Abstract
It is widely understood that commensal microbiota contributes to the maintenance of intestinal homeostasis through dynamic interactions with a body's immunity. And the immune regulation is important for the influenza vaccine's effectiveness after body injection, however, the mechanism between commensal microbiota and vaccine's effectiveness remains unknown. The impact that individual bacteria species have on the balance of the systemic immune system beyond the local intestinal mucosal tissues also remains less clear, and the related mechanism is still unknown. In this study, through the administration of various antibiotics, we examined the balance of helper T cell subsets in mice after inoculating them with the influenza virus and then, attempted to imitate the clinical practice in which patients are always prescribed with an antibiotic treatment in flu season. The data indicates that the mice in each group present differential immune responses in terms of the makeup of helper T cell subsets, although the Th17 cell activity seems to not be involved in the systemic immune modulation in the mice that are susceptible to the intervention of antibiotic. Th1, Th2, and anti-inflammatory regulatory T cells have been implicated in the contribution to the systemic immune response influenced by the antibiotic-induced dysbiosis. Thus we believe that the normal intestinal flora could maintain the immune balance and inhibit the inflammatory responses, which may be useful for clinical application to take intestinal flora into consideration when influenza vaccination was used.
Introduction
The complex interactions that take place between the human gut microbiota and host immune system are important components of bodily health. Over the millennia, our immune system has coevolved with an exceptional number of microorganisms and has since established a relatively stable environment in which both parties are profoundly benefited. These microbiota not only assist in providing the body with sufficient nutrients through aiding in digestion, but they also contribute to relative colonizing strength over any invading pathogens in addition to shaping immune system regulation, and likewise, the human gastrointestinal tract supplies its microbiota with adequate nutrition as well as a moderate environment that is required for their growth.Citation1 Moreover, intestinal flora may play an important role in the regulation of body reaction to vaccine injection. Recent research revealed that mice raised in a sterile environment and treated by antibacterial drug had a decreased response to influenza vaccination, while oral administration of colibacillus could rectify these responses. So these results implicated an positive role for gut microbiota in promoting immunity to vaccination.Citation2 Researches show that the activity of vaccine is closely related to the immune function of organism. The inactivated influenza vaccine may play its role by adjusting B cell to generate excessive interferon.Citation3 The intestinal flora plays a critical role in the maintenance of body immune adjustment. Once this intimate relationship's homeostasis is altered, the host often experiences detrimental health effects. Alterations in some distinct intestinal species, like firmicutes, bacteroidetes, or enterococcus, are commonly found in patients with inflammatory bowel disease (IBD),Citation4,5 which has also been associated with increased cytokines levels, like IFN-γ (interferon gamma) and IL-17 (interleukin 17) that are respectively secreted by Th1 (T helper cell 1) and Th17.Citation6,7 Accumulating evidence has demonstrated that distinct, individual microorganism species profoundly affect the balance of T lymphocytes subsets: more specifically, pro-inflammatory cells that include Th1 cells, Th17 cells, and Th2 cells as well as the anti-inflammatory Foxp3+ regulatory T cells (Treg).Citation8 A study has also demonstrated that a disorder affecting the mutualism between the microbiota and the mucosal immune system elicits the specific immune response of effector T cells, such as an increased population of Th17 and Th1 cells that can both engender excessive inflammation-mediated tissue damages.Citation9
T cell balance is associated with the development of respiratory diseases. Studies have shown that asthma attacks are closely associated with a decreased number of Treg cells and the activation of Th2 cells; additionally, Th17 cells were highly expressed in the lung in a murine model of asthma. It has been confirmed that allergic airway responses and asthma attacks are correlated with the changes in IgA induced by the dysbacteriosis of gut microbiota; therefore, a correlation has been proposed between gut microbiota and T cell balance.
