre-existing immunity due to previous vaccination or infection. Although some of the subjects might have been naturally exposed to influenza A (H3N2) virus during the study seasons, it is considered to be a very small proportion because the study were performed before the epidemic of influenza starts. Negative and positive correlations between antibody response and vaccination history were reported, and a number of studies have shown that previous vaccination did not significantly decrease antibody titer.Citation13-18 Because the influence of previous vaccination and infection in immune response was unable to evaluate in this study, we focused on the correlation between pre-vaccination titer and immune responses. The results showed that the antibody titer against the vaccine strain increased following utilization of the same vaccine for 2 consecutive years, and the fold increase following the first dose in the 2011/12 season was lower than that of the 2010/11 season. The second dose in the 2010/11 season had little impact regarding additional induction of antibodies. These phenomena might be due to high pre-vaccination antibody titer, as a negative correlation was observed between pre-vaccination titer and the fold increase.Citation19-21 Similarly, the fold increase against the circulating strains increased in both seasons, and the fold increase and seroresponse rate were lower in the 2011/12 season than in the 2010/11 season. These results indicate that antibody profiles depend on the pre-vaccination titer, and that negative correlation was also exerted against circulating strains.
Previous studies showed that protection from influenza increases with antibody titer and that HI antibody titers ≥1 :40 are considered to indicate a higher degree of protection than lower titers.Citation9,22,23 The seroprotection rates against vaccine strain in the present study met the criteria of the European Medicines Agency (EMA),Citation24 and vaccination increasing the seroprotection rate against circulating strains in both seasons. However, there are some limitations in this study. Because the sample size was insufficient, selection bias might have occurred. Furthermore, as the vaccine effectiveness of the subjects was not evaluated, protective antibody titer against circulating influenza virus was not estimated. Therefore, further investigation is required to assess the protective HI antibody titer and the effectiveness of TIV against circulating strains, as the antigenicity of circulating influenza viruses may change from year to year, and evaluation might be affected by vaccine antigen, age of subjects, number of subjects and study period.
The antigenicity of seasonal influenza vaccine strains does not always match circulating viruses due to antigenic drift.Citation25 Antigenicity of influenza viruses was generally measured by HI test with ferret reference antisera.Citation26 It was reported that the results from antigenic analyses with ferret antisera were in good agreement with those obtained using sera from vaccinees.Citation5 In our results, the HI assay with reference antisera showed that the antigenicity of the 3 circulating influenza strains in the 2010/11 season was close to the vaccine strain (1- to 2-fold difference) and that the antibody profiles of vaccinated human sera were similar among these strains. The antigenicity of both of the 2 circulating strains in the 2011/12 season was different from the vaccine strain (8-fold difference), and the antibody profiles of vaccinated human sera indicated that the 2 viruses were antigenically distinct. Homologous virus induces the highest titers of HI antibody,Citation9 and influenza vaccination is effective when the antigenic match is optimal.Citation6,27 Meanwhile, influenza vaccines were recently reported to provide cross-protection against mismatched strains,Citation28 with repeated vaccination increasing the affinity of antibodies.Citation14,15 In our present study, despite antigenic mismatch, the GMT against circulating strains in the 2011/12 season was higher than the prior season, and, notably, the seroprotection rate against A/Osaka/5/2012 reached 84% following vaccination. Because the antigenicity of circulating strains were different, it is difficult to directly compare the results between the seasons. However, the results in the 2011/12 season indicated that the antigenic match between circulating and vaccine strains that were measured by HI test with ferret antisera was not the sole reason for the increased seroprotection rates against circulating strains, and the reactivity of antibodies against influenza viruses arising from antigenic drift might have been enhanced by TIV.
In conclusion, the present study demonstrated an immune response to TIVs in 2010/11 and 2011/12 seasons, with the vaccination increasing the seroprotection rate against circulating H3N2 strains in healthy adults. Annual vaccination of TIV might have potential to induce reactive antibodies against antigenically distinct circulating influenza viruses.
