Abstract
The immune system exerts both tumor-destructive and tumor-protective functions. Mature dendritic cells (DCs), classically activated macrophages (M1), granulocytes, B lymphocytes, aβ and ɣδ T lymphocytes, natural killer T (NKT) cells, and natural killer (NK) cells may be implicated in antitumor immunoprotection. Conversely, tolerogenic DCs, alternatively activated macrophages (M2), myeloid-derived suppressor cells (MDSCs), and regulatory T (Tregs) and B cells (Bregs) are capable of suppressing antitumor immune responses. Anti-cancer vaccination is a useful strategy to elicit antitumor immune responses, while overcoming immunosuppressive mechanisms. Whole tumor cells or lysates derived thereof hold more promise as cancer vaccines than individual tumor-associated antigens (TAAs), because vaccinal cells can elicit immune responses to multiple TAAs. Cancer cell-based vaccines can be autologous, allogeneic or xenogeneic. Clinical use of xenogeneic vaccines is advantageous in that they can be most effective in breaking the preexisting immune tolerance to TAAs. To potentiate immunotherapy, vaccinations can be combined with other modalities that target different immune pathways. These modalities include 1) genetic or chemical modification of cell-based vaccines; 2) cross-priming TAAs to T cells by engaging dendritic cells; 3) T-cell adoptive therapy; 4) stimulation of cytotoxic inflammation by non-specific immunomodulators, toll-like receptor (TLR) agonists, cytokines, chemokines or hormones; 5) reduction of immunosuppression and/or stimulation of antitumor effector cells using antibodies, small molecules; and 6) various cytoreductive modalities. The authors envisage that combined immunotherapeutic strategies will allow for substantial improvements in clinical outcomes in the near future.
Abbreviations:
- Ab, antibodies
- ADCC, antibody-dependent cell cytotoxicity
- APC, antigen-presenting cell
- BCG, Bacillus Calmette-Guérin
- Breg, regulatory B cell
- CAR, chimeric antigen receptor
- COX, cyclooxygenase
- CTA, cancer/testis antigen
- CTL, cytotoxic T lymphocyte
- CTLA-4, cytotoxic T lymphocyte antigen-4
- DC, dendritic cell
- DTH, delayed-type hypersensitivity
- GITR, glucocorticoid-induced tumor necrosis factor receptor
- GM-CSF, granulocyte-macrophage colony stimulating factor
- HIFU, high-intensity focused ultrasound
- IDO, indoleamine-2, 3-dioxygenase
- IFN, interferon
- IL, interleukin
- LAK, lymphokine-activated killer
- M, macrophage
- M1, classically activated macrophage
- M2, alternatively activated macrophage, MDSC, myeloid-derived suppressor cell
- MHC, major histocompatibility complex
- NK, natural killer (cell)
- PD-1, programmed death-1
- PGE2, prostaglandin E2
- RFA, radiofrequency ablation
- RNS, reactive nitrogen species
- ROS
- reactive oxygen species
- TAA, tumor-associated antigen
- Th, T-helper cell
- TLR, toll-like receptor
- TNF, tumor necrosis factor
- Treg, regulatory T cell
- TGF, transforming growth factor
- VEGF, vascular endothelial growth factor
Introduction
The first line systemic anti-cancer treatment is based mainly on chemotherapy, which does not deliver an effective systemic treatment in many cases. Somatic cell genetic differences in tumor cells result in high proportion of drug-resistant cells. Furthermore, the proportion of resistant cells progressively increases during the course of treatment because of the selective growth advantages of drug-resistant cells, as compared with drug-susceptible cells. Another problem is that the cytotoxic activity of anticancer drugs is not selective, with drugs exerting their effects not only on tumors but also on normal cells. Therefore, chemotherapy may lead to serious potentially life-threatening side effects, which frequently require additional medical intervention. The development of drugs with targeted cytotoxic activity is unlikely in the foreseeable future because the key biochemical pathways are similar in tumor and normal cells. Nevertheless, tumor cells can be identified by quantitative and qualitative differences in potentially immunogenic markers (antigens) expressed on the cell surface. The current paradigm holds that tumor antigen-specific immune responses are capable of destructing tumor cells, and that the immune system functional status is related to cancer prognosis and clinical outcome.Citation1
Tumor-Associated Antigens
Tumor-associated antigens (TAAs) can be divided into 2 groups: (i) those consisting of the unique products encoded by mutated or viral genes, which are expressed exclusively in cancerous cells, and (ii) those containing shared antigens that can be expressed both by tumor and normal cells in a constitutive or a developmental stage-dependent pattern (e.g. during the perinatal period).Citation2 Some unique TAAs are directly linked to the development of cancer (e.g., products of DNA repair or apoptosis-related genes, products of tumor suppressor genes, altered proteins encoded by mutated proto-oncogenes), and in addition they could be relatively resistant to immunoselection due to their essential role in maintaining the neoplastic state. Other unique TAAs may have no direct or indirect association with malignant transformation, resulting from the general genetic instability of cancer cells. Unique TAAs can be considered as tumor-specific antigens. However, TAA-mediated specificity is imperfect, as these mutated antigens arise from normal proteins and can be also expressed in altered but non-malignant cells.Citation2
The vast majority of TAAs are shared with normal somatic cells. The shared TAAs are divided into 4 subgroups including cancer/testis, oncofetal, differentiation, and overexpressed antigens. Cancer/testis antigens (CTAs) Citation3 and oncofetal antigensCitation4 are 2 closely related categories of TAAs encoded by awakened 'silent' genes. In adult organisms, CTAs are normally expressed exclusively in immune privileged organs including the testis and placenta, and they can also be aberrantly expressed in tumor cells. The reasons underlying the expression of these genes in tumors have been examined for the MAGE genes, suggesting that the expression is triggered by promoter demethylation, which has a high CpG content.Citation4 CTAs are highly immunogenic because they are 'unknown' to the immune system and thus are not tolerated.Citation5 However, very low expression levels of oncofetal antigens have been shown in normal tissues (e.g., fetoprotein is expressed in the liver), in contrast with high expression levels in some cancers or during various non-malignant pathologies.Citation4,5 Overexpressed oncofetal antigens are less immunogenic than CTAs because they are presented to the immune system in the neonatal period during the establishment of immune tolerance. Differentiation TAAs exhibit tissue-specific and in some cases differentiation stage-dependent expression patterns. The expression of these proteins is generally increased in malignant cells, originating from a particular tissue. For example, gp100, and tyrosinase, Melan-A/MART-1 are expressed in normal melanocytes and overexpressed in melanoma cells.Citation6 Overexpressed antigens are widely distributed in many normal tissues at very low to moderate levels, while these antigens are overexpressed in a variety of histologically different tumor types. Approximately 20% of all TAAs identified so far belong to this antigenic subgroup, and such proteins, as Wilm's tumor-1, telomerase, Her2/neu, and survivin fall into this category.Citation2,5 TAAs (mainly shared) can also be of non-protein in origin, with some tumor-associated carbohydratesCitation7 and (glyco)lipidsCitation8 implicated in antitumor immune protection. Although these non-protein TAAs are not recognized in the context of conventional Major Histocompatibility Complex (MHC)-restricted T cells, they constitute targets for other components of antitumor immune responses, including natural killer (NK) cells, NK T cells, and ɣδ T cells.Citation2
Innate and Adaptive Mechanisms for Antitumor Protection
The immune system is composed of a multitude of cells that orchestrate coordinated responses against pathogens and cancer. Current paradigm holds that activation of innate immune cells (e.g., dendritic cells) paves the way to the development of adaptive immune responses, including that directed against tumors.Citation9
Mature dendritic cells (DCs) have been described to belong to myeloid or lymphoid haematopoietic lineages, with 2 major DC subsets being classical DCs (cDCs also referred to as myeloid or mDCs) and plasmacytoid DCs (pDCs). cDCs are composed of phenotypically and functionally diverse subsets. In human skin, the epidermis hosts only Langerhans cells, while the dermis contains 2 cDC subsets, CD1a+ DCs and CD14+ DCs. pDCs are the most common DCs in the blood playing a pivotal role in the secretion of Type I Interferons (IFNs) upon encountering viruses. Importantly, a subset of CD141+ DCs in humans have been reported to play a major role in cross-presentation and priming of anti-tumor immune response.Citation10 In addition, DCs secrete a wide variety of cytokines and chemokines (e.g. IFNs, tumor necrosis factor-α (TNF- α), IL-1, IL-4, IL-6, IL-10, IL-12, and IL-23), which control concomitant T-cell responses.Citation11 Three interactive signals are generally required for functional DС activation and subsequent development of innate and adaptive immunity against cancers, including adequate presentation of MHC-peptide complexes to DCs for T cell priming, upregulation of co-stimulatory molecules such as CD40, CD80, and CD86, and production of activating cytokines by T cells.Citation12
Classically activated macrophages (M1s)
Tumor-associated macrophages (Ms) constitute one of the major cellular compartments of the tumor microenvironment, exerting a significant functional influence over tumor development. In early stage tumors, Ms appear to have an inflammatory, tumoricidal (M1 or 'classically activated') phenotype. M1s exhibit phagocytic and antigen-presenting activity, produce proinflammatory cytokines and exert cytotoxic functions. М1s have the capacity to kill target cells via mechanisms dependent on reactive oxygen species (ROS), reactive nitrogen species (RNS), and IL-1β and TNF-α production.Citation9 M1s are known to promote also an indirect cytotoxicity by activating other immune cells, such as NK cells and T cells.Citation13
Granulocytes
Neutrophils have been shown to play a direct role in tumor regression, utilizing killing mechanisms such as Fas/FasLigand as well as ROS. Eosinophils are present in many cancer types, but despite their production of cytotoxic compounds including major basic protein, peroxidase and cationic protein, it remains unclear whether these cells exert pro- or anti-tumor effects.Citation9 Basophils are strongly associated with some malignancies, particularly acute and chronic myeloid leukemia. Basophils produce mediators involved in atopy and IL-4, and could potentially exert a powerful influence on tumor-induced immune responses via yet unknown mechanisms.Citation14
NK cells are potent contributors to the innate immune response being able to kill diseased cells, for instance via perforin- and granzymes-dependent mechanisms. NK cells express an array of different activating and inhibitory receptors facilitating recognition of stress ligands on tumor cells, which are characterized by the decreased or absent MHC expression.Citation9
B-cells express clonally diverse cell-surface immunoglobulin receptors capable of recognizing specific antigens. Upon antigenic and cytokine stimulation, B-cells differentiate into plasma cells, which produce antigen-specific antibodies (Abs). Tumor-specific Abs are capable of inducing antibody-dependent cell cytotoxicity (ADCC) and complement-dependent tumor cell lysis. In addition to their role in antibody generation, B cells mediate and regulate numerous other functions essential for immune homeostasis. For example, the antigen-presenting capacity of B cells is crucial for T-cell immune responses. B cells exogenously pulsed with an antigen can present MHC class II epitopes independently of their B-cell receptor specificity, and also are able to promote MHC class I cross-presentation.Citation15
Classical (aβ) T cells recognize small peptides presented by MHC molecules on the surface of antigen-presenting cells (APCs). Intracellular antigens are subjected to proteolysis, antigenic peptides are bound within the peptide-binding groove of the MHC molecule, and peptide-MHC complexes are transportd to the cell surface for subsequent T cell recognition. Two major classes of T cells and cognate MHC molecules have been demonstrated. CD4+ T cells recognize antigens in the context of MHC class II molecules primarily expressed by APCs. CD8+ T cells recognize peptides bound to MHC class I molecules expressed on nucleated cells including APCs.Citation16,17 After APC-dependent antigen presentation naïve CD4+ T cells differentiate into one of many types of CD4+ effector cells depending on the cytokine milieu of the microenvironment present during activation. One route involves T helper differentiation pathway releasing cytokines to 'help' activate B cells, NK cells, and CD8+ cytotoxic lymphocytes. A wide variety of T helper cell subsets with distinct roles have been described depending on the particular pathogen and the type of the downstream immune response (Th1, Th2, Th17, etc.). Th1 cells produce IFN-ɣ and several other cytokines, which predominantly promote cell-mediated immune responses. Conversely, Th2 cells produce IL-4, IL-5, and IL-13 and contribute predominantly to antibody-mediated responses.Citation9,18,19 A growing body of evidence suggests that Th1 rather than Th2 cells could inhibit tumor growth. Activation of Th1 cells promotes СTL generation, classical M activation, as well as activation of NK cells and other effector cells with cytotoxic potential. Characteristically, Th17 cells secrete IL-17 in response to bacterial pathogens and tumors, and the role of Th17 cells in cancer immunity is highly controversial, with studies reporting both pro-tumor and anti-tumor activity.Citation9 Following activation by APCs, CD8+ T cells exert a direct cell mediated cytotoxicity playing a pivotal role in tumor cell destruction. Upon activation and implementation of their functions, most T cells undergo programmed cell death to prevent over-activation of the immune system and limit potential collateral damage to the host cells. A small proportion (5–10%) of the activated cells enter a pool of long-lived memory T cells subdivided into CD45RA– CCR7+ central memory T (TCM) cells (traffic to lymphoid tissues), and CD45RA–CCR7– effector memory T (TEM) cells (migrate to multiple peripheral tissue sites). It has been shown that upon activation TCM cells produced more IL‑2 than TEM cells. Memory T cell exhibit enhanced sensitivity to membrane and cytokine costimulation, and can be effectively reactivated in the immunosuppressive tumor environment. The presence of memory cells could limit tumor regrowth and metastatic spread even months to years after the eradication of clinically evident disease,Citation11 therefore it stands to reason that eliciting memory responses constitutes a major goal of tumor immunotherapy.
