Abstract
NmenB vaccine (4CMenB) is now available, but studies on the cost-effectiveness of vaccine introduction in a country outbreak situation are lacking. The aim of this study was to evaluate the cost-effectiveness of 4CMenB in the context of a hypothetical epidemic outbreak in Chile. We analyzed the direct and indirect costs of acute disease, sequelae and death for each case of meningococcal disease (MD) based on information obtained during the latest NmenB outbreak in Santiago, Chile, occurring between 1993–1999, with an incidence of 5.9/100,000 inhabitants and a mortality of 7.3%. We analyzed the cost of a mass vaccination campaign, considering one dose of 4CMenB for population between 12 months and 25 y of age and 3 doses for infants. Cost-effectiveness analysis was based on 80% and 92% 4CMenB immunogenicity for individual's bellow and over 12 months respectively. Sensitivity analysis was applied to different vaccine costs. Results: The total cost of the epidemic was USD $59,967,351, considering individual cost of each acute case (USD$2,685), sequelae (USD$2,374) and death (USD $408,086). In Chile, the 4CMenB mass vaccination strategy would avoid 215 cases, 61 sequelae, and 16 deaths per year. The strategy would be cost-effective at a vaccine dose cost ≤ of USD$18. Conclusions: Implementation of a mass vaccination campaign to control a hypothetical NmenB outbreak in Chile would be cost-effective at a vaccine cost per dose ≤ of USD$18. This is the first report of a cost-effectiveness analysis for use of 4CMenB as a single intervention strategy to control an epidemic outbreak of NmenB.
Introduction
Meningococcal disease (MD) is a major cause of sepsis and meningitis in children and young adultsCitation1 with incidences ranging from 0.5 to 1,000 per 100,000 inhabitants worldwide.Citation2-4 Five Neisseria meningitidis (Nmen) serogroups - A, B, C, Y and W – account for the majority of cases, with a variable distribution among different regions and time periods.Citation3,4
In Latin America, serogroups B and C have been responsible for 80 to 90% of MD cases in the last 2 decadesCitation5 with an increase in serogroup W and Y during the last 3 y in the south cone.Citation6,7 The incidence of MD currently varies from 0.1 to 2 cases per 100,000 inhabitants among the different countries in the region with mortality rates ranging between 10 and 27%.Citation8
The development of vaccines against serogroup B has been difficult because of the similarity between bacterial and human neural tissue polysaccharides.Citation9,10 Using reverse vaccinology, protein based vaccines have been developed.Citation11-13 A multicomponent serogroup B meningococcal vaccine (4CMenB) has been developed including 4 recombinant proteins in addition to an outer membrane vesicle (OMV).Citation14,15 Vesikari et al.Citation11 demonstrated 84 to 100% protective immunogenicity of this vaccine in infants receiving immunization at 2, 4, and 6 months of age. The tolerability profile was acceptable when administered together with routine childhood vaccines. Santolaya et al.Citation12 assessed the immunogenicity of 4CMenB in different schedules of one, 2 or 3 doses in adolescents. The study showed a protective immune response in 92 to 97% adolescents after one dose, and 99 to 100% after 2 or 3 doses.
Serogroup B MD occurs in epidemic outbreaks with inter-epidemic periods of varying lengths differing to serogroup C which occurs in outbreaks and short waves and serogroup A which is associated with major cyclical epidemics in sub-Saharan countries.Citation9,16 The decision to incorporate a meningococcal vaccine to National Immunizations Programs should be determined by local epidemiology, disease impact and cost effectiveness evaluations of this measure. In low and middle-income countries, with limited resources, the decision to implement theses vaccines is prioritized for epidemic situations.
To date there are no models to study the cost-effectiveness of NmenB vaccine for the control of a MD outbreak. In order to develop these models, precise knowledge on the direct and indirect costs associated with management of acute illness and sequelae as well as the cost of each death for society is required.