Materials and Methods
Study subjects
The sera were obtained from healthy adults <65 y old residing in Japan who met the criteria for influenza vaccination in the 2010/11 and 2011/12 influenza seasons. Because it was the first time H1N1pdm09 was included in the 2010/11 TIV, the subjects in the season were injected 2 doses to examine immune response against H1N1pdm09 in another study (not published). The subjects in the 2010/11 season consisted of 54 adults aged 25 to 63 y (male percentage = 48 .1%, mean age = 39 .9), and the subjects in the 2011/12 season consisted of 31 adults aged 22 to 64 y (male percentage = 38 .7%, mean age = 42 .8). A total of 26 subjects in 2010/11 season attended in 2011/12 season, and 5 subjects who received TIV in the 2010/11 influenza season (fall of 2010) newly participated in 2011/12 season. Two subjects in 2010/2011 season dropped out before second dose because they did not want it. The subjects in the 2010/11 season received 2 subcutaneous injections of 0.5ml (15 μg of hemagglutinin antigen) of the commercially available TIV (Lot. HA101E; Biken) with a 4-week interval in the fall of 2010, while the subjects in the 2011/12 season received one subcutaneous injection of the TIV (Lot. HA119E) in the fall of 2011. The standard route of TIV is subcutaneous injection in Japan. The vaccine contained the following antigens: A/California/7/2009 (H1N1pdm09), A/Victoria/210/2009 (H3N2) and B/Brisbane/60/2008. Subjects completed a questionnaire regarding age and health status. All subjects provided written informed consent. The study was approved by the ethics committee of the Osaka Prefectural Institute of Public Health and Osaka City University Graduate School of Medicine.
Laboratory methods
Serum samples in the 2010/11 influenza season were collected before vaccination (S0), 4 weeks after the first dose (S1), and 4 weeks after the second dose (S2). Serum samples in the 2011/12 influenza season were collected before vaccination (S0) and 4 weeks after the dose (S1). Serum samples were treated with receptor-destroying enzyme (Denka Seiken) and inactivated for 1 h at 56°C to eliminate non-specific inhibitors. Serum anti-hemagglutinin antibody titers were measured using a standard HI assay with 0.75% guinea pig erythrocytes and antigens, including circulating influenza A (H3N2) strains and inactivated vaccine strain (A/Victoria/210/2009). The circulating influenza strains analyzed in the 2010/11 season were A/Osaka/67/2010, A/Kobe/357/2010, and A/Yokohama/79/2010, while those in the 2011/12 season were A/Osaka/110/2011 and A/Osaka/5/2012. The antigenicity of the circulating viruses was measured by HI assay with ferret antisera to the vaccine strain (A/Victoria/210/2009) provided from the Influenza Virus Research Center, National Institute of Infectious Diseases, Japan.
Statistical Analyses
GMT, seroprotection rate (post-vaccination HI titers of ≥1 :40) and seroresponse rate (4-fold increase in antibody level) were calculated to assess the immunogenicity of influenza vaccine. For calculation, titer values under 10 were assigned a value of 5. The significance of the fold increase within a category was assessed using Wilcoxon's signed-rank test. Analyses were performed using SAS, version 9.1.3 (SAS Institute Inc.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Chiharu Kawakami (Yokohama City Institute of Health) and Kyoko Akiyoshi (Kobe Institute of Health) for kindly providing the influenza virus strains. We also thank Jun Komano (Osaka Prefectural Institute of Public Health) for his helpful suggestions.
Funding
This study was supported by Research on Emerging and Reemerging Infectious Diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan (H23-Shinko-Ippan-017).
References
- Kilbourne ED, Arden NH. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, editors. Vaccines, 3rd ed., Philadelphia, PA: WB Saunders Company; 1999, p. 531-51.
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889-96; PMID:11259722; http://dx.doi.org/10.1056/NEJM200103223441204
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. Morbidity and Mortality Weekly Report 2011; 60:1128–32; PMID:21866086
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004; 59:1-15; PMID:14723616; http://dx.doi.org/10.1111/j.0300-9475.2004.01382.x.
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A (H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol 2000; 61:94-9; PMID:10745239; http://dx.doi.org/10.1002/(SICI)1096-9071(200005)61:1%3c94::AID-JMV15%3e3.0.CO;2-C.
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK, Marshfield Influenza Study Group. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis 2009; 199:159-67; PMID:19086915; http://dx.doi.org/10.1086/595861.
- Webster RG, Laver WG, Air GM, Schild GC. Molecular mechanisms of variation in influenza viruses. Nature 1982; 296:115-21; PMID:6174870; http://dx.doi.org/10.1038/296115a0.
- Pereira MS, Chakraverty P, Schild GC, Coleman MT, Dowdle WR. Prevalence of antibody to current influenza viruses and effect of vaccination on antibody response. Br Med J 1972; 4:701-3; PMID:4646846; http://dx.doi.org/10.1136/bmj.4.5842.701.