Recent compelling evidence also indicates that Th17 confer host immunity against a variety of microbes, including extracellular and intracellular pathogens. Therefore, it is necessary to analyze the relationships between gut microbiota and T cell subsets as well as between gut microbiota and immune recognition to elucidate the influences of gut microbiota on vaccine research and development and pulmonary diseases protection. In this study, we administer 3 commonly used antibiotics in clinical practice into mice, and then, following inoculation with the influenza virus, we demonstrate that the body's systemic immune homeostasis that is maintained by intact intestinal microbiota is largely attributed to the balance between pro-inflammatory cytokine-producing cells (Th1 and Th2) and the anti-inflammatory cytokine-producing cell (Foxp3+ Treg cells), rather than Th17 cells. Susceptible to the influenza virus infection, the makeup of the helper T cell subsets in mice that did not have antibiotic intervention does not seem to be altered as compared to the control group. Conversely, the antibiotic-sensitive microbiota species in the mice in which metronidazole, cephradine, and neomycin had been respectively administered play differential roles in the modulation of the immune system. So the intestinal flora plays important roles in the body immune responses and this may be taken into consideration in the clinical influenza vaccination application before influenza vaccination used in the future.
Results
Bacterial colony plate counts
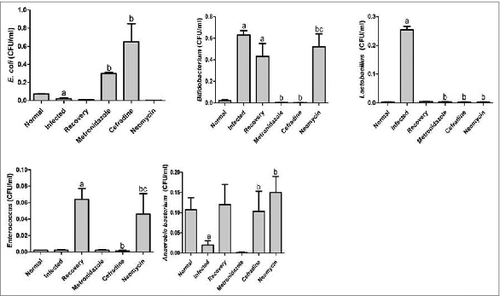
The bacterial colony plate counts represented significant differences between each group (). The plate counts of the enterococcus and the anaerobic bacteria were significantly lower; while the bifidobacterium and lactobacillus increased significantly in the viral infection group as compared with the normal group (P < 0.05). Additionally, the number of bifidobacterium and enterococcus increased in the recovery group as compared to the normal group (P < 0.05). The colony plate counts of E. Coli in the metronidazole and cefradine groups, the bifidobacterium and enterococcus of the neomycin group, and the anaerobes in the cefradine and neomycin groups increased significantly as compared to the infected group (P < 0.05). The bifidobacterium of the metronidazole and cefradine groups, enterococcus of the cefradine group, and finally lactobacillus in the metronidazole, cefradine, and neomycine groups decreased significantly when compared with the infected group (P < 0.05). The bifidobacterium in the neomycine groups increased when compared with the recovery group (P < 0.05). A comparative analysis of the colonies was conduct in the different experimental groups and we found that the viral inoculation and antibiotic intervention are capable of enhancing the unbalanced condition in the fecal flora mice as compared to both the normal group as well as the infected group respectively ().
Figure 1. Bacterial colony plate counts in each group. Bacterial colony plate counts in each group for 3 replications. a. P < 0.05 compared with the normal group; b. P < 0.05 compared with the infected group; and c. P < 0.05 compared with the recovery group. Bars indicate standard deviation by One-way ANOVA.
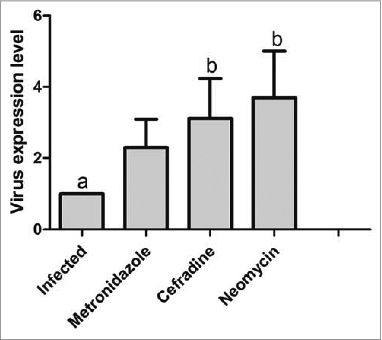
RT-PCR detection of virus amplification
The results of the RT-PCR experiment indicated that compared to the infected group, the amount of virus amplification increased in the metronidazole, cefradine, and neomycin groups of combined dysbacteriosis and viral infection; of these, compared to the infected group, the increases in virus amplification in the cefradine and neomycin groups were statistically significant (P < 0.05, ).
