Abstract
US common law recites a natural law, natural phenomenon or abstract idea as exceptions to the 4 statutory patentable categories to guard against the wholesale preemption of fundamental principles. The very recent evolutions of patent exceptions in the US may increase the difficulty of patenting and may create uncertainty in determining patent eligibility. To solve the thorny problem of eligibility, this study presents a flow chart based on the courts’ decisions that can serve as a set of guidelines for determining patent eligibility. A case related to prostate cancer immune therapy is presented for discussion.
The patentability mountainCitation1 () represents 5 upward tests for an invention to be patentable. The patent office is required by statute to examine every patent application to be certain it passes each of these tests.
Section 101 of the US Patent Code defines patent eligibility or subject matter requirement. According to the statute, a person who “invents or discovers any new and useful process, machine, manufacture or any composition of matter or any new and useful improvement thereof, may obtain a patent.” An invention that falls within one of the 4 above-mentioned statutory categories may be entitled to a patent. In recent decades, the patent system has demonstrated some permissiveness toward patent eligibility.Citation2 In particular, the US Supreme Court has suggested that the realm of patents encompasses “anything under the sun that is made by man."Citation3,4
Because these 4 statutory categories are deliberately broad, patent eligibility was seldom an obstacle during patent prosecution or litigation. However, very recent US Supreme CourtCitation5-7 and Federal Circuit Court of AppealsCitation8 (CAFC) cases have dramatically changed that situation. As the CAFC has commented, “short and unadorned §101 appears deceptively simple on its face, yet its proper application to computer-implemented inventions and in various other fields of technology has long vexed this and other courts.” Moreover, the US Supreme Court has noted that “the line between a patentable process and an unpatentable principle is not always clear.”Citation9 Fortunately, these courts’ opinion can serve as predictors of the answer to the thorny question of patent eligibility, and can therefore servce as guidelines during patent eligibility determination.
A Process for Determining Patent Eligibility
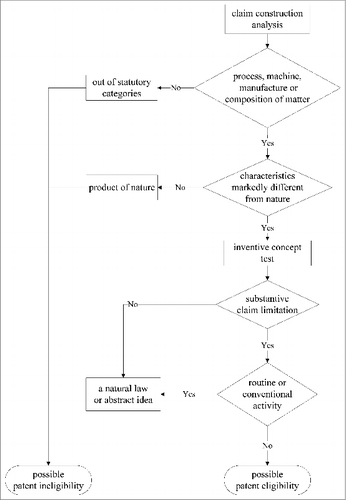
The process for determining patent eligibility can be represented as a flow chart (). Referring to the flow chart, the steps in determining patent eligibility can be itemized as follows.
Determine whether the claimed invention fits within one of the 4 statutory categories
The constitutional purpose for granting patents is to promote the progress of the useful arts. Section 101 of the US Patent Code clearly defines the 4 categories of patent eligibility as process, machine, manufacture or composition of matter. Among these categories, a process is a way to produce a desire result. The elements of a process or method claim must be steps or acts, expressed as verbal statements or phases.Citation10 It should be noted that a process patent is granted for discovering and disclosing a process, not for the scientific theory behind it.Citation2 Thus, the inventor may not realize exactly how the process works. On the other hand, the explanation of the process would be not patentable because an idea or a theory itself is not patent eligible. Moreover, the disclosure of the process mechanism of an invention that does not present a specific function may also lack specific and substantial utility and therefore not meet the enablement requirement.Citation11
A machine is an assembly of parts that transmit force, motion or energy to accomplish useful work. The term “apparatus” is used generically to denote various machines or devices, including mechanical, electrical, computer-related or hydraulic devices. While machines generally have moving parts and operations, the articles of manufactures usually have no moving parts. An article of manufacture may also refer to anything man-made that is not a machine or a composition of matter.Citation2 However, distinguishong between statutory manufacture and a machine is only of academic interest. Furthermore, claims to an article of manufacture differ little in principle from machine claims.Citation10
Compositions of matter are products for which the chemical nature or materials used, rather than the shape or form of a product, is the distinguishing characteristic. Very similarly to the above-mentioned machine versus manufacture distinction, it is also not necessary to determine the statutory difference between manufacture and composition. A claimable composition of matter may be a new molecule, compound, solution, mixture or even a living being with markedly different characteristics from any found in nature.Citation7
It should be noted that in contrast to the US statute, most other countries define patent eligibility in the negative excluding categories of inventions. For example, the European Patent Convention Article 52(2) recites (a) discoveries, scientific theories and mathematical methods; (b) aesthetic creations; (c) schemes, rules and methods for performing mental acts, playing games or doing business, and programs for computers; and (d) presentations of information as things not to be regarded as inventions. The China Patent Law excludes inventions that violate law or social ethics or harm public interests from being granted patents. Similarly, the Taiwan Patent Act excludes (a) animals, plants, and essential biological processes for the production of animals or plants, except processes for producing microorganisms; (b) diagnostic, therapeutic and surgical methods for the treatment of human; and (c) inventions contrary to public order or morality from being patented.