ɣδ T cells express a semi-invariant ɣδ TCR, which recognizes tumor-derived phosphoantigens or stress ligands, and can efficiently kill malignant cells from both hematological and solid tumors. Furthermore, ɣδ T cells produce Th1-type cytokines, TNF, IFN-ɣ, as well as other important powerful mediators, which armor ɣδ T cells to participate in the immune network attack on the tumor microenvironment.Citation9
NK T cells expressing both NK surface receptors and an invariant CD1d restricted TCR play an indirect role in tumor immunity via the secretion of IFNɣ and IL-4. NKT cells also efficiently lyse tumor cells through effector molecules, such as perforin, granzyme B and FasL.Citation9
To mount an effective anti-tumor immune response a coordinated action of the innate and adaptive immune responses is required (). Critical checkpoints include the activation of DCs and Ms to present TAAs, upregulation of multiple costimulatory ligand expression, cytokine and chemokine production s, induction of productive lymph node T cell responses, migration of activated T cells to the tumor microenvironment, and induction of the tumoricidal СTL activity. Tumor-induced T-cell reactivity also recruits effector cells to the tumor microenvironment, such as Ms, NK cells, and NKT cells, which are capable of destroying tumor cells escaping TСR-mediated T-cell recognition. In addition, T-cell-dependent Ab-mediated cytotoxic mechanisms can contribute significantly to the establishment of antitumor protection.
Table 1. Antitumor activities of immune cells
Natural and Tumor-Derived Immunosuppressive Mechanisms
The immune system is highly potent, as demonstrated in autoimmune disease settings and during excessive release of proinflammatory cytokines (a cytokine storm concept). To enable rapid expansion and pathogen-specific responses, the immune system possesses inherent positive feedback loops that promote activation. However, to maintain homeostasis and prevent potentially dangerous complications due to excessive immune activation, the immune system has also developed negative regulatory feedback loops, which are arguably more powerful. Unfortunately, aggressive tumor cells are able to hijack these mechanisms to evade the immune surveillance, presenting significant obstacles to the initiation and propagation of successful antitumor immune responses.Citation11 summarizes the immunosuppressive mechanisms capable of facilitating tumor development.
Table 2. Natural and tumor-derived immunosuppression
Tumor-Induced T-Cell Inactivation
T-cell anergy is a dysfunctional state caused by the insufficient TCR stimulation, e.g., when the TCR is ligated in the absence of important costimulatory signals, with the most important being CD28. Tumor-resident APCs and tumor cells usually express CD28 ligands B7–1 (CD80) and B7–2 (CD86) at low levels, suggesting that tumor milieu is characterized by the anergy- promoting context. A transcription factor EGR2 has recently been identified as a critical regulator of the anergic T cell phenotype, and evidence has been obtained that many CD8+ T cells infiltrating human tumors are EGR2+, providing more direct evidence for a functional contribution of T cell anergy to tumor immune escape.Citation20
T-cell exhaustion develops under conditions of antigen persistence caused by chronic infection or cancer. T cell exhaustion is characterized by a stepwise and progressive loss of T cell function, which could underlie the failure of the immune system to control cancers. Exhausted T cells are usually incapable of activation even in the presence of fully activated APCs. A major intrinsic factor of T cell exhaustion is an excessive and long-lasting antigenic stimulation of T cells, which leads to the up-regulation of inhibitory molecules, such as an alternative CD80/CD86 ligand cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and PD-L1/PD-L2 ligand PD-1. Recent reports showed that in addition to CTLA-4 and PD-1, other inhibitory receptors, such as lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin mucin 3 (TIM-3), natural killer cell receptor 2B4 (2B4, CD244), leukocyte immunoglobulin-like receptor superfamily B member 3 (LILRB3, PIRB) and 4 (LILRB4, GP49), and CD160, are co-expressed on exhausted T cells during tumor progression.Citation21 Some tumors are known to overexpress ligands for the receptors mentioned above, thus contributing to local immunosuppression. In addition, soluble immunosuppressive factors present in the tumor microenvironment could also support functional inhibition of exhausted T cells.Citation22
Tumor-Derived Immunosuppression
Immunosuppressive cytokines and chemokines
Tumor cells are often capable of secreting cytokines, such as transforming growth factor- β (TGF-β) and IL-10. TGF-β controls immune responses and maintains immune homeostasis by affecting proliferation, differentiation, and survival of multiple immune cell lineages.Citation21 IL-10 has been shown to reduce pro-inflammatory cytokine production, impede APC function, dampen T cell responses, and also affect B cell dysregulation.Citation21 Vascular endothelial growth factor (VEGF) is another cytokine that exerts a negative effect on the development of tumor-specific T-cell responses; specifically, it contributes to tumor immune escape by inducing immunosuppressive cells.Citation23 VEGF is known to stimulate tumor angiogenesis and vasculogenesis to support blood supply to the growing tumor. Tumor cells may also produce chemokines, such as CCL2 or CCL12, which downregulate effector T cell functions.Citation24 Importantly, these mediators are produced not only by tumor cells, but also by non-malignant stromal cells.Citation21
Immunosuppressive enzymes
A variety of tumors are characterized by elevated expression of the immunosuppressive tryptophan converting enzyme indoleamine-2,3-dioxygenase (IDO). In normal physiology, IDO metabolizes tryptophan and limits T- and NK-cell activation in local tissue microenvironments, such as the placenta.Citation25 T-cell proliferation is inhibited by IDO expressing cells via either the depletion of local tryptophan levels or apoptosis-inducing effects of its metabolites.Citation26 A second amino acid-catabolizing enzyme expressed in the tumor microenvironment is arginase, which catabolizes arginine, thus promoting the development of T cell dysfunction.Citation25 Сyclooxygenase (COX) is another immunosuppressive enzyme, which plays an important role in creating the immunosuppressive tumor milieu, with COX-1 isoform implicated in homeostasis, and COX-2 in inflammatory reactions and tumorigenesis. A COX-2 product prostaglandin E2 (PGE2) is abundantly produced by both tumor cells and tumor-infiltrating Ms. PGE2 acts directly on immune cells via 4 different subtypes of G protein-coupled E-prostanoid receptors (EP1–4), activating an intracellular cAMP-dependent protein kinase A (PKA) pathway. PGE2 decreases NK and ɣδ T-cell cytotoxicity and cytokine production, inhibits CD4+ T cell survival, and suppresses CD8+ T cell responses. In addition, COX2/PGE2 signaling induces regulatory T cell (Treg) activity, which could impede the development of antitumor immune responses.Citation27
Immunosuppressive Cells
Tolerogenic DCs support immune tolerance by modulating T cell activation. As central tolerance is incomplete, peripheral tolerance is needed. At steady-state, tissue-resident immature DCs readily internalize and present antigens from their environment; these cells are poorly immunogenic because they lack proinflammatory cytokine production and express low levels of co-stimulatory molecules. IL-10 or TGF-β produced by tolerogenic DCs are key soluble mediators inducing regulatory T-cell differentiation. IDO expression in DCs is considered to be an important mechanism of immune tolerance.Citation15
Alternatively activated macrophages (M2s) are generated from circulating monocytes upon IL-4 stimulation. M2s facilitate tumor angiogenesis and promote tumor invasion and metastasis. They also promote immunosuppression by producing IL-10, facilitating the development of IL-4-expressing Th2 cells, and providing a positive feedback for the generation of new M2s. CCL22 produced by M2 recruits Tregs to suppress CTL function. PD1L expressed by Ms induces apoptosis of activated T cells.Citation12
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with potent immunosuppressive activity. Human MDSCs can be induced by incubating peripheral blood mononuclear cells with GM-CSF and IL-6, or GM-CSF and IL-1β, PGE2, TNF-α and VEGF.Citation28 MDSCs can be either of monocytic or granulocytic origin. In the blood of cancer patients, MDSCs express LIN−HLADR−CD33+CD11b+ phenotype. A positive correlation between the frequency of MDSCs and advanced-stage tumors has been reported. MDSCs inhibit T cell activation via arginase, inducible nitric oxide synthase (iNOS), ROS or RNS. MDSCs deplete nutrients necessary for lymphocytes, disrupt IL-2 receptor signaling, interfere with lymphocyte trafficking, promote activation of Tregs by CD40-CD40L ligation and produce IL-10 or TGF-β. MDSCs have also been reported to induce contact-dependent mechanisms of T cell suppression.Citation12
Regulatory T cells (tregs)
There are 2 major populations of Tregs, those that develop naturally in the thymus and those that are induced in the periphery by TGF-β. In health, Tregs hinder the development of autoimmunity and curb bystander tissue destruction by limiting ongoing immune responses and maintaining tolerance to self-antigens. In cancer patients, circulating Treg numbers are increased compared with normal controls. In addition, high numbers of infiltrating Tregs have been observed in the tumor microenvironment.Citation25 To be classified as a Treg, a T cell must coexpress forkhead box P3 (Foxp3) and CD25 molecules. Tregs express a variety of inhibitory molecules such as LAG-3, glucocorticoid-induced tumor necrosis factor receptor (GITR), CTLA-4, and PD-1, which could be involved in the direct suppression of immune cells, including APCs. Tregs could suppress effector T cells indirectly by depleting local IL-2, which is required for the survival of actively dividing effector T cells. Indirect suppression may also occur via production of immunosuppressive cytokines, such as IL-10 or TGF-β.Citation21
Regulatory B cells (Bregs) secrete IL-10 and TGF-β shifting the balance of tumor-specific immune responses toward immunosuppression, thus sustaining tumor progression.Citation15
A Th1/Th2 imbalance is often established during tumor development, with the predominance of anti-inflammatory (Th2) cytokines.Citation29 This imbalance could reduce Th1-mediated immune anti-tumor responses. In addition, the prevalence of Th2 responses in tumor settings can promote alternative M activation and facilitate generation of MDSCs, thus favoring tumor growth.
Cancer Cell-Based Vaccines
There is considerable interest in developing therapeutic vaccines for cancer, as they hold promise of delaying or preventing cancer recurrence, particularly in early-stage disease patients. However, clinical application of cancer vaccines is complicated by the fact that most TAAs are non-mutated proteins, which are poorly immunogenic.Citation1,2 Moreover, immunizations with only one or several tumor-associated antigenic peptides frequently fail to control overall tumor development, creating favorable conditions for the growth of the tumor cell clones that lack the antigens present in the vaccine. Therefore, the use of whole tumor cells or lysates derived thereof as vaccines offers several advantages compared to individual TAAs. First, whole tumor cells elicit broad spectrum immune responses to different TAAs. In fact, a single histologically identical tumor consists of antigenically diverse cells.Citation30 Second, after internalization by APCs, tumor cell debris facilitate cross-presentation of antigens to CD4+ and CD8+ T cells, thus generating long-term CD8+ T cell memory with CD4+ T cell help.Citation31 Antigenic peptides expressed inside cells and/or on the cell surface are generally more immunogenic than the same peptides in a soluble unbound form. In fact, the immune system is better adapted to combat cells bearing an infectious or cancerous danger signals, compared with the relatively safe soluble products. Published data indicate that necrotic tumor cells can promote DC maturation,Citation32 possibly because dead cells release heat shock proteins (HSP), such as HSP 70 and 90, the pro-inflammatory factor high mobility group box 1 (HMGB1), and pro-inflammatory cytokines.Citation31 Furthermore, RNA and DNA of injured cells are rapidly degraded to purine bases, with subsequent convertion into uric acid (an end product of the purine metabolic pathway), which can serve as a critical endogenous danger signal driving DC maturation.Citation31,33 presents the characteristics of various types of cell-based vaccines.