Dysbiosis triggered by distinct antibiotic leads to inconsistent distribution of Th1 in mice
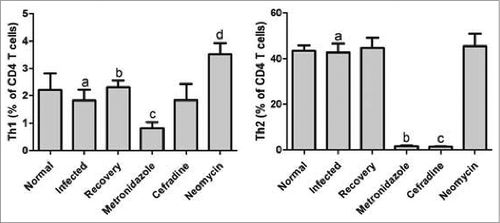
The results showed that the Th1-produced cytokines of IFN-γ in the antibiotic-treated mice spleen were markedly distinct from the untreated control groups. Interestingly, the CD4+IFN-γ+ T cells (Th1) in both the metronidazole and cephradine-intervened groups were severely reduced as compared to the control groups, whereas those in the neomycin group were appreciably more induced than in the control groups. The results also indicated that mice who are suffering from influenza and do not receive any antibiotics do not seem to have altered Th1 cell proliferation. Next, we focused on the Th1 cells multiplication between the individual antibiotic groups. According to the data analysis, comparing the 3 groups showed that the Th1 proliferation was remarkably activated following the elimination of neomycin-sensitive bacteria as compared with the metronidazole and cefradine group. Also noteworthy, between the 2 latter groups, there was no significant difference in the number of Th1 cells ().
Figure 3. Th1 and Th2 differentiation and polarization induced in antibiotic-treated mice. Comparative assessment of the percentages of CD4+ IFN-γ+ T cells between the metronidazole, cephradine, and neomycin-intervened groups. P < 0.05 for a vs c, a vs d, and b vs d in Th1 differentiation, and P < 0.05 for a vs b, and a vs c for Th2 differentiation by One-way ANOVA.
The effects of communal microbiota on the Th2 cell group
Similar to the aforementioned results in which the Th1 growth was induced by the antibiotic interventions, Th2 cells that produce IL-4 were also influenced by the gut microbiota. And there was not a significant difference between the mice in the control group and the influenza-infected mice. On the other hand, the expression levels of IL-4 in both the metronidazole and cephradine treatment groups were markedly reduced as compared to the 2 control groups ().
Critical role of gut flora in the regulation of Foxp3+ Treg cells’ immune responses
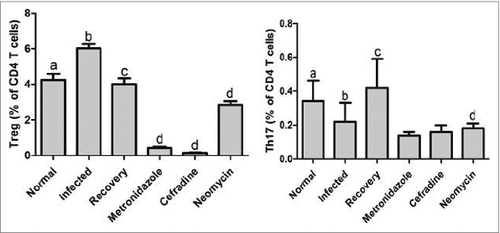
For the 2 control groups, the percentage of CD25+ Foxp3+ Treg cells out of the CD4+ cells in the flu-infected mice were analyzed as similar to the blank control group. Strikingly, we found that, although both the metronidazole and cephradine treatments caused a decline in the number of Treg cells in the mice splenocytes, the Foxp3 expression under the neomycin-induced dysbiosis was significantly reduced only when compared to those in the mice that were susceptible to the virus infection in the control group, rather than to the blank control group. To test the effects of the 3 antibiotic-induced dysbiosis on the Treg cell proliferation in order to explore the mutualism of commensal microbiota with the host immune system, we compared their individual roles in the Foxp3+ Treg cells. The results indicated that the neomycin-treated mice had obviously higher rates of Treg cell than did the other 2 antibiotic treatment groups (). In addition, it is worth noting that the number of Foxp3+ Treg cells in the mice spleen seemed to be depleted by removing the metronidazole and cephradine-sensitive bacteria, respectively.
Figure 4. The expression of Foxp3-positive Treg and Th17 cells immune responses triggered by gut flora variation. CD4+ CD25+ Foxp3+ Treg and Th17 cells in mice spleen were analyzed in each group. P < 0.05 for a vs b, b vs d, and c vs d in Treg cells, and P < 0.05 for a vs b, and c vs d in Th17 cells by One-way ANOVA.
Th17 cell development is not involved in the affected immune cells subsets by alteration in the gut microbiota
Inconsistent with the abovementioned phenomenon in which the Th1, Th2, and the Treg cells were correspondingly reacting to changes in the composition of intestinal microorganisms that were caused by either the intervention of an influenza virus inoculation or an antibiotic treatment, the Th17 cells in this study were not vary significantly in number among any of the experimental groups. Therefore, we reason that these results can correlate with recent reports that determine that active pro-inflammatory Th17 cells always contribute to diverse autoimmune diseases, such as arthritis and encephalomyelitis, while this self-immune protective mechanism often operates with few Th17 cells involved in the spleen and blood or other organs beyond the local mucosal tissues.