Determine the judicial exceptions to patent eligibility
Once an invention is determined to fall within one of the process, machine, manufacture or composition of matter categories, the determination must turn to the judicial exceptions to patent eligibility, which is the second step in determination of patent eligibility. The exceptions to patent eligibility cited in US common law guard against the wholesale preemption of fundamental principles. A patentable claim should not coexist with a natural law, natural phenomenon or abstract idea.
A natural law is a generalized description of the universe. Ohm's law, for example, describes the relationship between voltage, current and resistance, while Einstein's mass energy equivalence describes the relation between mass and energy. Almost all inventions can be described by the laws of nature. Thus, granting patents on these laws would slow technological progress, which certainly violates the principle that patent system is organized to encourage innovation.
The rule against patenting a law of nature has been applied to materials discovered in nature and to abstract idea. The US Supreme Court stressed that “the heat of the sun, electricity or the qualities of metals are part of the storehouse of all knowledge of all men. They are manifestations of laws of nature, free to all men and reserved exclusively to none.Citation12” For example, a discoverer cannot patent a new mineral she/he discovers. However, the use of the material in a particular application, such as the use of the mineral to treat cancer, is patent eligible.
Another general category of un-patentable subject matter is the category of abstract idea. An inventor cannot patent an idea that is without a material form or a practical application. Granting a patent to an abstract idea, rather than practical applications of the idea, would certainly discourage advancements in the technology. Furthermore, the CAFCCitation8 cited the US Supreme Court's Mayo case to stress that “patent law cannot inhibit further discovery by improperly tying up the future use of law of nature.”
Determining whether an invention claimed within judicial exceptions to patent eligibility is not straightforward.Citation8 What is crucial is that the US Supreme Court has abolished the CAFC's formulaic “machine or transformation” testCitation5 as the one and only rule and requires a flexible and pragmatic approach to the core principle underlying the exceptions to patent eligibility.Citation8 Therefore, to determine the judicial exceptions to patent eligibility, the procedures should be further broken down as described below.
Markedly differ from nature
A newly discovered natural product, such as a mineral in the Earth or a plant in the wild, lies beyond the scope of patent protection.Citation3,13 A recent US Supreme Court caseCitation7 clearly determined that “a naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated, but cDNA is patent eligible because it is not naturally occurring.” The determination was based on whether the invention claimed has “markedly different characteristics from any found in nature.”Citation3 An inventor who separates a gene from its surrounding material does not create anything. An isolated gene, even if it is important and useful, does not have any characteristic different from that in nature, and thus is not patent eligible. The US Supreme Court also stressed that “groundbreaking, innovative, or even brilliant discovery does not by itself satisfy the §101 inquiry.”Citation7
Inventive concept test
The patent system is organized to encourage innovation: a government grants the patentee the right to exclude others from practicing the technical development of an invention for a certain amount of time, in return for disclosing innovation. The government hopes other inventors will learn from the disclosure and use the knowledge as the basis for further innovation.Citation14 Based on the prerequisite to encourage innovation, granting a patent on the basic tools of scientific and technological work would, on the contrary, obstruct further innovation. Therefore, these fundamental tools of discovery should be free to allCitation12 and not be preempted. Indeed, the US Courts’ primary aim in applying the common law pertaining to patent eligibility is to guard against the wholesale preemption of fundamental principles.
To avoid the wholesale preemption of principles, the US Supreme Court created the inventive concept.Citation9 A claim that satisfies the inventive concept requirement is a process that focuses on the use of a natural law and contains other elements or a combination of elements to ensure that the patent amounts to significantly more than a patent on the natural law itself.Citation6 The CAFCCitation8 has interpreted the inventive concept in the context of §101 as a genuine human contribution, representing more than a trivial appendix to the underlying abstract idea, to the claimed subject matter.