Table 3. Characteristics of different types of cell-based vaccines
Autologous tumor cell vaccines are prepared from patient-derived tumor cells. These tumor cells are typically lysed or irradiated, combined with an immunostimulatory adjuvant (e.g., BCG), and then administered to the individual from whom the tumor cells were isolated. The major advantage of this type of vaccine is that all vaccine antigens are homologous to those of the patient's tumor. However, a sufficiently large tumor specimen is required to prepare autologous tumor cell vaccines, thus limiting this approach to certain tumor types or stages.Citation12
Allogeneic tumor cell vaccines typically contain 2 or 3 established human tumor cell lines potentially overcoming many of the limitations of autologous tumor cell vaccines. Potential advantages of this approach consist in a limitless source of tumor vaccine cells, standardized and large-scale vaccine production, reliable analysis of clinical outcomes, easy protocol to induce the expression of immunostimulatory molecules, and cost-effectiveness.Citation12
Xenogeneic tumor cell vaccines like allogeneic vaccines have no limitations related to their manufacture and standardization. An accumulating body of evidence suggests that xenogeneic vaccines can be very effective in breaking the immune tolerance to low immunogenic human TAAs. As TAAs are typically evolutionarily conserved molecules, strong homology is observed between human and animal TAAs. However, small interspecific structural differences in TAAs could present advantages for the construction of cancer vaccines, as xenogeneic antigens could represent an ‘altered self’, with sufficient differences from self-antigens to render them immunogenic, with sufficient similarities to allow reactive T cells to maintain self-recognition.Citation1,2
There is evidence to suggest that xenoepitopes can bind host MHC molecules more strongly compared to epitopes derived from native homologous proteins, resulting in the formation of longer-lived xenogeneic peptide/MHC complexes. Ultimately, this leads to more potent xenoantigen-induced T cell responses that are cross-reactive with self-protein-derived TAAs.Citation2,34 It is also important to note that all humans possess natural (preexisting) antibodies that provide acute rejection of any non-primate cells and function as a major barrier to the transplantation of animal organs in humans. A significant proportion of these Abs represent IgGs specific to the α-gal epitope abundantly expressed on the glycoproteins and glycolipids of non-primate mammals and New World monkeys.Citation35,36 By opsonizing xenogeneic tumor cells these natural Abs could promote internalization of antigenic material in APCs via a Fcɣ-receptor-mediated mechanism, thus greatly enhancing the immunogenic cross-presentation of TAAs to tumor-specific T lymphocytes.Citation1
Theoretically, poly-antigenic xenovaccination breaking immune tolerance could be associated with an increased risk of inducing pathogenic autoimmune reactions. However, our own data indicate that patients repeatedly immunized with murine melanoma and carcinoma antigens exhibited no clinical evidence of any systemic autoimmune disorders. Consistent with these data, serum rheumatoid factor levels and DNA-specific and cardiolipin-specific Abs remained at the base levels in those patients.Citation37,38
Potentiating Vaccine-Induced Immune Responses to Cancer Cell by Cell Manipulations
Genetic manipulations
Tumor cells do not typically produce immunostimulatory cytokines. Therefore, to enhance vaccine immunogenicity, tumor cell-based vaccines can be modified to secrete pro-inflammatory cytokines such as granulocyte-macrophage colony stimulating factor (GM-CSF), which could facilitate recruitment of DCs at the site of vaccine administration.Citation31 Vaccination with tumor cells genetically modified for IL-12 production (a key cytokine boosting Th1 immunity) has been shown to cause a significant tumor suppression in mice paralleled with high IFN-γ production and activation of CTL and NK cells.Citation12,39 Normally, tumor cells are devoid of molecules that could co-stimulate TAA-specific T cells. Therefore, transduction of tumor cell vaccines with co-stimulatory molecules such as B7–1 represents another possible approach to potentiate antitumor immune responses.Citation12,40 Viruses have been used to increase the immunogenicity of whole tumor cell vaccines via DC activation by 'danger signals' released by virally infected tumor cells, or via increased uptake of tumor lysate by DCs due to the expression of viral ligands on the surface of virally infected tumor cells.Citation31 However, in clinical settings the approaches based on the genetic modification of tumor cells are difficult to accomplish being technically complicated and time-consuming.Citation41
Oxidation of TAAs by hypochlorous acid (HOCl), a potent oxidant produced during acute inflammation with strong microbicidal activity, enhances the immunogenicity of protein antigens by several fold, both in vivo and ex vivo. Proteins oxidized by HOCl are more readily taken up and processed by APCs, leading to enhanced activation of antigen-specific T cells in vitro.Citation42 HOCl has also been used to potentiate the immunogenicity of whole tumor cells.Citation31
Dendritic cell vaccination
DCs are well known to play a critical role in determining both the qualitftive and quantitative parameters of T cell responses. For example, DC vaccines generated in vitro with GM-CSF and IFN-α are highly potent in priming CD4+ and CD8+ T cells.Citation43 DCs generated with GM-CSF and IL-15 display an enhanced ability to elicit tumor-specific CD8+ T cells.Citation44 Combination of BDCA3+ cDCs could be of special interest due to its potential to prime CD8+ T cell responses.Citation45 Mature DCs produce high levels of IL-12p70 and IFN-γ-inducible protein 10 (IP-10), thus providing conditions for Th1-polarization of antigen-induced immune responses.Citation31
Adoptive T-cell therapy is an effective approach to generate functional memory T cells for cancer immunotherapy. This modality consists of in vitro expansion of T cells from the patient's own peripheral blood or tumor in the presence of TAAs and cytokines, followed by the infusion of these cells back into the patient's bloodstream.Citation46 Before adoptive T cell transfer, patients are normally subjected to non-myeloablative leukoreductive therapy (e.g., сyclophosphamide plus fludarabine, with or without total body irradiation) to facilitate the long-term persistence and homeostatic proliferation of T cells. Following T cell administration, patients receive maintenance therapy with high dose IL-2.Citation11 Immunotherapies using monoclonal Ab and T cells have been recently merged in the development and clinical application of genetically engineered antibody-T cell chimeras. In this approach, chimeric antigen receptor T cells (CAR T cells) are combined with the unique antigen specificity of Ab. Results from early-phase CAR T-cell-based clinical trials demonstrate a significant antitumor potential for this approach, while revealing novel toxicities associated with immune over-activation.Citation47
NK cell-based immunotherapy
NK cells play a critical role in host innate immunity against several types of cancer, which in turn have developed mechanisms to escape NK cell attack or induce defective NK cells. Current NK cell-based cancer immunotherapies aim to overcome NK cell paralysis using several approaches. One approach uses expanded allogeneic NK cells, which are not inhibited by self histocompatibility antigens like autologous NK cells, for adoptive cellular immunotherapy. Another adoptive transfer approach utilizes stable allogeneic NK cell lines, which is more practical for quality control and large-scale production purposes. The third approach involves genetic modification of ex vivo NK cells or NK cell lines to overexpress cytokines, Fc receptors and/or chimeric tumor-antigen receptors. NK cells with therapeutic potential can be obtained from peripheral or cord blood cells or other sources, and a variety of protocols can be applied for their large-scale production, such as Abs, immobilized molecules or soluble growth factors, and/or other cellular activators.Citation48
Non-Specific Immunomodulators
Non-specific immunomodulators including mineral salts, emulsions, microparticles, and liposomes have been thoroughly investigated as cancer adjuvants.
Montanide ISA 51 and 720 are water-in-oil emulsions composed of mineral oil mixed with the surfactant mannide monooleate in a 1:1 ratio. The ISA 720 formulation is slightly different from that of ISA 51, as it permits more rapid antigen release.Citation31 Montanide is similar to incomplete Freund's adjuvant (IFA) in its physical characteristics, but its biodegradable nature makes it considerably less toxic than IFA.Citation49 The immune enhancement produced by the montanide emulsions is believed to result from the formation of a depot at the injection site.Citation50 Both Montanide ISA 51 and 720 have been shown to induce high Ab titers and CTL responses in a variety of animal species.Citation31
Alum, in the form of the particular aluminum salts such as Al(OH)3 and AlPO4, has been widely used in a variety of vaccine formulations. Antigens adsorbed to alum are presented in a multivalent form, which is more immunogenic and more efficiently internalized by APCs.Citation51 Alum is known to stimulate Th2-type responses characterized by the production of IL-4, IL-5, IL-6 and IgG1.Citation52 Recent data demonstrated that alum targets NOD-like receptor protein 3 (NLRP3), which interacts with the caspase recruitment domain (CARD)-containing adaptor protein ASC and protease caspase 1 to form a protein complex called inflammasome.Citation52 Once activated, the inflammasome is involved in processing pro-inflammatory cytokines, such as IL-1β, IL-18, and IL-33 into their mature form.Citation31
MF59 is an oil-emulsion consisting of small (160 nm in diameter) uniform and stable microvesicles. Each microvesicle consists of a drop of oil (i.e., squalene obtained from shark livers) surrounded by a monolayer of non-ionic detergents (i.e., Tween and Span 85). MF59 has been shown to stimulate the generation of CD4+ memory T cells and Ab responses.Citation31
QS21 is a highly purified saponin extracted from the bark of the South American tree Quillaja saponaria Molina. QS21 is a potent adjuvant for CTL induction and promotes the secretion of IL-2, IFN-γ, and IgG2a Abs.Citation53 While the mechanism of action of this adjuvant has not been yet fully elucidated, in vitro experiments suggest that it could improve antigen presentation by APCs and therefore optimize T cell responses. QS21 has also been demonstrated to stimulate B cell responses, although it remains to be established whether this occurs through a direct effect or via APC/T cell stimulation.Citation31
Keyhole Limpet Hemocyanin (KLH) is a natural protein isolated from the marine mollusk keyhole limpet. This immunogenic protein has been used as a hapten carrier for many small molecules including many chemicals (e.g., 2,4-dinitrophenol [DNP] and 2,4,6-trinitrophenol [TNP]), drugs, hormones, peptides, polysaccharides, lipids and oligonucleotides.Citation31
TLR Agonists
The immune system recognizes pathogen-associated molecular patterns (PAMPs) by means of pathogen-recognition receptors (PRRs), which include Toll-like receptors (TLR) expressed on the cell surface and endosomal compartments, as well as cytoplasmic receptors such as NOD-like receptors (NLRs) and retinoic acid inducible gene 1-like receptors (RLRs).Citation54 TLRs are the best studied PRRs, and their ligands have been studied as immunoadjuvants. Thus far, 11 human and 13 mouse TLRs have been identified. The TLR family members can be divided into 2 subpopulations with regard to their cellular localization. TLRs 1, 2, 4, 5, 6, and 11 are expressed on the cell surface, whereas TLRs 3, 7, 8, and 9 are localized in endosomes, lysosomes, or the endoplasmic reticulum.Citation55 TLRs can be also segregated into 3 groups based on the specific PAMPs they recognize. TLRs 1, 2, 4, 6, and 10 are involved in lipid recognition, TLRs 5 and 11 recognize proteins, and TLRs 3, 7, 8, and 9 detect nucleic acids.Citation56,57 Upon ligation, TLRs form homo- and heterodimers. TLR-mediated signals are transduced via 2 major signaling pathways dependent or independent of the Myeloid differentiation primary response protein (88) (MyD88).Citation58 MyD88 is an essential part of the signaling cascade for all TLRs except for TLR 3, which induces MyD88-independent signaling through a TRIF-mediated pathway.Citation55
TLR2 agonists
Recent results demonstrated that the peptidoglycan cell wall skeleton of BCG can act as an immunomodulator to activate DCs by binding to TLR2 and TLR4,Citation59 as well as to the nucleotide binding oligomerization domain (NOD)-like receptor (NLR). Another TLR2 agonist is macrophage activating lipopeptide (MALP)-2, which is a synthetic analog of M161antigen, a component of the mycoplasma cell wall lipoprotein, which signals through TLR2 and TLR6. (MALP)-2 can activate nuclear factor (NF)-κB to synthesize cytokines and chemokines such as MIP-1α, -2, and monocyte chemoattractant protein 1 (MCP-1), and can also induce DC maturation.Citation31,60
TLR3 agonists