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69-75; PMID:367490.
- Potter CW, Jennings R, Nicholson K, Tyrrell DA, Dickinson KG. Immunity to attenuated influenza virus WRL 105 infection induced by heterologous, inactivated influenza A virus vaccines. J Hyg (Lond) 1977; 79:321-32; PMID:270523; http://dx.doi.org/10.1017/S0022172400053158.
- Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 1996; 14:1597-602; PMID:9032887; http://dx.doi.org/10.1016/S0264-410X(96)00153-3.
- Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, Icardi G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59-and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010; 28:4123-29; PMID:20433807; http://dx.doi.org/10.1016/j.vaccine.2010.04.030.
- Ng S, Ip DK, Fang VJ, Chan KH, Chiu SS, Leung GM, Peiris JS, Cowling BJ. The Effect of Age and Recent Influenza Vaccination History on the Immunogenicity and Efficacy of 2009–10 Seasonal Trivalent Inactivated Influenza Vaccination in Children. PloS one 2013; 8:e59077; PMID:23554974; http://dx.doi.org/10.1371/journal.pone.0059077.
- Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol 2007; 79:320-5; PMID:17245715; http://dx.doi.org/10.1002/jmv.20801.
- de Bruijn IA, Remarque EJ, Jol-Van der Zijde CM, Van Tol MJD, Westendorp RGJ, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis 1999; 179:31-6; PMID:9841819; http://dx.doi.org/10.1086/314540.
- de Bruijn IA, Remarque EJ, Beyer WEP, Cessie SL, Masurel N, Ligthart GJ. Annually repeated influenza vaccination improves humoral responses to several influenza virus strains in healthy elderly. Vaccine 1997; 15:1323-9; PMID:9302738; http://dx.doi.org/10.1016/S0264-410X(97)00019-4.
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997; 15:1114-22; PMID:9269055; http://dx.doi.org/10.1016/S0264-410X(97)00003-0.
- Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, Murasko DM. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine 2001; 19:4610-7; PMID:11535308; http://dx.doi.org/10.1016/S0264-410X(01)00246-8.
- Hobson D, Baker FA, Curry RL. Effect of influenza vaccines in stimulating antibody in volunteers with prior immunity. Lancet 1973; 302:155-6; http://dx.doi.org/10.1016/S0140-6736(73)93106-1.
- Pyhälä R, Kumpulainen V, Alanko S, Forsten T. HI antibody kinetics in adult volunteers immunized repeatedly with inactivated trivalent influenza vaccine in 1990–1992. Vaccine 1994; 12:947-52; http://dx.doi.org/10.1016/0264-410X(94)90039-6.
- Ohfuji S, Fukushima W, Deguchi M, Kawabata K, Yoshida H, Hatayama H, Maeda A, Hirota Y. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: lowered antibody response by prior seasonal vaccination. J Infect Dis 2011; 203:1301-8; PMID:21459817; http://dx.doi.org/10.1093/infdis/jir026.
- Hirota Y, Kaji M, Ide S, Kajiwara J, Kataoka K, Goto S, Oka T. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine 1997; 15:962-7; PMID:9261942; http://dx.doi.org/10.1016/S0264-410X(96)00302-7.
- Miller DL, Reid D, Daimond JR, Pereira MS, Chakraverty P. Hong Kong influenza in the Royal Air force 1968-70. J Hyg (Lond) 1973; 71:535-47; PMID:4518353; http://dx.doi.org/10.1017/S0022172400046520.
- Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonization of requirements for influenza vaccines. London: European Agency for the Evaluation of Medicinal Products, Human Medicines Evaluation Unit, 12 March 1997, document: CPMP. 1997; BWP/214/96. Available from: http://www.emea.eu.int/pdfs/human/bwp/021496en.pdf
- Boni MF. Vaccination and antigenic drift in influenza. Vaccine 2008; 26:C8-C14; PMID:18773534; http://dx.doi.org/10.1016/j.vaccine.2008.04.011.
- Wright PF, Webster RG. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields virology, 4th ed., Philadelphia, PA: Lippincott Williams & Wilkins; 2001, p.1533-79.
- Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, Petrie JG, Lofthus G, Meece JK, Williams JV, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951-9; PMID:22843783; http://dx.doi.org/10.1093/cid/cis574.
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; http://dx.doi.org/10.1186/1741-7015-11-153.