Epidemiological and clinical information obtained from an outbreak can serve as a basis to generate a model of cost-effectiveness for implementation of NmenB vaccine strategy. Chile is a country with a population of near 16 million inhabitants with a gross domestic product (GDP) per capita of USD$ 15,636 (World Bank 2012). The last NMenB epidemic outbreak period in Chile occurred between 1993–1999 in Santiago, the main city of the country.Citation17,18 In 1993, rates for serogroup B MD in Santiago exceed the epidemic threshold of 5 per 100.000 inhabitants, with an incidence rate of 5.9 per 100,000 inhabitants (60% of the cases occurring among children under 5 y of age). The proportion of individuals suffering sequelae from MD in 1993 was 28.3% after one year of follow up and the lethality was 7.3%.Citation17,19,20 At that time, the Ministry of Health considered the outer membrane protein (OMP) meningococcal vaccine, but data suggested that this vaccine did not confer protection during a heterologous strain outbreak.Citation17
The aim of this study was to determine the cost-effectiveness of using 4CMenB in a one-time intervention strategy for the control of a hypothetical epidemic outbreak in Chile, a middle-income country.
Results
Costs of meningococcal disease
Direct, indirect and total acute illness costs can be found in Table 1. The mean direct cost at present value for management of an acute case of MD in public hospitals was USD $1,074. Hospital bed days accounted for most of the cost, followed by immediate post hospital follow-up, and by cost of laboratory tests. Mean indirect costs associated with acute illness was USD $1,611 adding to a total cost related to acute illness of USD $2,685. Details of acute illness costs and frequencies of services observed during MD in Public Hospitals are found in .
Table 1. Meningococcal Disease: Direct and indirect costs of acute illness, sequelae and indirect costs of death
Table 2. Frequencies and costs of services observed during MD in Public Hospitals, Santiago, Chile (1993)
Direct, indirect, and total costs of MD sequelae
The mean overall cost for management of MD sequelae was USD $2,374. The sequelae with the highest proportional cost was mild and moderate intellectual disability adding USD $ 1,389 per case. There were no case of severe mental retardation or profound intellectual disability recorded and thus for they were not included in the model ( and details in ).
Table 3. Number, frequencies and proportional costs of MD sequelaes observed in Santiago, Chile during the first epidemic year (1993)
Indirect costs of death
Productive years lost due to mortality multiplied by the average annual salary accounted for a mean indirect cost due to early MD associated death of USD $408,086 per case ().
Cost of the MD epidemic outbreak
The total estimated cost of the NMenB epidemic outbreak occurring between 1993 and 1999 was USD $59.967.351 calculated at present value with a 6% of rate discount ().
Table 4. Cost of Meningococcal Disease Epidemic Outbreak 1993–1999 Santiago, Chile
Intervention costs
Mass vaccination campaign costs
Logistics, advertising and cold chain associated costs for the proposed vaccination strategy was USD $ 355,568, USD $ 2,620,830 and USD $ 42,775 respectively. These were considered fixed costs independent of unit cost of the vaccine.
Vaccination campaign effectiveness analysis
The proposed vaccination strategy would theoretically prevented 215 cases yearly of MD, 60.9 sequelae and 15.7 deaths ( and ). The total averted costs (acute cases, sequelae and death) was estimated in USD $ 41,995,724. The net cost of the vaccination campaign include the cost of the vaccination strategy considering different values of the vaccine plus the cost of the cases derived from non-prevented MD cases, sequelae and death (USD $ 6,493,162) ().
Table 5. MD Outbreak Vaccination Theoretical Model: 4CmenB 3 doses in infants (<12 mo) and one dose given to population between 12 month and 25 y of age in Santiago, Chile
Table 6. Effectiveness of 4CmenB vaccination campaign for population under 25 y of age in a hypothetical NmenB MD outbreak in Santiago, Chile
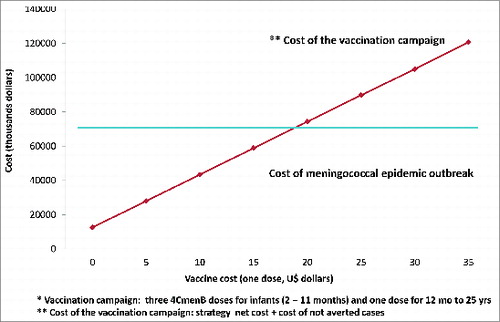
Table 7. Costs of the vaccination campaign with hypothetical costs of the vaccine compared to the cost of MD epidemic outbreak
We considered an additional period of 12 months of delay in the total cost of the outbreak (USD 9,900,168), in this scenario, the total cost of the MD outbreak increase from USD $59,967,351 to USD $69,867,519.