TLR3 is responsible for recognizing virus RNA by binding to double stranded RNA (dsRNA) and signaling via a Toll/IL-1R domain containing adaptor, inducing an IFN-β (TRIF)-dependent and MyD88-independent signaling pathway. This in turn activates an interferon regulatory transcription factor (IRF), leading to transcription and translation of genes encoding for IFN-α, IFN-β, as well as IFN-inducible genes encoding for chemokines, such as RANTES (CCL5) and IL-6. TLR3 agonists stimulate DC maturation and IL-12p70 production.Citation61 AmpligenR, poly(I:C12U) has been evaluated for its in vitro human DC maturation activity, and in vivo as an adjuvant in DC-based immunotherapy.Citation62 It was shown that poly(I:C12U) treatment was effective in upregulating the expression of MHC Class I and II, CD83, CCR7, CD86, and CD40 molecules on the cell surface of DCs, as well as in inducing high levels of IL-12 production, suggesting its usefulness as an adjuvant in DC-based whole tumor cell therapy.Citation31
TLR4 agonists
TLR4 is occupying a unique niche among TLRs because of its ability to activate both MyD88- and TRIF-dependent signaling pathways.Citation63 TLR4 together with TLR2 recognizes lipopolysaccharide (LPS), a glycolipid constituent of the outer membrane of Gram-negative bacteria. LPS-stimulated DCs produce high levels of Th1 cytokines, including IL-12p70 and IFN-γ -inducible protein 10 (IP-10), and stimulate potent anti-tumor T cell responses in vitro.Citation31
TLR7 and TLR8 agonists
TLR7 and TLR8 are involved in the recognition of single-stranded viral RNA during viral infection. Synthetic mixed TLR7/TLR8 agonists include imiquimod, midazoquinolin amines, resiquimod and 852A, which are analogs of DNA or RNA oligonucleotides.Citation64,65 These agonists are able to induce secretion of IFN-α, IL-6, IL-8, IL-12, and TNF-α by DCs and Ms, leading to stimulation of Th1 cell-mediated immune responses.Citation31
TLR9 agonists
TLR9 recognizes unmethylated cytosine-phosphateguanine (CpG) motifs of bacteria and DNA viruses.Citation66 CpG analogs (TLR9 agonists) represent short oligo-deoxynucleotides (CpG ODNs) stabilized by a phosphorothioate backbone. CpG ODNs are classified into class A, B and C based on their nucleotide sequence and length. Different classes of CpG ODNs are known to activate different cell types to produce different cytokine profiles. Class A and C CpG ODNs are directed to the lysosomal compartments of pDCs and B cells to induce IFN-α production, whereas class B CpGs ODNs are trafficked to the endosomal compartments and induce maturation of DCs, accompanied by low IFN-α production. Nevertheless, all classes of CpGs induce predominantly strong Th1 responses,Citation66 implying their high potential as adjuvants for cancer vaccines.Citation31
Cytokines and Chemokines
Cytokines have een used extensively in the clinical and preclinical settings to stimulate both cellular and humoral antitumor responses. For example, GM-CSF has been used to amplify DC generation, IL-12 and 18 to promote Th1 polarization, IL-2, IL-4, IL-7, IL-12, IL-15, and IL-21 to expand antigen-activated lymphocytes; and type I and II IFNs to enhance the tumoricidal activity of a variety of effector cells. Chemokines constitute a group of related chemoattractant peptides that are essential regulators of immune cell migration. Overall, the application of cytokines and chemokines as vaccine adjuvants aiming to enhance immune responses to cancer cells is a very promising clinical approach, which has been an active area of translational research over the last few decades.Citation67
Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) regulates haematopoietic progenitor cell differentiation and proliferation, and plays an important role in the maturation of professional APCs including DCs, monocytes and Ms.Citation68 GM-CSF also enhances the capacity of APCs to process and present antigens to T cells, leading to cytotoxic T cell activation, an increase in IFN-γ production and ultimately tumor regression.Citation68 As GM-CSF is able to recruit DC, stimulate DC maturation and enhance antigen-specific CD8+ T cell responses, GM-CSF holds promise as an immune adjuvant; therefore GM-CSF has been successfully combined with whole tumor cell vaccines, as well as DC-based vaccines.Citation31 However, high doses of GM-CSF could cause detrimental effects by virtue of inducing immune suppression via activation and expansion of MDSCs.Citation23
Interleukin 12 (IL-12) promotes IFN-ɣ release by IL-12R-expressing T, NK, and NKT cells, induces Th1 polarization, and contributes to CTL generation. IL-12 also exhibits anti-angiogenic activity. Because of its potency in inducing robust IFN-ɣ production by antigen-activated T cells, IL-12 may induce more pronounced effects when co-administered with a vaccine.Citation69 However, the systemic delivery of IL-12 has resulted in elevated toxicities, leading to the investigation of alternative modes for the local delivery of IL-12.Citation23
Interleukin-18 (IL-18) is an immunostimulatory cytokine belonging to the IL-1 family. The biologic effects of IL-18-based therapy include activation of Ms, NK cells, and T cells. IL-18 acts synergistically with other pro-inflammatory cytokines to promote IFN-γ production. It is produced by NK cells, T cells, and other cell types. Systemic administration of IL-18 has been shown to result in a significant antitumor immune activity in several preclinical animal models.Citation70
The ɣ-chain family of cytokines includes IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. IL-2 is a key growth factor involved in the clonal expansion of antigen-activated lymphocytes and the generation of immune memory. IL-2 has also been used in vitro to expand tumor-specific T-cells for adoptive immunotherapy. However, in vivo IL-2 therapy particularly in high-dose IL-2 regiment suffers from a high rate of associated toxicities.Citation23 Moreover, IL-2 stimulates proliferation of activated effector T-cells and NK cells, and also the development and homeostasis of Tregs, which constitutively express elevated levels of IL-2R α (CD25).Citation71 IL-15 is also a T-cell growth factor, which is however functionally different from IL-2. IL-15 is necessary for the development and homeostasis of memory CD8 T-cells and NK cells.Citation72 Unlike IL-2, IL-15 does not play a role in the proliferation of Tregs. Another T-cell growth factor IL-7, promotes the expansion of naïve T-cells and thus increases the diversity of the T-cell repertoire after lymphopenia.Citation72,73 Interestingly, IL-7 and IL-15 have been shown to reverse T cell anergy, suggesting that these cytokines may also restore the function of T cells rendered anergic in the tumor microenvironment.Citation25 Both IL-15 and IL-7 have demonstrated activity as adjuvant cytokines for cancer vaccine interventions in preclinical studies.Citation23 IL-21 is primarily produced by CD4+ T cells. IL-21 induces the differentiation of pro-inflammatory CD4+ Th17 cells, stimulates memory and naive CD8+ T-cell expansion synergistically with IL-7 or IL-15, and improves the degree of expansion and affinity of antigen-specific CTL clones generated in vitro.Citation74,75 The effects of IL-21 on NK cells include augmentation/differentiation and anti-tumor activity.Citation76 IL-21 also inhibits the activation and cytokine production of immature DCs. In preclinical in vivo models, IL-21 has been demonstrated to induce tumor rejection, prevention of metastatic process, and the induction of the immune memory.Citation77 Because of its effects on CD8+ T cells, IL-21 may be essential for the in vitro generation and expansion of tumor antigen-specific CTL lines/clones for subsequent adoptive immunotherapy. Moreover, it can be applied as a systemic immunomodulator combined with monoclonal antibody therapy to potentiate NK cell-mediated ADCC.Citation69
Interferons (IFNs) are a group of glycoproteins produced by a variety of cells stimulated by viral antigens and other factors, such as double-stranded RNA and mitogens. Ms and lymphocytes are responsible for the production of IFN-α, whereas fibroblasts and epithelial cells are involved in producing IFN-β. IFN-ɣ is produced by CD4+Th1 cells, CD8+ СTLs, CD8+ NK cells, and lymphokine-activated killer (LAK) cells. IFNs exert a variety of effects that contribute to antitumor surveillance, such as antiproliferative activity, stimulation of differentiation, alteration of antigen expression on the tumor cell surface, inhibition of oncogene activation, and inhibition of angiogenesis.Citation78 IFN-ɣ is an essential mediator responsible for the development of Th1-dependent immune responses, including the generation of tumor-specific CTLs. IFN-ɣ has been shown to potentiate DNA fragmentation and apoptotic cell death. Both IFN-α and IFN-ɣ potentiate TNF-dependent tumor cytotoxicity. Induction of MHC expression on tumor cell surfaces by IFNs can enhance the efficacy of host cell-mediated immunity and immunemediated tumor elimination.Citation78,79
Chemokines
To date, more than 50 chemokines and 18 chemokine receptors have been identified. These molecules are classified into 4 families (CC, CXC, C, and CX3C) based on the arrangement of the first 2 conserved cysteine residues, creating a structural motif. Functionally, chemokines are described as inflammatory (inducible) or homeostatic (constitutive) based on their pathophysiological effects. Inflammatory chemokines are secreted in inflamed tissues by resident and infiltrating cells after stimulation by pro-inflammatory cytokines or during contact with pathogens. Chemokines specialize in the recruitment of effector cells, particularly monocytes, granulocytes, and effector T cells to sites of inflammation or tissue destruction, or to the tumor microenvironment. Homeostatic chemokines are constitutively produced and regulate the physiologic trafficking of immune cells during hematopoiesis, antigen sampling in secondary lymphoid tissue and immune surveillance. Some chemokines have also been defined as angiogenic or angiostatic based on their role in promoting or suppressing tissue neovascularization, respectively.Citation67 One of the major challenges in the field of cancer vaccinology is an inadequate migration of immune cells into the vaccination site, as well as the hampered recruitment of the vaccine-mobilized immune cells with tumor-destructive potential to the tumor microenvironment. For this reason, chemokines might be used in an effort to improve immune cell recruitment and immunogenic antigen presentation. For example, CCL3 pre-treatment of mice has been shown to result in the recruitment of activated DCs in the peripheral blood. These DCs expressed a higher level of CCR7, displayed a pronounced chemotactic response toward secondary lymphoid tissue, and generated stronger CTL responses resulting in enhanced tumor rejection.Citation80 CCL21 has also been applied in the context of different DC vaccine strategies. Although considered to be a homeostatic chemokine, CCL21 influences T cell migration to secondary lymphoid organs during inflammation and enhances Th1-type T cell responses.Citation81 CCL5 is another chemokine that regulates T cell migration toward sites of tissue injury and inflammation, as well as Th1 differentiation.Citation82 Thus, the addition of chemokines to cancer vaccine formulations has the potential to provide great benefits in overcoming tumor-derived immunosuppression.Citation67
Hormones and Neuromediators
Neurohormonal mechanisms play a significant role in regulating adaptive immune responses. These mechanisms are often impaired with aging. Age-associated deterioration in the immune system referred to as immunosenescence contributes to an increased susceptibility to cancer in the elderly. As circulating melatonin levels decrease with age coinciding with the age-related decline of the immune system, much interest has been focused recently on melatonin's immunomodulatory effects. Antioxidant and immunopotentiating effects of melatonin have been reported, providing rationale for the potential application of this indoleamine as a 'replacement therapy' to limit or reverse some of the alterations occurring during immunosenescence.Citation83 The immunostimulatory role of melatonin manifests itself in the enhancement of Th1-mediated responses,Citation83-85 providing a rationale to the application of this hormone for potentiating antitumor effects of cancer vaccinations especially in elderly patients.
The elderly are also characterized by thymus involution. Therefore, administration of thymus hormones along with cancer vaccinations could contribute to differentiation of vaccine-induced T lymphocytes in patients of this age group. In support of this notion, the available data indicates that the administration of thymosin α as an adjuvant was effective in potentiating protective activity of an anti-influenza vaccine.Citation86
It has been previously shown that sex steroid ablation (SSA) rejuvenates the aging thymus, increases thymic export of naive T cells, and decreases the incidence of an inducible tumor in aged mice.Citation87 This data raises the possibility of effective application of various antisteroid modalities in cancer immunotherapies including those based on anticancer vaccinations.
Physiological levels of triiodothyronine (T3) have been demonstrated to enhance the expression of DC maturation markers (MHC II, CD80, CD86, and CD40), increase IL-12 secretion, and stimulate the ability of DCs to induce naive T cell proliferation and IFN-ɣ production in allogeneic T cell cultures.Citation88 It is worth mentioning that the production of thyroid hormones is often impaired in the elderly cancer patients. Therefore, combination therapy regiments of anticancer vaccinations with thyroid hormone replacement therapy could be justifiable in the elderly and deserves careful study.
An extensive body of evidence has been obtained to support the notion that hormones secreted by the neuroendocrine cells play an important role in communication and regulation of the immunocompetent cells. With special reference to protein hormones, this idea has been well documented for prolactin (PRL), growth hormone (GH), and insulin-like growth factor-1 (IGF-I).Citation89 Unfortunately, the effects of these hormones on cancer vaccine-induced immune responses remain unexplored.