Discussion
The intestinal immune system has developed under the dual pressure of protecting the host from pathogenic infections and coexistence with the myriad commensal organisms in the lumen. Recent studies have focused on revealing both the host receptors and the signaling pathways involved in sensing both commensal and pathogenic microbes. Gut microbiota are associated with the regulation of innate immunity in the body and pathogen-related pattern recognition receptors. Recent studies have confirmed that the intestinal flora plays a positive role in the maintenance of the vaccine effectiveness in the body, which was also related to the activation of immune system. However, the regulating mechanism was still unknown between the intestinal flora and vaccine potency. The intestinal flora dynamic balance plays important roles in the maintenance of mutualism between immune function in the body and gut microbiota.Citation10
However, the underlying mechanisms of these interactions remain uncertain.Citation11 Abnormal intestinal immune function usually causes an overgrowth of gut bacteria, bacterial translocation, and induction or aggravation of multiple organ failure. Research shows that to vaccinate before or during antibiotic therapy may damage the antibody response of certain vaccine in the human body. The immune response to influenza vaccine, to some extent, relies on the signal of the enteric microorganism, which indicates that the intestinal flora has a strong immune response on the seasonal influenza and inactive poliomyelitis vaccine. Therefore, the enteric microorganism may exert great influence on human immunization vaccination, or even result in the decline of vaccine immunity.Citation2 As a result, the effectiveness of vaccine declines and affects the prevention of illness. Further knowledge of these microbial sensing pathways is important since they are related to the immune system and host-microbial homeostasis as well as antimicrobial defense mechanisms. In our previous work, we had founded that the T cell balance was associated with the development of respiratory diseases and host-microbial homeostasis.Citation12 So it is necessary to analyze the relationships between gut microbiota and T cell subsets as well as gut microbiota and vaccine vaccination recognition to elucidate the influences of gut microbiota on pulmonary diseases and T cell balance.
In this study, mice were treated with antibiotics to successfully establish an animal model of dysbacteriosis. Viral infection was then conducted to investigate the influences of dysbacteriosis on T cell balance in the body. And the results showed that the number of plate counts was different between each of the groups after 3 different antibiotic treatments and this treatment could induce the unbalance of intestinal microorganisms. And the results revealed that viral infection caused changes in the gut microbiota and reductions in the numbers of enterococcus, anaerobic bacteria, bifidobacterium, lactobacillus and E. coli, which indicated that changes in the gut microbiota caused by viral infection might induce inflammatory responses in the systems related to the intestinal mucosa.
T helper cells are important immune regulatory cells in the body that participate in cell-mediated immune responses, regulate humoral immune responses, defend against bacterial infection, and inhibit autoimmune diseases and chronic inflammatory diseases. It has been known that some specific microbiota species differentially contribute to the generation and development of the local mucosal immunity and that gut flora DNA has promoted intestinal homeostasis through the modulation of the Treg/Teff cell (IL-17 and IFN-γ-producing effector T cells) equilibrium. The destruction of Th/Treg balance will cause the development of respiratory diseases. Th1/Th2 imbalance causes Th2 cells to not be able to effectively inhibit Th1 cell proliferation, thus causing an increase in the serum TNF-α content, stimulating lung tissues to release inflammatory mediators, damaging lung tissues, and promoting the aggravation of airway inflammatory responses. The reduction of the number of peripheral blood Treg cells in asthma patients causes the activation of effector CD4+ T cells, especially Th2 cells, which produce a large amount of pro-inflammatory factors, thus resulting in persistent asthma attacks. In vitro studies confirmed that IL-17 could induce the expression of many airway mucin genes in airway epithelial cells, regulate neutrophils, and mediate the occurrence of asthmatic inflammatory responses. Gut microbiota have important influences on CD4+ T cell differentiation in intestinal mucosal tissues. Studies have shown that Th17 cells only aggregate when commensal bacteria are present.Citation13 After neonatal mice were treated with antibiotics, the number of Th17 cells exhibited a significant decreasing trend.Citation13 Treg cells were abundant