For the sensitivity analysis we compared the net cost of vaccination campaing (mass vaccination campaign plus the costs of not adverted cases, sequelae and death considering the hypothetical effectiveness of the vaccine) with costs of the MD epidemic outbreak plus 12 months of delay, resulting in a cost effective vaccination campaign at a vaccine cost of equal or less than 18 USD ( and ).
Discussion
This study provides an estimate of the cost-effectiveness of a vaccination campaign using the novel 4CMenB vaccine for control of a new hypothetical outbreak of MD from NmenB in a middle-income country. A strategy considering 3 vaccine doses in infants younger than 1 y and one dose for individuals aged 12 months to 25 y could avert 215 acute MD cases, 61 sequelae and 16 deaths. 4CMenB mass vaccination in this hypothetical epidemic situation resulted cost-effective at a vaccine cost per dose of USD$18 or less.
The decision to vaccinate the population in an epidemic outbreak depends on factors such as the impact of the disease, the number of cases averted, the mortality rate, the availability of a vaccine against predominant serogroup and costs.Citation21-23
A novel vaccine for NmenB, approved by the European Medicines Agency (EMA) in January 2013Citation24 has become available. In the UK the incorporation of this vaccine to the national immunization program was evaluated. A theoretical model of 4CMenB vaccine cost-effectiveness was performed in infants under one year, describing that the vaccine was cost-effective with a value of less than USD$ 13.7.Citation25 Pouwels et al.,Citation26 showed that vaccination with 4CMenB for Dutch infants in a primary 3-dose scheme and a booster dose at 11 months, was not cost-effective with the current incidence of MD in the country of less than 1 per 100,000 at a vaccine cost above USD $50 per dose. Preliminary data of the costs of MD during endemic periods in some Latin American countries have been reported only in small-circumscribed outbreaks. The total cost (direct and indirect) of the acute illness in one study conducted in Chile was USD $ 3,785, in Colombia USD $ 5,108 and Panama USD $ 5,327.Citation27
Vaccination strategies during an outbreak have been widely studied with vaccines in different regions of the world, especially in sub-Saharan Africa.Citation28,29 This strategy proved to be suboptimal because of lack of capacity for appropriate surveillance. However, in higher-income countries with better surveillance systems, the use of strategies of vaccination against serogroup C outbreaks, such as New Zealand,Citation30 United Kingdom,Citation31 CanadaCitation32 and BrazilCitation33 have shown to be effective for the control of MD cases.
After mass vaccination with the monovalent conjugate vaccine C in Canada in 2002, there was an increase in the proportion of NmenB cases. In recent years, serogroup B has been responsible for 88% of all cases of reported in surveillance laboratories in Canada.Citation32 It is important to note, that changes in the predominant serogroup of Nmen (capsular switch) can be determined by vaccination programsCitation34,35 changing immunoprevenible serogroups to another not immunoprevenible serogroups.
Recently in Chile, an increase of MD cases by serogroup W was reported. The Ministry of Health started a vaccination campaign for all children from 9 month to 5 y of age with the tetravalent conjugate vaccine ACWY in the country, without a previous cost-effectiveness study because of the rapid emergence of the outbreak associated with high mortality.Citation36 Since the beginning of vaccination strategy there have been no reported cases of MD in the vaccinated group. Serogroup B is currently the second serogroup in frequency in our country and it seems interesting to known the costs of MD in case that its reemerge, knowing the variable epidemiology of Nmen in time.
An appropriate estimate of disease impact is relevant for the adoption of better decisions once an epidemic outbreak occurs, including the cost-effectiveness of a vaccination strategy.