Immunomodulatory Antibodies
Immunoactivating antibodies
CD40 is a member of the TNF receptor superfamily (TNFRSF), which is expressed on many cell types including Ms, DCs, B cells, activated CD8+ and CD4+ T cells, endothelial cells and fibroblasts. CD40 binds to its ligand CD40L (CD154), which is primarily expressed by activated T and B cells, as well as and platelets. CD40 expressed on APCs such as DCs plays a key role in priming and expansion of antigen-specific CD4+ T cells, principally by regulating the expression of costimulatory molecules such as CD80 and CD86 on the cell surface of APC, and production of cytokines such as IL-8, MIP-1α, TNF-α, and IL-12. CD40 expressed on B cells controls various B cell functions, including IgG class switching, survival, germinal center formation, memory B cell generation and production of IL-1, IL-6, IL-8, IL-10, IL-12, TNF-α, and MIP-1α. As CD40 agonist therapy showed promising activity in improving tumor immunity, several anti-CD40 Abs with agonist properties have been developed.Citation90 Four–1BB is another member of the TNF receptor superfamily and a marker of T-cell activation. Preclinical studies suggested that 4–1BB enhanced antigen-specific T-cell activity, and that agonist anti-4–1BB Ab facilitated the regression of established murine tumors.Citation90 A third member of the TNF receptor superfamily GITR is a type I transmembrane protein expressed on inactive T-cells, which is up-regulated following T-cell activation. Ligation of GITR enhances immunity by providing a costimulatory signal that enhances T-cell proliferation and effector functions and inhibits the immune suppressive effects of Tregs.Citation91 Enhanced primary and recall CD8+ T-cell responses, along with associated increases in tumor immunity was observed when vaccination with tumor antigens was combined with GITR ligation.Citation92 In addition to CD-40, 4–1BB, and GITR, OX-40 (CD134) is another costimulatory molecule belonging to the TNF receptor superfamily. OX40-OX40 ligand interaction augments T-cell function and inhibits the suppressive activity of T regulatory cells. Preclinical data has shown that agonistic Ab against OX40 may potentiate the antitumor activity elicited by TAA immunization.Citation90
Antibodies blocking co-inhibitory signals
An alternative strategy to enhance immune reactivity has been developed aiming to eliminate negative regulation of T-cells by coinhibitory molecules, such as CTLA-4, B7- H1, B7-H4 and others.Citation23,93 In combination with tumor vaccines, anti- CTLA-4 Ab has been shown to enhance antitumor T-cell responses.Citation94 B7-H1 is constitutively expressed in many types of human tumors, and it has been shown to contribute to immune evasion by tumors by promoting apoptosis of activated effector T-cellsCitation95 and tumor resistance to T cell- mediated lysis.Citation96 Blockade of B7-H1 with specific Abs results in enhanced antitumor immune responses.Citation96,97 B7-H4 is another member of the B7 family that is implicated in the negative regulation of T-cell immunity. Ligation of B7-H4 has been demonstrated to inhibit T-cell proliferation and IL-2 production, while inhibition of B7-H4 in preclinical animal models resulted in enhanced cytotoxic alloantigen-specific T-cell responses.Citation98 Administration of anti- PD1 Ab has been found to have an impact on overall survival in patients with advanced melanoma.Citation99 It should be noted however that Ab-mediated blockade of coinhibitory signals may be accompanied by adverse events in the form of autoimmune reactions.Citation23
Antibodies blocking immunosuppressive cytokines
In order to evade immunosurveillance, tumors developed several direct/indirect strategies including secretion of inhibitory cytokines or modulation of various immune cells toward secretion of cytokines associated with reduced immune responses. Thus, TGF-β can be directly secreted by many types of tumor cells, and inhibition of TGF-β can reverse the immunosuppressive effects of the tumor microenvironment, as was demonstrated in several preclinical studies.Citation23,100 As IL-10 and IL-13 could also be involved in tumor-induced immunosuppression, these cytokines are considered to be promising targets for antibody-mediated blockade aimed at stimulating antitumor immune responses.Citation101 In addition, VEGF has been shown to downregulate the development of antitumor T-cell responses. Therefore, conceptually immunotherapeutic strategies targeting VEGF or its receptors will not only disrupt angiogenesis and growth in tumor settings, but also upregulate antitumor immunity, resulting in enhanced responses to cancer vaccines. Consistent with this idea, an anti-VEGF Ab enhanced the efficacy of an antitumor DC-based vaccine, resulting in a prolonged and pronounced antitumor effect in preclinical studies.Citation102 However, anti-VEGF therapies in the clinical settings have induced toxicities including wound healing complications and adverse vascular effects.Citation23
Depletion/Inhibition of Immunosuppressor Cells
Depletion/inhibition of regulatory T cells
Studies in several experimental models have demonstrated that the depletion of Tregs using an anti CD25 mAb enhanced the development of antitumor immunity, leading to tumor rejection.Citation103,104 Previously, other modalities were shown in preclinical studies to reduce Tregs and to enhance antigen-specific immune responses, such as a fusion protein of IL-2 and diphtheria toxin - denileukin diftitox, (DAB389IL-2),Citation23. The TLR7/8 agonist resiquimod has also shown promise due to its ability to enhance the induction of innate cytokines such as IL-12 and TNF-α and suppress Treg function.Citation105
Depletion/Inhibition of Myeloid-Derived Suppressor Cells
All-trans-retinoic acid (ATRA) has been used to target MDSCs. ATRA exerts a direct effect on MDSCs by inducing PMN-MDSC apoptosis and differentiating M-MDSCs to Ms and DCs.Citation106 Indeed, a number of clinical pharmacological agents currently in use (e.g., PDE5 inhibitors, COX-2 inhibitors, ARG1 inhibitors, bisphosphonates, gemcitabine, and paclitaxel) and other agents currently in preclinical testing may play a fundamental role in promoting anti-tumor immune responses by inhibiting MDSC functionality.Citation11
Small Molecules
Inducers of tumor cell sensitivity to apoptosis
Modulation of tumor necrosis factor-inducing ligand (TRAIL) receptors preferentially expressed by cancer cells may act synergistically with vaccination therapy. Histone deacetylase inhibitors have been shown to up-regulate the TRAIL death receptor DR5, leading to enhanced sensitization of cancer cells to TRAIL-induced death and the activity of vaccine-induced immune effector cells. Furthermore, a demethylating agent decitabine has been shown to sensitize melanoma cell lines to the cytotoxic effects of IFNs by inducing the expression of proapoptotic and growth-inhibitory genes.Citation107 The anti-apoptotic members of the Bcl-2 family are potential targets for immunosensitizing drugs. Bcl-2 is also upregulated in many cancer cells, and inactivating its function may result in a proapoptotic cancer cell inflammatory milieu facilitating cytotoxic activity of vaccine-induced immune cells. ABT-737 is a small molecule inhibitor of the anti-apoptotic proteins Bcl-2, Bcl-XL, and Bcl-w. Several studies revealed that ABT-737 did not directly initiate the apoptotic process, but rather upregulated various death signals, including immune-mediated signals.Citation69,108 Apoptotic pathways could also be targeted by altering the transcription of apoptosis regulators. Thus, inhibition of nuclear factor-ǩB in melanoma sensitizes cells to TRAIL-induced apoptosis.Citation69,109 Another approach involves a proteasome inhibitor bortezomib, known to inhibit nuclear factor-ǩB and down-regulate the antiapoptotic molecule FLICE-inhibitory protein (FLIP).Citation69,110 Certain small molecules, such as B-raf inhibitor and a tyrosine kinase inhibitor sunitinib have been shown to upregulate T cell activity and antitumor efficacy in preclinical studies. Recent studies also showed that an mTOR inhibitor rapamycin promotes IL-12 production and the development of memory CD8+ T cells leading to enhanced vaccine potency.Citation12
Inhibitors of indoleamine 2,3- dioxygenase (IDO)
IDO is an immunosuppressive enzyme, which is highly expressed in the microenvironments of various tumors. Two IDO inhibitors have entered clinical trials a tryptophan analog 1-methyl-tryptophan (1-MT) and an enzymatic inhibitor of IDO INCB024360. In preclinical studies, both IDO inhibitors were in effective attenuating tumor growth in wild type, but not immunodeficient animals.Citation111
Inhibitors of cyclooxygenase (COX)
An immunosuppressive COX-2 product PGE2 is secreted abundantly by tumor cells and by tumor-infiltrating macrophages. Selective COX-2 inhibitors celecoxib and rofecoxib and a non-selective agent indomethacin have shown promising results not only in the treatment of primary tumors but also in the prevention of metastatic spread in both preclinical models and clinical settings. When used in combination, these treatments synergistically/additively enhanced the efficacy of vaccines including DC-based vaccines.Citation112
Histamine type-2 receptor antagonists
Cimetidine is the oldest H2 receptor antagonist. An accumulating body of evidence suggests that this drug may improve the survival of patients with different malignancies, and its antitumor effect is mediated at least in part by immune mechanisms. In particular, cimetidine-mediated immunomodulation is based on the the enhancement of antigen-presenting DC activity,Citation113 inhibition of suppressor T lymphocyte activityCitation114 and MDSC activity,Citation115 stimulation of NK cell activityCitation116 and increase in IL-2 production in helper T lymphocytes.Citation117 Another H2-antagonist famotidine has been found to stimulate IL-2-driven LAK activity beneficial for the treatment of renal cancer.Citation118 Combination protocols of cancer vaccinations with H2 antagonists have not been extensively studied yet.
Aptamers
The first nucleic acid molecules tested as therapeutic agents were used in antisense strategies. Antisense compounds are single-stranded nucleic acids, which disrupt synthesis of a targeted protein by hybridizing in a sequence-dependent manner to the cognate mRNA, and which can be generated against most targets. Aptamers directly inhibit the activity of existing proteins, thus functionally being more similar to mAb or small molecule drugs than to antisense compounds. The specificity and avidity of aptamers are comparable to or exceeds that of mAbs, but unlike mAbs, aptamers are synthesized chemically, thus offering significant advantages in terms of reduced production cost and more straightforward regulatory approval processes.Citation69 An VEGF-specific aptamer became the first drug of this kind approved for human therapy to treat age-related macular degeneration.Citation119 First in vivo modulation of immune responses by aptamers was described for murine CTLA-4, which enhanced tumor immunity. Bivalent and multivalent configurations of other immunomodulatory aptamers binding to 4–1BB or OX40 costimulated T-cell activation in vitro and promoted tumor rejection in vivo.Citation120 The application of immune-modulating aptamers as immunoadjuvants takes advantage of the inherent lack of immunogenicity of these agents.Citation69
Antioxidants
A chronic inflammatory condition associated with increased oxidative stress has been suggested as one of the mechanisms responsible for tumor-induced immune suppression. Indeed, macrophage-derived nitric oxide was shown to downregulate phosphorylation and activation of JAK3/STAT5 signal transduction proteins, thus inhibiting proliferative T cell responses to IL-2. Furthermore, micromolar levels of hydrogen peroxide selectively inhibited Th1 cytokine production in memory/activated T cells, and this inhibition correlated with the blockade of nuclear factor-ǩB activation.Citation121 Altogether, these observations provide support for the application of antioxidants in combination therapy protocols with cancer vaccines. Accordingly, the immunomodulatory and antitumor activity of vitamins C, E, and D, as well as some anti-inflammatory secondary plant metabolites, such as resveratrol (a polyphenol from grapes), genistein (an isoflavone in soybean), quercetin (a flavonol in vegetables and fruits), and shikonin (a naphthoquinone from Lithospermum erythrorhizon) were recently attributed to their antioxidant properties.Citation123 No serious side effects were observed for these agents, which justifies wide immunotherapeutic application of antioxidants in various combination therapy settings and treatment regimens.
Physical Treatments
Radiation therapy is a standard modality for many types of cancer due to its direct cytotoxic effect on the tumor cells and its palliative effects on the patient. A recent report demonstrated that local irradiation of tumors with subtumoricidal doses could result in changes in tumor cell phenotypes including the upregulation of MHC, Fas, ICAM-1, and various TAAs.Citation23 Local radiation not only debulks the tumor but also generates an inflammatory microenvironment, thereby promoting presentation of dying tumor-released TAAs by DCs and subsequent T cell priming. Radiation also renders tumor cells more susceptible to attack by tumor-specific CTLs.Citation12
Radiofrequency ablation (RFA) is another approach to induce immune-dependent tumor cell death, in which radiofrequency waves are converted into heat to achieve local temperatures sufficient to induce tissue destruction. Recent data indicates that RFA enhanced DC maturation, increased the frequency of tumor-specific T-cells, and reduced Treg numbers, making RFA plus immunotherapy an appealing combination approach for cancer therapy.Citation123,124
High-intensity focused ultrasound (HIFU) is a technique in which a transducer is used to generate high-frequency ultrasound waves focused on a single point in the tumor. HIFU can deliver a large amount of energy that is subsequently converted to thermal energy, resulting in tumor cell destruction with minimal damage to the surrounding tissue.Citation125 The results also demonstrate that immunogenic TAAs remain available in the tumor debris after HIFU ablation for effective presentation by DCs, increasing the number and activity of TILs.Citation126
Cryoablation
Early clinical studies using cryoablation demonstrated the regression of untreated metastatic lesions and activation of antitumor immunity. Recent clinical studies revealed the upregulation of serum cytokine levels and generation of tumor-specific T-cells following cryoablation.Citation126,127 The simplicity of cryoablation coupled with its minimal negative effects on the immune system make this procedure an attractive candidate for combination regiment with immunotherapy.Citation124
Chemotherapy
Long-standing dogma suggested that myelosuppressive effects of chemotherapy would prevent its application in combination with immunotherapy. However, this assumption was challenged by a large amount of experimental data obtained in recent years.Citation128 Similar to the radiotherapy, the application of various chemotherapy regiments in combination with tumor cell vaccines was shown to stimulate antitumor immune responses. Widespread chemotherapy application for cancer treatment substantiates the development of combinatorial approaches using vaccines plus standard chemotherapeutic agents.Citation23 Mechanisms underlying chemotherapy-mediated upregulation of the anti-tumor immunity include upregulation of tumor cells immunogenicity, reduction of immune dysfunction by impairing the functionality of immunosuppressive cells, or induction of apoptotic cancer cell death, which activates immune responses.Citation129 It has been demonstrated that growing tumor cells can be much more sensitive to T-cell attack compared to the quiescent cells.Citation26 Therefore, chemotherapy could potentiate the efficiency of immunotherapy, at least in part, by stimulating compensatory tumor cell proliferation. In addition, as a high tumor burden is immunosuppressive, cytoreduction before immunotherapy can improve outcomes in the metastatic settings. It should also be noted that multidrug resistance (MDR) to chemotherapy renders cancer cells relatively insensitive to treatment with many standard cytotoxic anti-cancer agents. Therefore, cancer immunotherapy could be an important modality to treat MDR positive cancers, as resistance to immunotherapy is generally unrelated to mechanisms of resistance to cytotoxic agents.Citation26
Rational Immunotherapeutic Strategies
The combination of traditional therepeutic approaches with cancer vaccines provides an opportunity to further improve the efficiency of anti-cancer vaccines. Unlike other modalities, cancer vaccines have demonstrated thus far no associated toxicities, and therefore their use could lead to increased patient survival and improved quality of life. We envisage that combination immunotherapy should achieve the following 3 aims: (i) breaking immune tolerance to TAAs and increasing the frequency of tumor-specific lymphocytes; (ii) stimulating immune cell migration into the tumor site and eliciting cytodestructive inflammation in the tumor microenvironment; (iii)activating tumor- specific T-lymphocytes and non-specific cytotoxiс effector cells, as well as reducing the activity of local and systemic immunosuppressive mechanisms.
