Abstract
A live attenuated influenza vaccine (LAIV) was offered during the 2012-13 influenza season in Quebec, Canada, to children aged between 2 and 17 years with chronic medical conditions. Despite the offer, uptake of the vaccine was low. We assessed the perceptions and opinions about seasonal influenza vaccination and LAIV use among vaccine providers who participated in the 2012-13 campaign. More than 70% of them thought that LAIV was safe and effective and more than 90% considered that the vaccine was well-received by parents and healthcare professionals. According to respondents, the most frequent concerns of parents about LAIV were linked to vaccine efficacy. LAIV is well-accepted by vaccine providers involved in influenza vaccination clinics, but more information about the vaccine and the recommendations for its use are needed to increase vaccine uptake.
Introduction
In Canada, immunization programs are under the responsibility of provinces and territories. In the province of Quebec, influenza vaccination is recommended for people at high risk of serious complications as well as for their contacts.Citation1 The Quebec publicly funded influenza vaccination program targets infants aged 6–23 months; adults aged ≥60 years; people having frequent contact with people at higher risk of complications from infection (e.g. healthcare professionals) and individuals aged ≥2 years with chronic medical conditions (e.g., cardiac and pulmonary disorders, diabetes, immune-compromised conditions, renal disease, asthma, etc.).Citation2
Trivalent inactivated influenza vaccines (TIV), which are administered intramuscularly, are routinely used in Quebec to vaccinate children and adolescents against influenza. However, since 2010, a live attenuated influenza vaccine (LAIV) administered by intranasal spray (FluMist®) has been approved for use in healthy children aged between 2 and 17 years in Canada.Citation1 In 2012, the Quebec Immunization Committee recommended the preferential use of LAIV over TIV for all children aged between 2 and 17 years, including children with underlying chronic medical conditions without immunosuppression.Citation3
In the fall of 2012, the Quebec Ministry of Health bought 100,000 doses of LAIV. Given the limited quantity of doses, only children aged between 2 and 17 years with chronic medical conditions were targeted to receive LAIV and no large promotional activities were made. In the early weeks of the 2012–13 vaccination campaign, due to a lack of demand, the recommendations on LAIV use were expanded to include all children aged between 2 and 17 years targeted by the publicly-funded influenza vaccination program. However, only half of the available doses were administered during the vaccination campaign.
Vaccine providers' recommendations are an important determinant of vaccine acceptance among patients.Citation4-7 For instance, in a review published in 2012, nurses' knowledge and attitudes about influenza vaccine were highly associated with their own vaccine uptake, their intention to recommend the vaccine to their patients and the vaccine uptake of their patients.Citation8 Since LAIV will be used routinely in future seasonal influenza vaccination campaigns, a survey was conducted to explore vaccine providers’ knowledge, attitudes and practices (KAP) regarding seasonal influenza immunization and use of LAIV.
Results
The survey invitation was sent to 427 vaccine providers and 314 of them completed it (76% filled out the questionnaire online and the others completed the paper version). The respondents’ socio demographic and professionals’ characteristics are presented in . Almost all respondents (94%) were nurses, and the majority were working in local community service centers (known by their French acronym CLSCs) (79%). Fifty one percent (51%) of the respondents had more than 20 years of practice.
Table 1. Characteristics of survey participants
Almost 85% of respondents reported having received the seasonal influenza vaccine during the 2012-13 campaign. Main reasons for having been vaccinated were to protect themselves and others (patients or family members). A low perceived vulnerability to influenza and a low perceived severity of the infection were the main reasons for not having received the vaccine (mentioned by the 63 unvaccinated respondents).
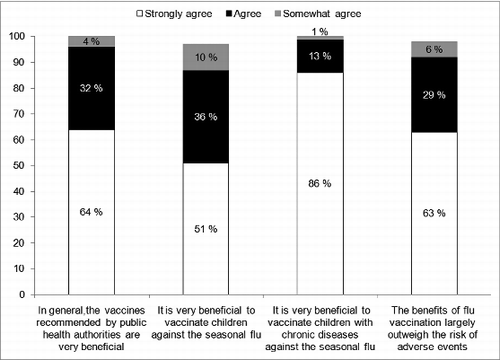
In general, opinions about seasonal influenza vaccination were very positive and more than 90% of respondents agreed with statements included in the survey (). Respondents who received the vaccine for the 2012-13 season were more likely to believe that it was very useful to vaccinate children against seasonal influenza than unvaccinated respondents (99% vs. 92%; P = 0.01).