In most developing countries health care costs are underestimated, in which private health system has significantly higher costs compared to public health system. In our study, when we estimated the cost of a case of MD in the private health system, a value of USD $ 11,958 was obtained.Citation37,38
The costs of sequelae also appear low in our model. This was calculated by the frequency of occurrence of each particular sequelae. The most common sequelae detected were those with lower cost for treatment and follow - up (borderline and mild intellectual disability). There were no cases of severe mental retardation or profound intellectual disability and for this reason it was not included in the model. As we showed in the , severe cases of sequelae were as follows: intellectual disability (n = 0), hearing loss (n = 1), spastic paresis (n = 0), paralysis (n = 0), amputation (n = 1). The case of amputation did not present functional limitations since only the second and third toe were involved. In our study the frequency of the calculations were consistent with those observed in other countries.Citation39
We considered a vaccination strategy with 3 doses of 4CmenB in infants younger than 12 months and one dose in the group aged 12 months to 25 years, because there is no data with only one dose in infants less than 1 y of age. First data reported in infants suggested that this vaccine take over 73 to 87% of the NmenB strains in 5 European countries.Citation35,40 That's why we used an immunogenicity theorical value of 80%. In adolescents (11–17 years) protective titers are observed (over 90%) with one vaccine dose,Citation12 so this immunogenicity was extrapolated in this study to subjects between 1–25 y
One limitation of our study was that we have to estimate the vaccine effectiveness from immunogenicity data, because the lack of clinical studies on NmenB vaccine efficacy. We used a theoretical model based on data collected from a real outbreak of NmenB MD, determining MD cases treated in the public hospital network of Santiago, assuming that the characteristics of a new outbreak would be similar. This model could be extrapolated to other Latin American and middle-income countries with similar health care and living costs but as mentioned previously, in developing countries the health care costs are underestimated compared to the real costs of the health services. At higher cost of the disease could show a better cost-effectiveness of the intervention against epidemic outbreak, and make vaccination look more favorable. Another limitation of our model was that at that time we did not consider the costs of adverse reactions of the vaccine and the waning immunity years post-vaccination. We consider the same immunogenicity of the vaccine for the entire period. Recently, Santolaya et al.Citation41 reported the persistence of antibodies in adolescents 18–24 months after immunization with one, 2, or 3 doses of 4CMenB meningococcal serogroup B vaccine. They showed that after 18–24 months, 62–73% of subjects receiving one dose had titters ≥ 4 against the 3 antigens, 77–94% after 2 doses and 86–97% after 3 doses. To our knowledge, there is not available information in infants and the impact of 4CmenB in control of massive outbreaks. We also considered that our model had the limitation to assess the impact of disability only in a 5 y period, nevertheless death costs were calculated as years of productive life lost due to mortality multiplied by the average salary, considering the life expectancy in Chile.
The immunogenicity of 4CMenB could be underestimated because the clinical effectiveness of the vaccination and herd immunity effect is unknown.Citation42,43 Evidence from large-scale vaccination programs in Canada, Spain and the UKCitation43-45 suggest that there may be benefits on the herd immunity associated with meningococcal vaccination, at least in some populations,Citation46 situation that theoretically could occur with the NmenB vaccine improving its cost effectiveness.
In conclusion, 4CMenB mass vaccination in a hypothetical epidemic situation to control an NmenB outbreak in Chile resulted cost-effective when the cost per dose were USD$18 or less. This report is the first approach to 4CMenB cost-effectiveness in the context of an epidemic outbreak of serogroup B. The present study might become of interest to countries where Nmen B is predominant since epidemic outbreaks can occur due to the cyclical nature of MD.
Methods
To assess the cost-effectiveness of 4CMenB we extrapolated data from the last NmenB epidemic outbreak occurred in Chile between 1993–1999. Data was obtained from the Epidemiology and Surveillance Division of the Ministry of Health, Chile; which collects all cases of MD reported in our country (MD is a mandatory report illness). We selected the total notified cases per year of MD in Santiago, between January 1st, 1993 and December 31st, 1999. The case definition was a MD -like syndrome plus NmenB isolation from cerebrospinal fluid (CSF), and/or capsular antigen positivity in CSF, and/or gram negative diplococci in CSF, and/or ≥ 100 leukocytes/mm3 CSF associated with disseminated petechiae or purpuric eruptions. A non-meningitis MD case was defined when Nmen strain was isolated from a normally sterile body sites (blood, synovial fluid or skin lesions) or the individual had symptoms consistent with bacterial sepsis including purpuric eruptions and/or a Waterhouse-Friderichsen syndrome in the absence of a meningeal syndrome.