In our opinion, xenogeneic cell-based vaccines could constitute the most effective approach to breaking immune tolerance in cancer settings. This notion is based on the observations that xenogeneic proteins are highly immunogenic and effective in breaking immune tolerance to the analogous human self-proteins. In addition, a wide variety of animal tumor cell lines are available, which could be exploited to design vaccines with maximal antigenic overlap with the target tumors. Such vaccines could elicit strong polyclonal tumor-specific immune responses with minimal immunological side effects. An arsenal of tools can be used to potentiate antitumor effects of cancer vaccinations ().
Table 4. Immune-based interventions and their mechanisms of action
Immunoadjuvants used to enhance the efficacy of cancer vaccines stimulate vaccine-specific immune responses and define their Th1/Th2 bias. It is generally accepted that Th1 responses are more protective against cancer than Th2 responses. The application of TLR agonists and IFNs as adjuvants could promote predominantly Th1-mediated immune responses elicited by vaccines, while cytokines of the ɣ-chain family (IL-2, IL-4, IL-7, IL-9 and IL-15) could promote the expansion of vaccine-specific T cells. Adoptive T-cell therapy proved to be an effective approach to expand tumor -specific T cells. This technology facilitates the propagation and differentiation of antitumor T cells in favorable growth conditions in the absence of tumor immunosuppression, followed by the infusion of the generated memory T cells into the patient. Compared to naïve T cells, memory T cells are far less sensitive to tumor-mediated immunosuppression, and thus could remain functionally active even in patients with advanced cancer.
To effectively control growth of cancer cells in the body, TAA-specific T cells and other immune cells with antitumor potential must gain access to, and function within, the tumor microenvironment. An accumulating body of evidence suggests the existence of 2 types of immune resistance within the tumor microenvironment: (i) deficiency of immune cell trafficking due to low levels of inflammation and chemokine deficient milieu, and (ii) dominant suppression due to immune inhibitory mechanisms. In general, tumor microenvironment can be allocated to ‘non-inflamed’ and ‘inflamed’ groups (), with one of the major immunotherapy goals being to promote tumor inflammation and to recruit immune cells within the tumor.Citation130 T cells are known to be the major orchestrators of inflammation. Hence, it is widely believed that patients with tumors that fail to recruit and activate T cells may be resistant to all current immunotherapy approaches.Citation130
Figure 1. Non-inflamed versus inflamed tumor microenvironments with implications for immunotherapy. Potential barriers to developing antitumor immune responses, and possible contra interventions are indicated for non-inflamed (left) and inflamed tumors (rights). This figure is partially adapted from Spranger S, Gajewski T., 2013 Citation25.
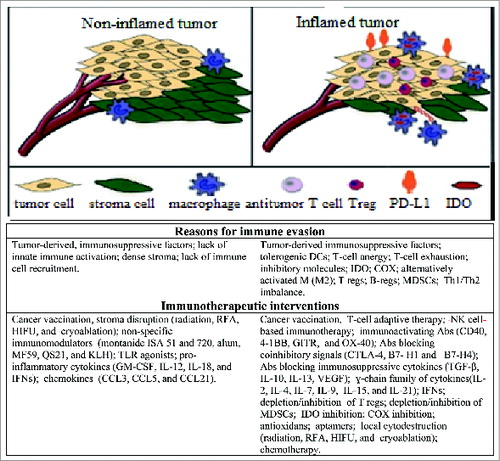
Typically, tumor cells fail to express both MHC II and costimulatory molecules (CD28 and others), and consequently are deficient in CD4 T cell activation. To be fully activated, tumor-specific CD4 cells need APCs expressing both TAA/MHC II complexes and costimulatory molecules. In contrast to CD4+ T cells, tumor-specific CD8 cells are able to directly recognize tumor cells via TAA/MHC I complexes. However, to be fully activated, these cells also require costimulation by activated DCs, Ms or B cells. In the absence of such costimulation, tumor- reactive CD8+ T cells become anergic. Therefore, strategies to promote robust signaling via APCs in the tumor microenvironment could greatly facilitate the activation of both CD4+ and CD8+ T cells, thus improving overall tumor control.
Reversal of T-cell anergy can be achieved by using homeostatic cytokines IL-7 and IL-15 or by transferring T cells into lymphopenic recipients leading to the liberation of endogenous IL-7 and IL-15.Citation130 Another feasible approach to prevent anergy of vaccine-elicited T cells is to apply immunoactivating Abs capable of directly costimulating TAA-activated T cells. In addition, costimulatory Abs directed against CD28, CD40, 41-BB, GITR, and OX-40 could be useful in enhancing immune-mediated antitumor effects.Citation90
Radiation, RFA, HIFU and cryoablation could be useful both in debulking of tumors and in stimulating tumor-destructive inflammation via APCs activation.
As noted above, chemotherapeutic conditioning could be useful for subsequent immunotherapy by virtue of niche formation in lymphoid tissues and triggering homeostatic cell proliferation mechanisms. Under such conditions, vaccine-elicited lymphocytes actively proliferate, increasing their frequencies in the blood and lymphoid tissues. It is important to note that in contrast to the high-dose cytoreductive chemotherapy, which causes profound immunosuppression, immunomodulatory therapies are typically characterized by lowdose protocols and do not cause severe complications due to immunosuppression. This justifies the combination of chemotherapy with vaccinotherapy to achieve the optimal antitumor effect. Importantly, timing of cytotoxic agent administration can also modulate immune effects. For example, maximum tolerated doses of cyclophosphamide, doxorubicin or paclitaxel administered with an experimental cancer vaccine in a mouse model reduced vaccine efficacy, whereas the same doses given prior to vaccination improved vaccine efficacy.Citation129,131
Immunostimulating interventions often activate immunosuppressive feedback mechanisms, which prevent excessive immune responses. For example, on the one hand TLR agonists induce production of type I IFN and pro-inflammatory cytokines, such as IL-12, IL-1β, TNF-α and IL-6, but on the other hand these agents induce anti-inflammatory molecules, such as IL-10 and PGE2.Citation132 The ability of TLR agonists to induce Tregs has also been shown.Citation133 Therefore, an attractive strategy could be combining tumor vaccines in adjuvant formulations with interventions reducing immunosuppression and overcoming T cell exhaustion. To this end, Abs or small molecules blocking coinhibitory signals, immunosuppressive cytokines could be applied. Cancer vaccinations could be also effectively combined with the strategies aimed at deleting/inhibiting immunosuppressor cells, such as Tregs or MDSCs. Therefore, clinical studies of different anti-cancer therapies applied in combination with tumor vaccinations are required.
Immunological and Clinical Monitoring of Cancer Vaccine-Treated Patients
In view of the diversity and complexity of the immune system, it is essential to assess immune responses to vaccine antigens before vaccination onset. Vaccination with antigens, incapable of inducing antitumor immune responses provide no clinical benefits. Furthermore, vaccines could inhibit beneficial antitumor immunity through stimulating immunosuppressive mechanisms including those mediated by inducible T regs. Therefore, specific criteria should be elaborated in order to select appropriate patients or adequate "personalized’ vaccine antigens based on the ascertainment of the individual immunological reactivity.Citation134
Implementation of correlation studies of specific immune responses and clinical outcomes constitutes another ambitious goal for immune monitoring. Results of the delayed type hypersensitivity response (DTH) assay were shown to correlate directly with peripheral blood antigen-specific T cell responses, such that this test has been used as a measure of antigen recall or memory responses for a long time. Over a decade ago, DTH responses were shown to correlate with the survival of melanoma patients treated with autologous tumor cell lysate vaccines. It is also known that the magnitude of Th1 immune responses and epitope spreading in cancer vaccine-treated patients could be associated with clinical responsiveness to treatment.Citation135 A serologic assessment of tumor-specific immunity in cancer patients demonstrated that the presence of IgG Abs specific for selected TAAs in the blood is consistent with an improved survival, as compared to patients without such Abs. Moreover, immunity to multiple cancer antigens, rather than immunity restricted to a single antigen, appeared to impart a more favorable prognosis.Citation136
It is well known that the development of autoimmunity is associated with clinically effective cancer immunotherapy.Citation137 Thus, treatment of patients with anti-CTLA-4 antibodies has been shown to induce autoimmunity, which predicts clinical response in cancer settings. The development of autoimmunity was found to be an independent predictor of overall survival in patients with melanoma treated with IFN or IL-2.Citation135
Aging is accompanied by an increased susceptibility to infectious agents and cancer, and by an inability to mount protective immune responses to vaccines. Although regulatory T cell numbers tend to increase with age, autoimmunity is more common in the elderly, suggesting that inhibitory pathways may become impaired in this age group. Immune responses in the elderly patients are by far more variable than those in young individuals and therefore should be interpreted with greet caution.Citation138 Autoimmune responses in such patients are not likely to be associated with a favorable prognosis in all cases.
Historically, most immunoassays currently employed in clinical trials are adjusted to evaluate vaccine potency, but are not well suited to assess immunotherapy-mediated antitumor activity with a notable exception of the assessment of immune cell infiltrates in cancer biopsies. Such 'immune signatures' can serve as independent prognostic predictors for survival.Citation12 For example, in several studies infiltration of CD3+ T cells into the tumor bed appeared to be a critical predictor of survival.Citation136
The classic World Health Organization (WHO) and Response Evaluation Criteria In Solid Tumors (RECIST) criteria that are applied to measure the efficacy of cytotoxic chemotherapy depend on tumor shrinkage, and any increase in tumor size beyond a certain level, as well as the appearance of new lesions is considered as a treatment failure. However, there is now ample evidence that these criteria do not apply to immunotherapy. Immunotherapy-induced tumor regressions have been well documented after initial progression and even after the appearance of new lesions, which are likely to be caused by the lymphocyte infiltration into tumors.Citation128,139 Over a long period of time, vaccine-induced antitumor activity could result in a slower tumor growth rate and improved overall survival, even though patients do not show significant reductions in tumor burden and an improvement in relapse-free survival.Citation12,140 In contrast to cytotoxic chemotherapy, tumor vaccine-based strategies could permit the host to reach a steady state status with the malignant process, in which the net result of tumor growth and destruction is close to a zero. Achieving this balance might lead to more significant survival benefits than a rapid destruction and rapid regrowth of the tumor following cytotoxic therapy. Therefore, traditional response criteria may not be adequate for evaluating clinical responses to a given immunotherapy, and new 'immune response criteria' should be developed to optimize classification and evaluation of clinical responses in immunotherapeutic patients.Citation12,141
Conclusion
It is generally accepted that the immune system has an inherent capacity to prevent tumor development. Xenovaccination with poly-antigenic cells can effectively break immune tolerance to multiple TAAs, and this approach may become a mainstream strategy in the treatment of most cancers. However, vaccination alone is often insufficient to mount effective antitumor immune responses. To improve clinical outcomes, vaccinations should be combined with other intervention strategies that target different pathways. Clinical application of immunotherapeutic agents is devoid of serious side effects, which permits their use in different combinations and in various treatment regimens. An attractive protocol would be to combine vaccinations in an adjuvant formulation with an immunosuppression-blocking modality. Another feasible variant could include a cytoreductive treatment followed by vaccinations along with immunostimulating and/or immunosuppression-blocking modalities. Multiple examples of successful immunotherapeutic combinations have been evaluated in preclinical models, and the results are consistent with the above described logic framework. To be clinically effective, the particular immunotherapy strategy should be undoubtedly personalized and developed based on a thorough analysis of both the course of disease and patient's immune reactivity. In our view, the lack of personalization in the immunotherapy treatment, which is usually focused on treatment standardization could at least partially account for the failure of many completed immunotherapeutic trials. A variety of internal and external factors can affect variably both immune responses and tumor development in different patients. Nevertheless, we anticipate that novel immunotherapeutic strategies in the form of highly immunogenic poly-antigenic vaccines (e.g., xenogeneic cell vaccines) along with other agents and/or approaches aiming to reinforce antitumor immunity will make a great progress in cancer treatment and considerably improve clinical outcomes already in the not too distant future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study has been supported by the Neurotechnology company (Vilnius, Lithuania).