in the intestinal mucosal tissues; however, their percentage increased in bacteria-free mice and displayed a negative correlation with the percentage of Th17 cells. Recent compelling evidence indicates that Th17 confer host immunity against a variety of microbes, including extracellular and intracellular pathogens. Therefore, understanding mechanisms for the induction and activation of Ag-specific Th17 is important for the rational design of vaccines against pathogens.Citation14 Research shows that to vaccinate before or during antibiotic therapy may damage the antibody response of certain vaccine in the human body. The immune response to influenza vaccine, to some extent, relies on the signal of the enteric microorganism, which indicates that the intestinal flora has a strong immune response on the seasonal influenza and inactive poliomyelitis vaccine. Therefore, the enteric microorganism may exert great influence on human immunization vaccination, or even result in the decline of vaccine immunity. As a result, the effectiveness of vaccine declines and affects the prevention of illness.Citation2
In this study, our results revealed that, due to administering different antibiotics into the mice's gastrointestinal tracts, neomycin, metronidazole, and cephradine-sensitive bacteria are separately required for the multiplication of distinct T cells subsets. And we have found that metronidazole and cephradine-sensitive bacteria both suppress the secretion of cytokines of IFN-γ, IL-4, and Foxp3 that each feature Th1, Th2, and Treg cells, respectively. The neomycin treatment could enhance the IFN-γ excretion without stimulating any significant differences in IL-4 and Foxp3 in comparison to the control group, which indicated that it may be involved into a delicate regulation of the immune response in partnership with the commensal microbiota that automatically adapt and tailor themselves to attain mutual homeostasis.
It has been found that there was a tight correlation between changes in the commensal bacteria composition and IBD in children who have had 5 or more courses of antibiotic treatment.Citation15 The relationship between the host-gut microbiota and systemic immunity attracting more and more attention, however, the mechanism for this phenomenon remains unknown.
The results indicated that the mice who suffered from an influenza virus infection also encountered a drastic variation in the makeup of the systemic T cells subsets after being administered antibiotics. This highlights the critical roles played by Th1, Th2, and Treg cells in the modulation of the systemic immunity in the mice with antibiotic-induced dysbiosis. Although, the Th17 cells seem to not be implicated in the mice's systemic immunity regulation following the alteration of the commensal bacteria (which is also reflected by the discovery that CD4+ IFN-γ-positive cells could be similarly found in the mice's spleen and gut lamina propria, whereas CD4+ IL-17-producing cells are rarely observed in the spleen), the roles of Th17 cells have been proven to be essential for performing the effector function in the immunity of the intestine coupled with certain microbiota-associated intervention.Citation16 We speculate that the Th17 involvement in mediating the homeostasis of the host-commensal microbiota relationship might be restricted only to some specific bacteria species, like the segmented filamentous bacteria Citation13,17,18 and the altered-schaedler flora,Citation19 rather than to a wide range of alteration in gut microorganisms. Many recent findings have focused on how commensal microbiota affects the immune cell subsets, such as regulatory T cells, Th17, Th1, and Th2; in addition, their close link has been explored in terms of inflammation.Citation20
In this study, we investigated the commensal bacteria-influenced immune balance by exploring the Th and Treg cells equilibrium in antibiotic-treated mice, a homeostasis of the host-microbiota mutualism. And from this research we speculated that dysbacteriosis aggravated inflammatory exudates in the lung tissues of mice after infection with FM1 viruses, thereby causing increased virus amplification in the lungs, disrupting the Th/Treg immune balance in the body and increased the risk of inflammation in the body and disrupted the immune balance. Furthermore, dysbacteriosis of gut microbiota maybe down-regulate the immune recognition mechanisms in the lungs and induce an abnormal innate immune mechanism in the body; therefore, immune response functions could not be exerted, reducing the ability to clear viruses from the lungs. And from this research we got a conclusion that the normal gut microbiota had an important significance in the maintenance of immune function and recognition mechanisms in the body and these finding also showed that the clinical application should take intestinal flora into consideration before influenza vaccination was used.