The majority of respondents agreed that LAIV was effective (34% strongly agreed and 37% agreed) and safe (38% strongly agreed and 39% agreed). Compared to unvaccinated respondents, a higher proportion of respondents who were vaccinated against seasonal influenza strongly agreed with the statements about the efficacy and safety of LAIV (efficacy: 89% vs. 65%, P < 0.0001; safety: 92% vs. 76%, P < 0.0001). When asked to compare LAIV and TIV, the majority of respondents estimated that safety and efficacy of both vaccines were similar (). However, 12% and 20% of the respondents did not know. A higher proportion of respondents self-reported having sufficient knowledge about TIV (50% strongly agreed and 39% agreed) than about LAIV (24% strongly agreed and 37% agreed). Only 7% and 9% of respondents thought that the vaccine had not been well-accepted by parents and other healthcare professionals.
Table 2. Opinions about comparison of the live attenuated seasonal flu vaccine with the trivalent inactivated seasonal influenza vaccine
More than 90% of the respondents reported recommending seasonal influenza vaccination to parents for themselves (89%) or to parents for their child (88%). More vaccinated respondents than unvaccinated ones made those recommendations (100% vs 91%, and 100% vs 93% respectively; P < 0.006). Seventy percent (70%) indicated having recommended LAIV only to children aged between 2 and 17 years with chronic medical conditions whereas 64% of respondents indicated having recommended LAIV to children in contact with people at high risk of developing influenza-related complications. Thirty percent (30%) of respondents indicated having recommended LAIV to all children.
During the vaccination campaign, 71% of respondents reported having used LAIV. Almost all of these respondents indicated that it was easy to vaccinate children with the vaccine (57% strongly agreed). Overall, respondents felt that they had received enough information regarding LAIV (26% strongly agreed and 39% agreed) and were aware of the recommendations regarding its use (37% strongly agreed and 39% agreed). The majority of respondents considered that it was useful to vaccinate with LAIV (48% strongly agreed and 31% agreed). However, the majority of respondents thought the target groups were poorly informed about LAIV (46% strongly agreed and 33% agreed), and less than half of respondents considered LAIV doses have been well-used (15% strongly agreed and 26% agreed).
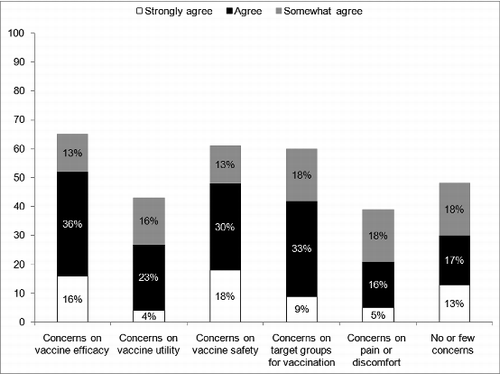
Respondents have also been asked about frequent questions or concerns raised by parents in regard of LAIV (). According to them, the most common concerns and questions raised by parents were regarding vaccine efficacy and vaccine safety. Almost one-third of the respondents mentioned that parents had little or no concerns about LAIV.
Discussion
This survey highlighted the perceptions of vaccine providers in Quebec in regards of LAIV which is preferentially recommended for seasonal influenza immunization of children aged between 2 and 17 years, including those with chronic medical conditions. Findings of this study indicate that vaccine providers considered LAIV to be safe and effective and were supportive of its use. The majority of respondents mentioned having received enough information about recommendations for LAIV and considered having sufficient knowledge about the vaccine. However, the fact that many respondents did not know how to answer some questions indicated that vaccine providers could benefit from additional information. In addition, almost one-third of respondents mentioned having recommended LAIV to all children which is not supported by the actual recommendations.