References
- Seledtsov VI, Shishkov AA, Seledtsova GV. Xenovaccinotherapy for cancer. in:ozdemir O, editor. current cancer treatment – novel beyond conventional approaches. InTech 2011; 415-28.
- Strioga MM, Darinskas A, Pasukoniene V, Mlynska A, Ostapenko V, Schijns V. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use? Vaccine 2014; 32:4015-24; PMID:24837511; http://dx.doi.org/10.1016/j.vaccine.2014.05.006
- Lim SH, Zhang Y, Zhang J. Cancer-testis antigens: the current status on antigenregulation and potential clinical use. Am J Blood Res 2012; 2:29-35; PMID:22432085
- Malati T. Tumour markers: an overview. Indian J Clin Biochem 2007; 22:17-31; PMID:23105677; http://dx.doi.org/10.1007/BF02913308
- Lucas S, Coulie PG. About human tumor antigens to be used in immunotherapy. Semin Immunol 2008; 20:301-7; PMID:18395462; http://dx.doi.org/10.1016/j.smim.2008.02.001
- Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL. Comparative analysis of the in vivo expression of tyrosinase, MART-1/melan-A,and gp100 in metastatic melanoma lesions: implications for immunotherapy. J Immunother 1998; 21: 27-31; PMID:9456433; http://dx.doi.org/10.1097/00002371-199801000-00003
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malig-nancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol 2001; 491: 369-402; PMID:14533809; http://dx.doi.org/10.1007/978-1-4615-1267-7_24
- Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with α-galactosylceramide leads topotent and long-lived T cell mediated immunity via dendritic cells. J Exp Med 2007; 204:2641-53; PMID:17923500; http://dx.doi.org/10.1084/jem.20070458
- Darcy PK, Neeson P, Yong CS, Kershaw MH. Manipulating immune cells for adoptive immunotherapy of cancer. Curr Opin Immunol 2014; 27: 46-52; PMID:24534448; http://dx.doi.org/10.1016/j.coi.2014.01.008
- Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol 2011; 186:1325-31; PMID:21248270; http://dx.doi.org/10.4049/jimmunol.0902539
- Raval RR, Sharabi AB, Walker AJ, Drake CG, Sharma P. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J Immunother Cancer 2014; 2:14; PMID:24883190; http://dx.doi.org/10.1186/2051-1426-2-14
- Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 2013; 119: 421-75; PMID:23870514; http://dx.doi.org/10.1016/B978-0-12-407190-2.00007-1
- Fridlender ZG, Albelda SM. Modifying tumor-associated macrophages: an important adjunct to immunotherapy. Oncoimmunology 2013; 2: e26620; PMID:24498549; http://dx.doi.org/10.4161/onci.26620
- Cromheecke JL, Nguyen KT, Huston DP. Emerging role of human basophil biology in health and disease. Curr Allergy Asthma Rep 2014; 14:408; PMID:24346805; http://dx.doi.org/10.1007/s11882-013-0408-2
- Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C. B cell-regulated immune responses in tumor models and cancer patients. Oncoimmunology 2013; 2:e25443; PMID:24073382; http://dx.doi.org/10.4161/onci.25443
- Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today 1994; 15:362-6; PMID:7916949; http://dx.doi.org/10.1016/0167-5699(94)90174-0
- Seledtsov VI, Seledtsova GV. An 'antigenic ligand competition' model for antigen receptor-mediated lymphocyte selection. Biomed Pharmacother 1996; 50:170-7; PMID:8881375; http://dx.doi.org/10.1016/0753-3322(96)85293-0
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996; 17:138-46; PMID:8820272; http://dx.doi.org/10.1016/0167-5699(96)80606-2
- Seledtsov VI, Seledtsova GV. A possible role of pre-existing IgM/IgG antibodies in determining immune response type. Immunol Cell Biol 1997; 75:176-80; PMID:9107571; http://dx.doi.org/10.1038/icb.1997.24
- Gajewski TF. Cancer immunotherapy. Mol Oncol 2012; 6:242-50; PMID:22248437; http://dx.doi.org/10.1016/j.molonc.2012.01.002
- Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep 2011; 44:217-31; PMID:21524346; http://dx.doi.org/10.5483/BMBRep.2011.44.4.217
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011; 239:27-44; PMID:21198663; http://dx.doi.org/10.1111/j.1600-065X.2010.00979.x
- Palena C, Schlom J. Vaccines against human carcinomas: strategies to improve antitumor immune responses. J Biomed Biotechnol 2010; 2010: ID 380697; PMID:20300434; http://dx.doi.org/10.1155/2010/380697
- Lasaro MO, Ertl HC. Targeting inhibitory pathways in cancer immunotherapy. Curr Opin Immunol 2010; 22:385-90; PMID:20466529; http://dx.doi.org/10.1016/j.coi.2010.04.005
- Spranger S, Gajewski T. Rational combinations of immunotherapeutics that target discrete pathways. J Immunother Cancer 2013; 1:16; PMID:24829752; http://dx.doi.org/10.1186/2051-1426-1-16
- Melenhorst JJ, Barrett AJ.Tumor vaccines and beyond. Cytotherapy 2011; 13:8-18; PMID:21067312; http://dx.doi.org/10.3109/14653249.2010.530649
- Hussain M, Javeed A, Ashraf M, Al-Zaubai N, Stewart A, Mukhtar MM. Non-steroidal anti-inflammatory drugs, tumour immunity and immunotherapy. Pharmacol Res 2012; 66:7-18; PMID:22449788; http://dx.doi.org/10.1016/j.phrs.2012.02.003
- Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol 2011; 11:802-7; PMID:21237299; http://dx.doi.org/10.1016/j.intimp.2011.01.003
- Agarwal N, Padmanabh S, Vogelzang NJ. Development of novel immune interventions for prostate cancer. Clin Genitourin Cancer 2012; 10:84-92; PMID:22409862; http://dx.doi.org/10.1016/j.clgc.2012.01.012
- Ward S, Casey D, Labarthe MC, Whelan M, Dalgleish A, Pandha H, Todryk S. Immunotherapeutic potential of whole tumor cells. Cancer Immunol Immunother 2002; 51:351-7; PMID:12192534; http://dx.doi.org/10.1007/s00262-002-0286-2
- Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int Rev Immunol 2011; 30:150-82; PMID:21557641; http://dx.doi.org/10.3109/08830185.2011.572210
- Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000; 191:423-34; PMID:10662788
- Hu D-E, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res 2004; 64:5059-62; PMID:15289304
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NPgp100/pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med 1998; 188:277-86; PMID:9670040
- Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-a-galactosyl specificity. J Exp Med 1984; 160:1519-31; PMID:6491603
- Galili U. Interaction of the natural anti-gal antibody with a-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today 1993; 14:480-2; PMID:7506033
- Seledtsov VI, Shishkov AA, Surovtseva MA, Samarin DM, Seledtsova GV, Niza NA, Seledtsov DV. Xenovaccinotherapy for melanoma. Eur J Dermatol 2006; 16:655-61; PMID:17229606
- Seledtsov VI, Niza NA, Surovtseva MA, Shishkov AA, Samarin DM, Seledtsova GV, Seledtsov DV. Xenovaccinotherapy for colorectal cancer. Biomed Pharmacother 2007; 61:125-30
- Asada H, Kishida T, Hirai H, Satoh E, Ohashi S, Takeuchi M, Kubo T, Kita M, Iwakura Y, Imanishi J., et al. Significant antitumor effects obtained by autologous tumor cell vaccine engineered to secrete interleukin (IL)-12 and IL-18 by means of the EBV/lipoplex. Mol Ther 2002; 5:609-16; PMID:11991752
- Fishman M, Hunter TB, Soliman H, Thompson P, Dunn M, Smilee R, Farmelo MJ, Noyes DR, Mahany JJ, Lee JH., et al. Phase II trial of B7–1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J Immunother 2008; 31:72-80; PMID:18157014
- Parmiani G, Pilla L, Maccalli C, Russo V. Autologous versus allogeneic cell-based vaccines? Cancer J 2011; 17:331-6; PMID:21952283; http://dx.doi.org/10.1097/PPO.0b013e3182337a76
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol 2002; 3:1129-34; PMID:12447370
- Lapenta C, Santini SM, Logozzi M, Spada M, Andreotti M, Di Pucchio T, Parlato S, Belardelli F. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J Exp Med 2003; 198:361-7; PMID:12874266
- Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol 2007; 37:1678-90; PMID:17492620
- Palucka K, Ueno H, Banchereau J.Recent developments in cancer vaccines. J Immunol 2011; 186:1325-31; PMID:21248270; http://dx.doi.org/10.4049/jimmunol.0902539
- Salem ML. Triggering of toll-like receptor signaling pathways in T cells contributes to the anti-tumor efficacy of T cell responses. Immunol Lett 2011; 137:9-14; PMID:21352854; http://dx.doi.org/10.1016/j.imlet.2011.02.019
- Kenderian SS, Ruella M, Gill S, Kalos M. Chimeric antigen receptor T-cell therapy to target hematologic malignancies.Cancer Res 2014; 74:6383-89; PMID:25371415; http://dx.doi.org/10.1158/0008-5472.CAN-14-1530
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:23604045; http://dx.doi.org/10.1038/cmi.2013.10
- Scalzo AA, Elliott SL, Cox J, Gardner J, Moss DJ, Suhrbier A. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatible adjuvant (montanide ISA 720). J Virol 1995; 69:1306-9; PMID:7815511
- Miles AP, McClellan HA, Rausch KM, Zhu D, Whitmore MD, Singh S, Martin LB, Wu Y, Giersing BK, Stowers AW., et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 2005; 23:2530-9; PMID:15752840; http://dx.doi.org/10.1016/j.vaccine.2004.08.049
- Hem SL, HogenEsch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines 2007; 6:685-98; PMID:17931150; http://dx.doi.org/10.1586/14760584.6.5.685
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008; 453:1122-6; PMID:18496530; http://dx.doi.org/10.1038/nature06939
- Kensil C, Kammer R. QS-21: A water-soluble triterpene glycoside adjuvant. Expert Opin Investig Drugs 1998; 7:1475-82; PMID:15992044; http://dx.doi.org/10.1517/13543784.7.9.1475
- Dubensky TW Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol 2010; 22:155-61; PMID:20488726; http://dx.doi.org/10.1016/j.smim.2010.04.007
- Akira S. Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci 2009; 85, 143-56; PMID:19367086; http://dx.doi.org/10.2183/pjab.85.143
- Rakoff-Nahoum S, Medzhitov R, Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008; 73:555-61; PMID:18605980; http://dx.doi.org/10.1134/S0006297908050088
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22:240-73; PMID:19366914; http://dx.doi.org/10.1128/CMR.00046-08
- Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut 2009; 58:704-20; PMID:19359436; http://dx.doi.org/10.1136/gut.2008.156307
- Uehori J, Matsumoto M, Tsuji S, Akazawa T, Takeuchi O, Akira S, Kawata T, Azuma I, Toyoshima K, Seya T. Simultaneous blocking of human toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by mycobacterium bovis bacillus calmette-guérin peptidoglycan. Infect Immun 2003; 71:4238-49; PMID:12874299; http://dx.doi.org/10.1128/IAI.71.8.4238-4249.2003
- Schneider C, Schmidt T, Ziske C, Tiemann K, Lee KM, Uhlinsky V, Behrens P, Sauerbruch T, Schmidt-Wolf IG, Mühlradt PF., et al. Tumour suppression induced by the macrophage activating lipopeptide MALP-2 in an ultrasound guided pancreatic carcinoma mouse model. Gut 2004; 53:355-61; PMID:14960515; http://dx.doi.org/10.1136/gut.2003.026005
- Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol 1999; 163:57-61; PMID:10384099
- Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, Mason M, Adams M. A clinical grade poly I:C-analogue (ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine 2009; 27:107-15; PMID:18977262; http://dx.doi.org/10.1016/j.vaccine.2008.10.024
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R.TRAM couples endocytosis of toll-like receptor 4 to the induction of interferon-β. Nat Immunol 2008; 9:361-8; PMID:18297073; http://dx.doi.org/10.1038/ni1569
- Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VFI. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist 2008; 13:859-75; PMID:18701762; http://dx.doi.org/10.1634/theoncologist.2008-0097
- Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs 2008; 17:1051-65; PMID:18549341; http://dx.doi.org/10.1517/13543784.17.7.1051
- Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117:1184-94; PMID:17476348
- Bobanga ID, Petrosiute A, Huang AY. Chemokines as cancer vaccine adjuvants. Vaccines (Basel) 2013; 1:444-62; PMID:24967094
- Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev 2002; 188:147-54; PMID:12445288
- Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Critical Rev Clin Lab Sci 2009; 46:167-189; PMID:19650714; http://dx.doi.org/10.1080/10408360902937809
- Kuppala MB, Syed SB, Bandaru S, Varre S, Akka J, Mundulru HP. Immunotherapeutic approach for better management of cancer: role of IL-18. Asian Pac J Cancer Prev 2012; 13:5353-61; PMID:23317183