Materials and Methods
Mice and antibiotic treatment
4 weeks old National Institute of Health (NIH) mice weighed 20∼22 g were purchased from the Animal Experiment Center, Guangdong Academy of Medical Sciences. Mice were respectively treated with metronidazole (15 mg/ml), cefradine (30 mg/ml), and neomycin sulfate (15 mg/ml) through an intragastric administration for 4 weeks (0.2 ml/d). All animal experiments were conducted in accordance with the principles and procedures outlined in the animal experiments committee of Jinan University.
Animal grouping and Influenza virus inoculation
48 mice were randomly divided into 6 groups of 8. The 6 groups are as follows: a normal group and a virus-infected group and 3 groups that were initially treated with different antibiotic treatments and then treated with the A/FM1/1/47 virus for another 4 days, named as metronidazole group, cefradine group, and neomycin group. The remaining group was the recovery group in which the mice were only treated with neomycin. The viral titer was determined on the same batch normal NIH mice as the 50% median lethal dose (LD50) for the whole experiment use. The mice were infected with 20%LD50 indicated virus after intranasal administration in the last week for 4 days after mice being fully anesthetized.
Bacterial colony plate counts
10-fold serial dilutions of a 1 g sample from cecum were performed and then plated onto Pfizer selective enterococcus agar, violet red bile agar-MUG, MRS agar, and BBL agar (Huankai Microbial Technology Co., Ltd., Guangzhou), respectively, for the cultivation of enterococcus, Escherichia coli, bifidobacterium and lactobacillus. The anaerobic agar (Oxoid Ltd., Basingstoke, Hampshire, UK) was used for the anaerobic bacteria plate count. The enterococcus, bifidobacterium, and lactobacillus plates were incubated at 37°C for 24 h under aerobic conditions, whereas the anaerobic bacteria and bifidobacterium plates were incubated at 37°C for 48 h under anaerobic conditions (AN0035, AnaeroGen sachet, Oxoid AnaeroGen System, Oxoid). The plate counts were conducted with 3 replications, and the growth number of each bacterial type was determined as log CFU/ml.
Virus quantification detection
Viral RNA was isolated from the lung tissue with a Beyozol reagent (Beyotime Biotechnology, Haimen), quantified with a Nanodrop ND-1000 spectrophotometer (Rockland, Delaware, United States), and then reverse transcribed with a reverse transcription kit (Tiangen Biotech Co. Ltd, Beijing). The virus RNA levels were examined using the real master mix (Tiangen Biotech Co. Ltd, Beijing), and polymerase chain reaction (PCR) reactions were performed and analyzed on a Bio-rad mini-opticon detection system. The forward primer sequence was 5′-GACCAATCCTGTCACCTCTGAC-3′, and the reverse was 5′- GGGCATTTGGACAAACGTCTACG-3′. The primers were synthesized in Generay Biotech Co., Ltd, Shanghai.
Cell isolation and flow cytometry
Lymphocytes were isolated from the spleens by using the ficoll according to the manufacture's instruction. The lymphocytes were washed with PBS (phosphate buffered saline) and then aspirated for 4 h with 50 ng/ml PMA (Propylene glycol monomethyl ether acetate), 1 μM ionomycin, and 500 ng/ml monesin (MultiSciences Biotech Co., Hangzhou, China). The intracellular expression of IFN-γ, IL-4, IL-17, and Foxp3 were stained by the relevant antibodies following the surface staining of CD4 and CD25 (eBioscience, Germany) and then the cellular fix/perm procedure using the cytofix/cytoperm kit (BD Bioscience, US). All of the cytokine staining procedures were conducted according to the manufacturer instruction. The stained cells were analyzed via a flow cytometric analysis using a FACScan cytometer equipped with cell quest software (BD Bioscience, US).
Statistics
Data analysis was performed in SPSS for Windows (version 18). The significant difference between the groups was analyzed using one-way ANOVA method, and the differences were considered statistically significant at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the National Natural Science Foundation of China (81473557 and 81273616), the Natural Science Foundation of Guangdong Province (S2013010013434), the Natural Science Foundation of Shandong Province (ZR2014HQ051) and the Science and Technology Program of Guangzhou (2014J4100106).