To determine whether a claim meets the inventive concept requirement, we need to determine whether the claim has substantive limitations that are not just routine or conventional activities.
Substantive claim limitations
The US Supreme Court has clearly indicated that a patent-eligible claim should be significantly more than a basic principle.Citation6 Thus, a claim must include one or more substantive limitations to avoid wholesale preemption of the principle. The CAFCCitation8 has further noted that the field-of-use, monitoring, adjusting and computer limitations are trivial.
Before the Supreme Court's Bilski case,Citation5 the CAFC had utilized only the machine-or-transformation test, tying a process to a particular apparatus or transforming a particular article into a different state, to determine the patentability of the process. Now, the CAFCCitation8,15 holds that if a claim requires a computer to perform operations that are not merely accelerated or more accurate calculations, the computer itself does not render the claim patent eligible. For example, a claim for a data processing system, comprising a data storage unit and a computer but not reciting a meaningful limitation beyond generally linking the usage of the method to a technology would not satisfy the §101 inquiry.
Determining whether a claim with a substantive limitation meets the inventive concept requirement depends on whether the claim would wholly preempt the principle. A claim preempting a principle or equation would not have substantive limitations. In contrast, a claim targeting a specific purpose may not necessarily pass the inventive concept test. As the Court of Customs and Patent Appeals (CCPA) indicated,Citation16 "if a claim is directed essentially to a method of calculating, using a mathematical formula, even if the solution is for a specific purpose, the claimed method is nonstatutory." Quoting the CCPA's decision, the US Supreme CourtCitation9 found that the patent specification in question did not explain how to select variables, nor did the specification contain any disclosure relating to the processes at work, the monitoring of process variables or the means of setting off a system. Based on the findings, the Supreme CourtCitation9 determined that granting the patent would preempt the equation and thus the subject matter was not patentable under §101, even though the claim had a specific end use to pass the wholesale preemption test.
Well-understood, routine or conventional activities
In the Mayo case,Citation6 a claim recited steps for administering the drug thiopurine, determining the resulting metabolite concentration in a patient's blood, and adjusting the drug dose based on the concentration. The US Supreme Court's held that the claim failed the patent eligibility test because the substantive limitation was a well-understood, routine or conventional activity previously engaged in using the technology.
The Supreme Court further stressed that upholding the claim's patents eligibility would risk disproportionately tying up the use of the underlying natural laws, inhibiting their use in the making of further discoveries. Indeed, the patent system is organized to encourage innovation, in the hope that other inventors will learn from the disclosure and use the knowledge as the basis for further innovation.Citation14 Granting a patent on a well-understood, routine or conventional activity may cause any method involving the activity to infringe on the patent. Granting such a patent would slow technological progress and certainly violate the principle that the patent system is organized to encourage innovation.
The Validation of the Flow Chart
To demonstrate the feasibility of the flow chart developed, this study retrospectively reviews the US Courts’ determinations related to patent eligibility. In the Benson case,Citation17 the claims in dispute were not limited to any particular technology, apparatus or end use. Based on the flow chart, the claims would fail the inventive concept test because they did not meet the substantive claim limitation.
In the Diehr case,Citation18 the claimed method included constantly determining the temperature inside a mold, repetitively calculating the necessary cure time using a mathematical equation, and opening the rubber molding press when the elapsed cure time equaled the calculated cure time. The Supreme Court held the claim to be patent eligible because that the claimed process incorporated the mathematical equation with other steps and did not seek to preempt the equation. Furthermore, a step in the process was new in the art. Following the flow chart developed, the claim passed the inventive concept test because the claim had substantive limitations and the limitations were not just routine or conventional activities.
In the Supreme Court's Kappos case,Citation5 one main claim recited a series of instructions on how to hedge risk that included transactions between a commodity provider and consumers and between the commodity provider and other market participants in counter-risk positions to those of consumers. The other main claim put the concept articulated above into a mathematical formula. The Court held the claims to be patent ineligible because they covered the abstract idea of hedging against risk and granting a patent would preempt the use of this approach in all fields. Based on the flow chart developed, the claims failed the inventive concept test because they lacked substantive claim limitations.
The Mayo caseCitation6 involved a claimed method for optimizing thiopurine administration in a patient based on a correlation between the therapeutic efficacy of a particular dose of a thiopurine and the resulting concentration of the drug metabolites in the patient's blood. The Supreme Court held that claims to be patent ineligible because that they did not transform unpatentable natural laws into patent-eligible applications of those laws. Following the flow chart developed, the claims did not meet the inventive concept test because the claims’ limitations were well-understood, routine or conventional activities.