Over 70% of the respondents reported believing that LAIV was safe and effective. However, only 51% thought that LAIV was as effective as TIV and 20% did not know. The fact that many vaccine providers were unsure about the efficacy of LAIV is worrying as respondents also reported that one of the most frequent concerns of parents was about vaccine efficacy. Although this study did not investigate how vaccine providers handled parental questions or concerns, others studies have shown that providers are one of the most trusted sources of information about the benefits and safety of vaccines and play a critical role in influencing parents’ decision to vaccinate their child.Citation4,9-11 In this study, we only investigated the perspective of vaccine providers about parental concerns for LAIV and other studies have illustrated discrepancies between parental beliefs that may influence immunization behavior, and provider assumptions about those beliefs.Citation12,13
Although the intranasal spray delivery method is believed to be less stressful for young children and easier to administer,Citation14 the mode of administration of LAIV also raised questions and concerns for some parents according to the respondents. Other studies have shown that fear of needles and pain is a frequent reason for parents to decline influenza immunization for their child.Citation15,16 Results of a recent study have shown that parents prefer the nasal spray over the injection because their child's discomfort due to injection or their child's fear of injections.Citation14 However, in 3 other studies, the nasal spray was not preferred by parents over the injection for the immunization of their children.Citation13,17,18 In the light of these results, parents’ views regarding the safety and efficacy of influenza vaccination for children and their preference for different modes of administration should be further explored. In this study, despite the fact that almost all vaccine providers surveyed had very positive attitudes toward influenza vaccination and LAIV, only half of the doses bought for the 2012-13 vaccination campaign were administered. Most respondents considered that the sub-optimal use of LAIV was due to a lack of demand for the vaccine by parents, which might be explained, at least partially, by the absence of informational and promotional activities for this vaccine by the public health authorities. The majority of vaccine providers considered that the target groups were poorly informed about this vaccine. It is also possible that the changes in the recommendations for use of LAIV during the campaign generated some confusion on the ground and decreased vaccine providers’ willingness to recommend the vaccine. In this study, more than half of the respondents mentioned frequent concerns and questions of parents regarding the efficacy and safety of LAIV, which could also be an explanation for the low uptake. Other studies have also shown that new vaccines are usually generating more doubts and hesitancies in the public than vaccines routinely used for a longer time.Citation4,11,19 Results of this study should be interpreted in the light of some limitations. First, although more than 70% of the invited vaccine providers responded, we cannot exclude potential response bias. Respondents were identified by public health professionals at the regional level, and we don't have any information on vaccine providers who did not respond or who were not invited. Almost 80% of respondents were working in public health immunization clinics, and few respondents were from private clinics. However, in Quebec, the organization of mass vaccination campaigns against influenza is under the responsibility of the public health sector. In addition, respondents in this survey had very positive opinions regarding seasonal influenza vaccination. For instance, almost 85% of nurses reported having received the influenza vaccine in this study whereas other Quebec survey's results indicated that around 60% of nurses are vaccinated against influenza.Citation20 Although a social desirability bias cannot be totally excluded, it is encouraging to see that nurses who are actively involved in seasonal influenza vaccination campaigns are strongly supportive of influenza immunization. Health professionals’ knowledge and attitudes about vaccines have previously been shown to be an important determinant of their own vaccine uptake, their intention to recommend the vaccine to their patients and the vaccine uptake of their patients.Citation8,21-25
To conclude, results of this study indicate an overall high acceptability of seasonal influenza vaccination among Quebec vaccine providers. Despite the fact that vaccine providers held positive attitudes toward LAIV, the low uptake for LAIV observed in Quebec despite free vaccines and preferential recommendation to use LAIV over TIV warrants further studies. Our findings indicate that vaccine providers’ level of knowledge regarding LAIV safety and efficacy could be improved. The absence of promotional activities as well as the change in the recommendations for LAIV in the middle of the campaign are potential explanations for the low uptake. Further studies will be needed to assess parents’ opinions regarding seasonal vaccination and LAIV as well as to explore how vaccine providers address parents’ concerns and questions about this vaccine.
To ensure an optimal use of LAIV, public health authorities will have to remind to parents about the importance of seasonal influenza vaccination for children with chronic medical conditions and inform vaccine providers about the safety, efficacy and recommendations about LAIV.
Material and Methods
Healthcare providers who were actively involved in the 2012-13 seasonal influenza vaccination campaign in 17 of the 18 regions of Quebec (one northern region was excluded) were invited to participate in the survey. Potential participants were identified by public health professionals at the regional level.