References
- Kelly D, Conway S, Aminov R. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol [Internet] 2005; 26:326–33. Available from: http://www.sciencedirect.com/science/article/pii/S1471490605001079; PMID:15922949
- Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity [Internet] 2014; 41:478–92. Available from: http://www.cell.com/immunity/abstract/S1074-7613(14)00303-3; PMID:25220212; http://dx.doi.org/10.1016/j.immuni.2014.08.009
- Tarride J-E, Burke N, Von Keyserlingk C, O'Reilly D, Xie F, Goeree R. Cost-effectiveness analysis of intranasal live attenuated vaccine (LAIV) versus injectable inactivated influenza vaccine (TIV) for Canadian children and adolescents. Clin Outcomes Res CEOR [Internet] 2012; 4:287–98. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3468276/; PMID:23055756; http://dx.doi.org/10.2147/CEOR.S33444
- Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol [Internet] 2010; 28:623–67; PMID:20192812; http://dx.doi.org/10.1146/annurev-immunol-030409-101330
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology [Internet] 2008; 134:577–94. Available from: http://www.sciencedirect.com/science/article/pii/S0016508507021579; PMID:18242222; http://dx.doi.org/10.1053/j.gastro.2007.11.059
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity [Internet] 2010; 33:279–88. Available from: http://www.sciencedirect.com/science/article/pii/S1074761310002931; PMID:20732640; http://dx.doi.org/10.1016/j.immuni.2010.08.010
- Kaser A, Zeissig S, Blumberg RS. Genes and environment: How will our concepts on the pathophysiology of IBD develop in the future? Dig Dis [Internet] 2010; 28:395–405. Available from: http://www.karger.com/DOI/10.1159/000320393; PMID:20926863; http://dx.doi.org/10.1159/000320393
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Sci [Internet] 2012; 336:1268–73. Available from: http://www.sciencemag.org/content/336/6086/1268.abstract; PMID:22674334; http://dx.doi.org/10.1126/science.1223490
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagán AJ, Pepper M, Maynard CL, Elson CO, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Sci [Internet] 2012; 337:1553–6. Available from: http://www.sciencemag.org/content/337/6101/1553.abstract; PMID: 22923434
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MAE, Geuking MB, Beutler B, Tedder TF, Hardt W-D. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science (80) 2009; 325:617–20; PMID:19644121
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science (80) 2010; 327:291–5; PMID:20075244
- Wu S, Jiang Z-Y, Sun Y-F, Yu B, Chen J, Dai C-Q, Wu X-L, Tang X-L, Chen X-Y. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza a virus infection. Curr Microbiol 2013; 67:414–22.
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch S, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–98; PMID:19836068; http://dx.doi.org/10.1016/j.cell.2009.09.033
- Duluc D, Joo H, Ni L, Yin W, Upchurch K, Li D, Xue Y, Klucar P, Zurawski S, Zurawski G, et al. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J Immunol [Internet] 2014; 192:5776–88; Available from: http://www.jimmunol.org/content/early/2014/05/16/jimmunol.1301661.abstract; PMID:24835401; http://dx.doi.org/10.4049/jimmunol.1301661
- Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011; 60:49–54; PMID:20966024; http://dx.doi.org/10.1136/gut.2010.219683
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474:298–306; PMID:21677746; http://dx.doi.org/10.1038/nature10208
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity [Internet] 2009; 31:677–89. Available from: http://www.sciencedirect.com/science/article/pii/S107476130900404X; PMID:19833089
- Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol 2010; 3:209–12; PMID:20147894; http://dx.doi.org/10.1038/mi.2010.3
- Geuking MB, Cahenzli J, Lawson MAE, Ng DCK, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011; 34:794–806; PMID:21596591; http://dx.doi.org/10.1016/j.immuni.2011.03.021
- Chinen T, Rudensky AY. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev 2012; 245:45–55; PMID:22168413; http://dx.doi.org/10.1111/j.1600-065X.2011.01083.x