Inventors discovered the precise location and sequence of the BRCA1 and BRCA2 genes. Mutations in these genes can dramatically increase an individual's risk of developing breast and ovarian cancer. The claims asserted a patent on isolated DNAs coding for BRCA1 and BRCA2 polypeptides with the amino acid sequence sets found. The US Supreme CourtCitation7 held that claim to be patent ineligible because the inventors did not create or alter any of the genetic information encoded in these genes. Following the flow chart developed, the claims did not have characteristics markedly different from nature and therefore were patent-ineligible product of nature.
Another invention disclosed a computerized method, computer-readable media and systems for conducting financial transactions using third party to settle obligations between the first and second party as to mitigate settlement risk. The claimed method recited as components of the methods creating shadow records, using a computer to adjust and to maintain these records, reconciling recordings and corresponding exchange institution accounts through end-of-day transactions. The CAFC en bancCitation8 held the claim to be patent ineligible because the limitations of this claim were not substantive. The CAFC also determined that the claimed computer program and data processing system did not have meaningful limitation beyond generally linking the program and system to the use of the method, even though the claims were associated with certain computer components. Following the flow chart developed, the claims did not pass the inventive concept test because of the lack of substantive claim limitations.
A Case Study Related to Immune Therapy
Immunotherapy has been investigated in both preclinical studies and clinical trials as a new therapy for prostate cancer. For example, Sipuleucel-T is the first immunotherapy approved by the US FDA in 2010 for men with castrate-resistant prostate cancer,Citation19,20 However, unlike radiation and chemotherapy, immunotherapies are not immediately cytotoxic and can not promptly decrease tumor burden. Thus, therapies to combine immunotherapies with chemotherapy, radiation and androgen deprivation therapy are highly recommended.Citation19
An invention identifies a method that can assist physicians to better select drug treatments and in particular combination drug compositions for individual patients with prostate cancer. The independent claim was drafted as:
“A method for monitoring the efficacy of an anti-cancer therapy in a patient, wherein the anti-cancer therapy comprises an immune cell activating agent and antigen display enhancing agent that is able to stimulate or enhance the presentation of a cancer specific antigen on the surface of cancer cells, the method comprising:
incubating a first plurality of immune cells obtained from the patient prior to treatment with the immune cell activating agent to provide activated immune cells;
incubating cancer cells from an established cancer cell line with the antigen display enhancing agent, thereby providing treated cancer cells;
contacting the activated immune cells with the treated cancer cells and determining the ability of the activated immune cells to kill the treated cancer cells; and
repeating steps (b)-(c) with a second plurality of immune cells obtained from the patient at a subsequent time interval following administration of the anti-cancer treatment,
wherein a reduction in the ability of the activated immune cells prepared from the second plurality of immune cells to kill the treated cancer cells compared to the ability of activated immune cells prepared from the first plurality of immune cells to kill the treated cancer cells is indicative of a reduction in the efficacy of the candidate anti-cancer therapy.”
The dependent claim was written to add the limitation of monitoring the levels of at least of one cancer specific biomarker and/or the levels of at least of marker of immune health in the patient.
The patent examiner rejected both claims under 35 USC 101 because the claimed invention is not a patent eligible subject matter. The examiner explained that the correlation of a cancer specific biomarker and the immune response of a patient with cancer is a natural law. Furthermore, the steps of treating a patient with an anti-cancer therapy and then measuring the levels of a cancer specific biomarker to monitor the efficacy of the treatment are routine or conventional activities in the field.
Based on the rejection, the patentee amended the “cancer” term in above independent claim specific to “prostate cancer.” The amendment argument included that the claims integrated a natural principle into the claimed invention such that the natural principle is practically applies; the steps were sufficient to ensure that the claims amounts to significantly more than the natural principal itself; and the claims did not cover every substantial practical application of a law of nature. Following the amendment and argument, the examiner allowed the claims.
Even though the claims were allowed by amending to prostate cancer, the authors still believe that these steps claimed are routine or conventional activities in the field. Moreover, the claims include all kinds of immune cells and antigens for prostate cancer. Certainly, the dependent claim will cover every substantial practical application of the prostate cancer treatment, including a brand new biomarker. Tracing back to the flow chart for determining patent eligibility (), the claim would be determined as without substantive claim limitation or routine or conventional activities.