Between March 15 and April 3 2013, a link to an online questionnaire was sent by email to nurses and physicians using “Survey Monkey” software. A paper version of the questionnaire was also available upon request. Responses were collected anonymously. The survey instrument was developed based on previous studies and was validated by a panel of experts.Citation26,27 The questionnaire included 21 questions to collect information about KAP regarding seasonal influenza vaccination in general and use of LAIV, in particular barriers and enabling factors regarding its use; seasonal influenza vaccine status and socio demographic and professional characteristics of the respondent. Most questions were close-ended and could be answered on a 6-point Likert scale (ranging from “strongly agree” to “strongly disagree”). The survey instrument is available upon request.
Descriptive statistics were generated for all variables. Univariate analysis was performed. Comparison between responses according to professional and demographic characteristics (respondents’ vaccine status against seasonal influenza, number of years of practice, primary specialization, etc.) were done using χ2 or Fisher exact tests as appropriate. Knowledge, attitudes and practices about LAIV were analyzed in 3 categories: positive opinions (responses “strongly agree” and “agree”); negative opinions (responses “strongly disagree” and “disagree”) and neutral opinions (responses “somewhat agree” and “somewhat disagree”). Missing responses were excluded from the analysis. For univariate analysis, a probability level of P < 0.05 was considered statistically significant. Data were analyzed with SAS statistical software (version 9.3).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We would like to thank the Quebec ministry of Health and Social Services for the study funding.
References
- National Advisory Comittee on Immunization. Statement on seasonal influenza vaccine for 2014-2015. Public Health Agency of Canada, 2014:68.
- Ministère de la Santé et des Services sociaux. Protocole d'immunisation du Québec. Québec: Ministère de la Santé et des Services sociaux, 2013:485.
- Boulianne N, Quach C. Utilisation du vaccin à virus vivant atténué contre l’influenza (VVAI), Flumist® chez les enfants et les adolescents âgés de 2-17 ans avec maladies chroniques. Québec: Institut national de santé publique du Québec, 2012:5.
- Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics 2008; 122:718-25; PMID:18829793; http://dx.doi.org/10.1542/peds.2007-0538
- Ridda I, Motbey C, Lam L, Lindley IR, McIntyre PB, Macintyre CR. Factors associated with pneumococcal immunisation among hospitalised elderly persons: a survey of patient's perception, attitude, and knowledge. Vaccine 2008; 26:234-40; PMID:18054818; http://dx.doi.org/10.1016/j.vaccine.2007.10.067
- Stefanoff P, Mamelund SE, Robinson M, Netterlid E, Tuells J, Bergsaker MA, Heijbel H, Yarwood J. Tracking parental attitudes on vaccination across European countries: the vaccine safety, attitudes, training and communication project (VACSATC). Vaccine 2010; 28:5731-7; PMID:20558250; http://dx.doi.org/10.1016/j.vaccine.2010.06.009
- Schmitt HJ, Booy R, Aston R, Van Damme P, Schumacher RF, Campins M, Rodrigo C, Heikkinen T, Weil-Olivier C, Finn A, et al. How to optimise the coverage rate of infant and adult immunisations in Europe. BMC Med 2007; 5:11; PMID:17535430; http://dx.doi.org/10.1186/1741-7015-5-11
- Zhang J, While AE, Norman IJ. Knowledge and attitudes regarding influenza vaccination among nurses: a research review. Vaccine 2010; 28:7207-14; PMID:20804802; http://dx.doi.org/10.1016/j.vaccine.2010.08.065
- Mergler M, Omer S, Pan W, Navar-Boggan A, Orenstein W, Marcuse E, Taylor J, deHart M, Carter T, Damico A, et al. Association of vaccine-related attitudes and beliefs between parents and health care providers. Vaccine 2013; 31:4591-5; PMID:23896424; http://dx.doi.org/10.1016/j.vaccine.2013.07.039