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+CD25hi foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409-14; PMID:16304057
- Waldmann TA.The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6:595-601; PMID:16868550
- Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest 2005; 115:1177-87; PMID:15841203
- Spolski R, Leonard WJ. The yin and yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol 2008; 20:295-301; PMID:18554883; http://dx.doi.org/10.1016/j.coi.2008.02.004
- Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/ cytokine receptor system that has come of age. J Leukoc Biol 2008; 84:348-56; PMID:18467657; http://dx.doi.org/10.1189/jlb.0308149
- Wilk E, Kalippke K, Buyny S, Schmidt RE, Jacobs R. New aspects of NK cell subset identification and inference of NK cells’ regulatory capacity by assessing functional and genomic profiles. Immunobiology 2008; 213:271-83; PMID:18406373; http://dx.doi.org/10.1016/j.imbio.2007.10.012
- Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res 2007; 13:6926-32; PMID:18056166; http://dx.doi.org/10.1158/1078-0432.CCR-07-1238
- Bekisz J, Sato Y, Johnson C, Husain SR, Puri RK, Zoon KC. Immunomodulatory effects of interferons in malignancies. J Interferon Cytokine Res 2013; 33:154-61; PMID:23570381; http://dx.doi.org/10.1089/jir.2012.0167
- Balachandran S, Adams GP. Interferon-γ-induced necrosis: an antitumor biotherapeutic perspective. J Interferon Cytokine Res 2013; 33:171-80; PMID:23570383; http://dx.doi.org/10.1089/jir.2012.0087
- Cao Q, Jin Y, Jin M, He S, Gu Q, Qiu Y, Ge H, Yoneyama H, Zhang Y. Therapeutic effect of MIP-1alpha-recruited dendritic cells on preestablished solid and metastatic tumors. Cancer Lett 2010; 295:17-26; PMID:20202744; http://dx.doi.org/10.1016/j.canlet.2010.02.009
- Serra HM, Baena-Cagnani CE, Eberhard Y. Is secondary lymphoid-organ chemokine (SLC/CCL21) much more than a constitutive chemokine? Allergy 2004; 59:1219-23; PMID:15461605; http://dx.doi.org/10.1111/j.1398-9995.2004.00531.x
- Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol 2003; 15:5-14; PMID:12495636; http://dx.doi.org/10.1016/S1044-5323(02)00123-9
- Espino J, Pariente JA, Rodríguez AB. Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev 2012; 2012:670294; PMID:23346283; http://dx.doi.org/10.1155/2012/670294
- Garcia-Mauriño S, Gonzalez-Haba MG, Calvo JR, Rafii-El-Idrissi M, Sanchez-Margalet V, Goberna R, Guerrero JM.“Melatonin enhances IL-2, IL-6, and IFN gamma production by human circulating CD4+ cells: a possible nuclear receptor mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol 1997; 159:574-81; PMID:9218571
- Currier NL, Sun LZ, Miller SC. Exogenous melatonin: quantitative enhancement in vivo of cells mediating non-specific immunity. J Neuroimmunol 2000; 104:101-8; PMID:10713348; http://dx.doi.org/10.1016/S0165-5728(99)00271-4
- Ershler WB, Gravenstein S, Geloo ZS. Thymosin α 1 as an adjunct to influenza vaccination in the elderly: rationale and trial summaries. Ann N Y Acad Sci 2007; 1112:375-84; PMID:17600281; http://dx.doi.org/10.1196/annals.1415.050
- Heng TS, Reiseger JJ, Fletcher AL, Leggatt GR, White OJ, Vlahos K, Frazer IH, Turner SJ, Boyd RL. Impact of sex steroid ablation on viral, tumour and vaccine responses in aged mice. PLoS One 2012; 7:e42677; PMID:22880080; http://dx.doi.org/10.1371/journal.pone.0042677
- Mascanfroni I, Montesinos Mdel M, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, Masini-Repiso AM, Targovnik HM, Rabinovich GA, Pellizas CG. Control of dendritic cell maturation and function by triiodothyronine. FASEB J 2008; 22:1032-42; PMID:17991732; http://dx.doi.org/10.1096/fj.07-8652com
- Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun 2007; 21:384-92; PMID:17198749; http://dx.doi.org/10.1016/j.bbi.2006.11.010
- Postow M, Callahan MK, Wolchok JD. Beyond cancer vaccines: a reason for future optimism with immunomodulatory therapy. Cancer J 2011; 17:372-8; PMID:21952288; http://dx.doi.org/10.1097/PPO.0b013e31823261db
- Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA 2003; 100:15059-64; PMID:14608036; http://dx.doi.org/10.1073/pnas.2334901100
- Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP., et al. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res 2006; 66:4904-12; PMID:16651447; http://dx.doi.org/10.1158/0008-5472.CAN-05-2813
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002; 3:611-8; PMID:12087419; http://dx.doi.org/10.1038/ni0702-611
- Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005; 174:5994-6004; PMID:15879092; http://dx.doi.org/10.4049/jimmunol.174.10.5994
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/10.1038/nm0902-1039c
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99:12293-7; PMID:12218188; http://dx.doi.org/10.1073/pnas.192461099
- Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G., et al. Blockade of B7- H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65:1089-96; PMID:15705911
- Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003; 18:849-61; PMID:12818165; http://dx.doi.org/10.1016/S1074-7613(03)00152-3
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/10.1200/JCO.2013.53.0105
- Terabe M, Ambrosino E, Takaku S, O'Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin Cancer Res 2009; 15:6560-9; PMID:19861451; http://dx.doi.org/10.1158/1078-0432.CCR-09-1066
- Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. Immunol 2011; 186:1325-31; PMID:21248270; http://dx.doi.org/10.4049/jimmunol.0902539
- Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 1999; 5:2963-70; PMID:10537366
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res 1999; 59:3128-33; PMID:10397255
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999; 163:5211-8; PMID:10553041
- Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Curr Opin Pharmacol 2011; 11:404-11; PMID:21501972; http://dx.doi.org/10.1016/j.coph.2011.03.004
- Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013; 62:909-18; PMID:23589106; http://dx.doi.org/10.1007/s00262-013-1396-8
- Gollob JA, Sciambi CJ. Decitabine up-regulates S100A2 expression and synergizes with IFN-gamma to kill uveal melanoma cells. Clin Cancer Res 2007; 13:5219-25; PMID:17785578
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N., et al. Functional and physical interaction between bcl-X(L) and a BH3-like domain in beclin-1. Embo J 2007; 26:2527-39; PMID:17446862
- Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T, Hersey P. The role of NF-kappa B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol 2001; 166:5337-45; PMID:11313369
- Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, Blazar BR, Zhang X, Elliott PJ, Murphy WJ. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAILmediated apoptosis by reducing levels of c-FLIP. Blood 2003; 102:303-10; PMID:12637321
- Fehres CM, Unger WW, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol 2014; 5:149; PMID:24782858; http://dx.doi.org/10.3389/fimmu.2014.00149
- Hussain M, Javeed A, Ashraf M, Al-Zaubai N, Stewart A, Mukhtar MM. Non-steroidal anti-inflammatory drugs, tumour immunity and immunotherapy. Pharmacol Res 2012; 66:7-18; PMID:22449788; http://dx.doi.org/10.1016/j.phrs.2012.02.003
- Kubota T, Fujiwara H, Ueda Y, Itoh T, Yamashita T, Yoshimura T, Okugawa K, Yamamoto Y, Yano Y, Yamagishi H. Cimetidine modulates the antigen presenting capacity of dendritic cells from colorectal cancer patients. Br J Cancer 2002; 86:1257-61; PMID:11953882
- Osband ME, Hamilton D, Shen Y-J, Cohen E, Shlesinger M, Laven P, Brown A, McCaffrey R. Successful tumour immunotherapy with cimetidine in mice. Lancet 1981; 1:636-38; PMID:6110864
- Zheng Y, Xu M, Li X, Jia J, Fan K, Lai G. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol Immunol 2013; 54:74-83; PMID:23220070; http://dx.doi.org/10.1016/j.molimm.2012.10.035
- Hellstrand K, Hermodsson S. Histamine H2-receptor-mediated regulation of human natural killer cell activity. J Immunol 1986; 137:656-60; PMID:3722819
- Gifford RRM, Tirberg AF. Histamine type-2 receptor antagonist immune modulation II. cimetidine and ranitidine increase interleukin-2 production. Surgery 1987; 102:242-47; PMID:3497460
- Quan WD, Gagnon GA, Walker PR, Quan FM. Pulse interleukin-2 with famotidine inducesCD56+ lymphocytes in the peripheral blood of patients with metastatic melanoma or kidney cancer. Cancer Biother Radiopharm 2011; 26:65-7; PMID:21348776; http://dx.doi.org/10.1089/cbr.2010.0879
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr., Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 2006; 5:123-32; PMID:16518379
- McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest 2008; 118:376-86; PMID:18060045
- Malmberg KJ. Effective immunotherapy against cancer: a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother 2004; 53:879-92; PMID:15338206
- Aravindaram K, Yang NS. Anti-inflammatory plant natural products for cancer therapy. Planta Med 2010; 76:1103-17; PMID:20432202; http://dx.doi.org/10.1055/s-0030-1249859
- Fagnoni FF, Zerbini A, Pelosi G, Missale G. Combination of radiofrequency ablation and immunotherapy. Front Biosci 2008; 13:369-81; PMID:17981554
- Higgins JP, Bernstein MB, Hodge JW. Enhancing immune responses to tumor-associated antigens. Cancer Biol Ther 2009; 8:1440-9; PMID:19556848
- Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: a temperature's story. Cancer Lett 2008; 271:191-204; PMID:18597930; http://dx.doi.org/10.1016/j.canlet.2008.05.026
- Osada S, Imai H, Tomita H, Tokuyama Y, Okumura N, Matsuhashi N, Sakashita F, Nonaka K. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol 2007; 95:491-8; PMID:17219394
- Si T, Guo Z, Hao X. Immunologic response to primary cryoablation of high-risk prostate cancer. Cryobiology 2008; 57:66-71; PMID:18593573; http://dx.doi.org/10.1016/j.cryobiol.2008.06.003
- Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy-revisited. Nat Rev Drug Discov 2011; 10:591-600; PMID:21804596; http://dx.doi.org/10.1038/nrd3500
- Curiel TJ. Immunotherapy: a useful strategy to help combat multidrug resistance. Drug Resist Updat 2012; 15:106-13; PMID:22483359; http://dx.doi.org/10.1016/j.drup.2012.03.003
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:24048123; 10.1038/ni.2703
- Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance theantitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res 2001; 61:3689-97; PMID:11325840
- Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Curr Opin Pharmacol 2011; 11:404-11; PMID:21501972; http://dx.doi.org/10.1016/j.coph.2011.03.004
- Lu H. TLR Agonists for cancer immunotherapy: tipping the balance between the Immune stimulatory and inhibitory effects. Front Immunol 2014; 5:83; PMID:24624132; http://dx.doi.org/10.3389/fimmu.2014.00083
- Sasada T, Komatsu N, Suekane S, Yamada A, Noguchi M, Itoh K. Overcoming the hurdles of randomised clinical trials of therapeutic cancer vaccines. Eur J Cancer 2010; 46:1514-9; PMID:20413296; http://dx.doi.org/10.1016/j.ejca.2010.03.013
- Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 2011; 60:433-42; PMID:21221967; http://dx.doi.org/10.1007/s00262-010-0960-8
- Dang Y, Disis ML. Identification of immunologic biomarkers associated with clinical response after immune-based therapy for cancer. Ann N Y Acad Sci 2009; 1174:81-7; PMID:19769740; http://dx.doi.org/10.1111/j.1749-6632.2009.04937.x
- Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, Fierro MT, Savoia P, Bernengo MG. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a singleinstitution hospital-based observational cohort study. Ann Oncol 2010; 21:409-14; PMID:19622589; http://dx.doi.org/10.1093/annonc/mdp325
- Lasaro MO, Ertl HC. Targeting inhibitory pathways in cancer immunotherapy. Curr Opin Immunol 2010; 22:385-90; PMID:20466529; http://dx.doi.org/10.1016/j.coi.2010.04.005
- Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 2009; 58:823-30; PMID:19198837; http://dx.doi.org/10.1007/s00262-008-0653-8
- Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. The oncologist 2010; 15:969-75; PMID:20798195; http://dx.doi.org/10.1634/theoncologist.2010-0129
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G., et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412-20; PMID:19934295; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624