Conclusions
Unlike the patent codes of most other countries, which define patent eligibility in the negative excluding categories of inventions, the US Patent Code circumscribes process, machine, manufacture and composition of matter as patentable subject matter. Moreover, US common law cites exceptions to patent eligibility, including a natural law, natural phenomenon or abstract idea, to guard against the wholesale preemption of fundamental principles. Granting patents on these principles would slow technological progress and thus violate the doctrine that the patent system is organized to encourage innovation. The recent evolution of patent exceptionsCitation6-8 in the US may increase the difficulty of patenting and may create uncertainty in determining patent eligibility.
To determine patent eligibility, the flow chart presented in this study demonstrates that, according to the recent US Supreme CourtCitation5-7 and CAFCCitation8 cases, a claim with characteristics not markedly different from nature, without substantive limitations or with limitations that are well-understood, routine or conventional activities previously engaged in with the technology are not patent eligible. These decisions may create difficulties in patenting procedures for clinical diagnosis, natural products, and agbiotech products or processes and generally for the patenting of isolated proteinsCitation21 Natural gene patents are considered to be less valued than they were in the USCitation22 Furthermore, the CAFC's machine-or-transformation test is no longer the sole test for determining patent eligibility. The CAFCCitation8 holds that a claim, that does not recit a meaningful limitation on computer or data storage unit within the claim, is not patent eligilbe. This assessment also has implications for the software industry, including bioinformatics.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported in part by the National Science Council (101-2221-E-075-001) and Taipei Veterans General Hospital (V102C-068, V103 C-208), Taiwan.
References
- Pressman D. Patent it yourself, ed. t. edition. 2000, Berkeley, CA: Nolo.com.
- Schechter RE, Thmoas JR. Principles of Patent Law. 2004, St. Paul: West, a Thmoson business
- Diamond v. Chakrabarty. 1980 The US Supreme Court.
- Durham A. Patent Law Essentials. 1999, Westport: CT Greenwood Publishing Group, Inc.
- Bilski v. Kappos. 2010 The US Supreme Court.
- Mayo v. Prometheus. 2012, The US Supreme Court.
- Association for Molecular Pathology v. Myriad Genetics. 2013, The US Supreme Court.
- CLS Bank International v. Alice Corporation Pty. Ltd. 2013, Federal Circuit Court of Appeals.
- Parker v. Flook 1978 The US Supreme Court.
- Faber RC. Landis on mechanics of patent claim drafting. fourth edition ed.; 2001 New York: PLI Press.
- In re Fisher. 2005, the United States Court of Appeals for the Federal Circuit.
- Funk Brothers Seed Co. v. Kalo Inculant Co. 1948, The US Supreme Court.
- Adelman M, et al. Cases and Materials on Patent Law,. American casebook series. 1998, ST. Paul: West Group
- Wang S-J. Designing around patents: a guideline. Nat Biotechnol 2008; 26(5):519–522; PMID:18464780; http://dx.doi.org/10.1038/nbt0508-519
- Dealertrack, Inc. v. Huber. 2012 Federal Circuit Court of Appeals.
- In re Richman. 1977 The Court of Customs and Patent Appeals
- Gottschalk v. Benson. 1972 The US Supreme Court.
- Diamond v. Diehr. 1981 The US Supreme Court.
- Uhlman MA, Bing MT, Lubaroff DM. Prostate cancer vaccines in combination with additional treatment modalities. Immunol Res 2014; 59(1-3):236–242; PMID:24838261; http://dx.doi.org/10.1007/s12026-014-8532-1
- Dawson NA, Roesch EE. Sipuleucel-T and immunotherapy in the treatment of prostate cancer. Expert Opin Biol Ther 2014; 14(5):709–719; PMID:24620782; http://dx.doi.org/10.1517/14712598.2014.896897
- Ratner M. Myriad decision aftershocks ripple through biotech. Nate Biotechnol 2013; 31(8):663–664; PMID:23929321; http://dx.doi.org/10.1038/nbt0813-663
- Matthijs G, Huys I, Van Overwalle G, Stoppa-Lyonnet D. The European BRCA patent oppositions and appeals: coloring inside the lines. Nat Biotechnol 2013; 31(8):704–710; PMID:23929344; http://dx.doi.org/10.1038/nbt.2644