- Smith P, Kennedy A, Wooten K, Gust D, Pickering L. Association between health care providers' influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics 2006; 118:e1287-e92; PMID:17079529; http://dx.doi.org/10.1542/peds.2006-0923
- Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics 2010; 125:654-9
- Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions–Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med 1995; 149:845-9; PMID:7633536; http://dx.doi.org/10.1001/archpedi.1995.02170210019003
- Healy C, Montesinos D, Middleman A. Parent and provider perspective on immunization: are providers overestimating parental concerns? Vaccine 2014; 32:579-84; PMID:24315883; http://dx.doi.org/10.1016/j.vaccine.2013.11.076
- Flood EM, Ryan KJ, Rousculp MD, Beusterien KM, Divino VM, Block SL, Hall MC, Mahadevia PJ. Parent preferences for pediatric influenza vaccine attributes. Clin Pediatr (Phila) 2011; 50:338-47; PMID:21196417; http://dx.doi.org/10.1177/0009922810391247
- Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, Sovran J, Stephens D, Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012; 30:4807-12; PMID:22617633; http://dx.doi.org/10.1016/j.vaccine.2012.05.011
- Bhat-Schelbert K, Lin CJ, Matambanadzo A, Hannibal K, Nowalk MP, Zimmerman RK. Barriers to and facilitators of child influenza vaccine—perspectives from parents, teens, marketing and healthcare professionals. Vaccine 2012; 30:2448-52; PMID:22300721; http://dx.doi.org/10.1016/j.vaccine.2012.01.049
- Kaaijk P, Kleijne D, Knol M, Harmsen I, Ophorst O, Rots N. Parents' attitude toward multiple vaccinations at a single visit alternative delivery methods. Hum Vaccin Immunother 2014; 10:2483-9; PMID:25424960; http://dx.doi.org/10.4161/hv.29361
- Shono A, Kondo M. Parents' preferences for seasonal influenza vaccine for their children in Japan. Vaccine 2014; 32:50-71-5076; http://dx.doi.org/10.1016/j.vaccine.2014.07.002
- Bedford H, Lansley M. More vaccines for children? Parents' views. Vaccine 2007; 25:7818-23; PMID:17920170; http://dx.doi.org/10.1016/j.vaccine.2007.08.057
- Dubé E, Defay F, Kiely M, Guay M, Boulianne N, Sauvageau C, Landry M, Markovski F, Turmel B, Hudon N, et al. Enquête québécoise sur la vaccination contre la grippe saisonnière, le pneumocoque et la rougeole en 2012. Québec: Institut national de santé publique du Québec et ministère de la Santé et des Services sociaux, 2013:137.
- Clark SJ, Cowan AE, Wortley PM. Influenza vaccination attitudes and practices among US registered nurses. Am J Infect Control 2009; 37:551-6; PMID:19556035; http://dx.doi.org/10.1016/j.ajic.2009.02.012
- Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals–a review of studies on attitudes and predictors. Vaccine 2009; 27:3935-44; PMID:19467744; http://dx.doi.org/10.1016/j.vaccine.2009.03.056
- Posfay-Barbe KM, Heininger U, Aebi C, Desgrandchamps D, Vaudaux B, Siegrist CA. How do physicians immunize their own children? Differences among pediatricians and nonpediatricians. Pediatrics 2005; 116:e623-33; PMID:16263976; http://dx.doi.org/10.1542/peds.2005-0885
- Katz-Sidlow RJ, Sidlow, R. A look at the pediatrician as parent: experiences with the introduction of varicella vaccines. Clin Pediatr (Phila) 2003; 42:635-40; PMID:14552523; http://dx.doi.org/10.1177/000992280304200710
- Connors CM, Miller NC, Krause VL. Universal hepatitis B vaccination: hospital factors influencing first- dose uptake for neonates in Darwin. Aust N Z J Public Health 1998; 22:143-5; PMID:9599867; http://dx.doi.org/10.1111/j.1467-842X.1998.tb01159.x
- Dube E, Gilca V, Sauvageau C, Bettinger JA, Boucher FD, McNeil S, Gemmill I, Lavoie F, Ouakki M, Boulianne N. Clinicians' opinions on new vaccination programs implementation. Vaccine 2012; 30:4632-7; PMID:22580354; http://dx.doi.org/10.1016/j.vaccine.2012.04.100
- Kiely M, Defay F, Sauvageau C, Gilca V, Guay M, Boulianne N, Dubé E. Grippes A(H1N1) et saisonnière: bilan de campagnes. Perspect Infirm 2012; 9:61-4