To assess the cost-effectiveness model we considered 3 points: 1. the costs of MD (direct and indirect costs of the acute illness and sequelae, indirect costs of deaths), 2. the costs of the epidemic outbreak and 3. the intervention costs (mass vaccination campaign and the hypothetical effectiveness of the vaccine) and the cost-effectiveness analysis.
All costs were estimated in local currency values and later converted into USD values using the value of the dollar at the time of analysis (USD $1 = 480 CLP).Citation47 We considered an annual discount rate of 6% (corresponding to the inflation rate during the year 1993) and the general consumer price index from Chile to adjust all costs into 2013 US dollars equivalent.
Costs of meningococcal disease
We considered direct, indirect and total costs of the acute illness; direct, indirect and total costs of sequelae, and the indirect costs of deaths.
Direct, indirect and total cost of acute illness
Eigthy % of the MD cases was treated in the public hospital network of Santiago during the NmenB epidemic outbreak and data were available for cost evaluation during the first epidemic year (1993). The total costs of health services considered in each MD case of the 1993 cohort were calculated. These costs were applied to the cases detected by surveillance in the following years, 1994 to 1999. The costs of the acute MD from other years were available, but did not differ from that described in the cohort of 1993.
For calculations of direct costs we included the costs of consultation prior to hospitalization, treatment-related expenses, hospital bed days, drugs administered, laboratory tests, and contact chemoprophylaxis. We used the average costs provided by the Chilean public health care system for each item specifically adjusted to each individual case (). The average fee established by the National Health Fund (FONASA) according to the Institutional Assistance Regulations (MAI 2013) multiplied the total number of events per item per patient.
Indirect costs were calculated for 2 age groups: ≤17 y and ≥ 18 y as 18 y is the age of legal adulthood. If the patient was ≤17 y we calculated transportation expenditures of the patient and costs associated with transportation and work absenteeism of the caregiver. If the patient was ≥18 y we considered the transportation expenditures and the cost of absenteeism of the patient. Public transportation in Santiago averages USD $1.28 per ticket and the loss of one workday is equivalent to USD $55.6 (based on Chile per capita income, World Bank March 2013).
Direct, indirect and total costs associated with sequelae from MD
We defined sequelae from MD as one or more of the following: intellectual disability with intellectual coefficient (IQ) <80 (borderline 79–70, mild 69–55, moderate 54–40, severe 39–25 and profound <25); unilateral or bilateral hearing loss of ≥30 dB; paralysis; spastic paresis; seizures; and amputation. The frequency of MD sequelae was obtained from the 1993 cohort and applied to the cases detected by surveillance in the following years, 1994 to 1999.Citation17 Patients analyzed during 1993 were asked for an informed oral consent and the total frequency of sequelae reported in 1993 was 28.3%. Seventeen % had some degree of intellectual disability, 7% any level of hearing loss, 1.5% paresis and/or spasticity, 1.2% seizures and 1.2% amputation. Based on these data the frequencies of sequelae for the full outbreak period were estimated.Citation17
For direct costs of MD sequelae estimation, treatment and follow up indications from specialists (neurologists, psychologists, otolaryngologists, and orthopedic physicians) were collected.Citation17 They defined a therapy guideline for a 5-years follow up after the acute illness. Cost assessment was done based on assistance fees established by FONASA according to MAI 2013. The proportional costs of each sequelae were estimated according to its frequency of occurrence ().
The indirect costs of MD sequelae correspond to patient and caregiver transportation expenses, together with absence from work costs (either caregiver or the patient). We performed a 5-year post-acute illness projection of these costs using an estimated number of visits to the specialists. Cases were divided into 2 age groups: ≤17 y and ≥18 y
Indirect costs of death
The observed mortality was 7.3%. Indirect costs of death were calculated as years of productive life lost due to mortality multiplied by the average annual salary in Chile (USD$ 14,394)) (Per Capita Income World Bank 2013). We considered a life expectancy in Chile of 82.2 y for women and 76 y for men (National Institute of Statistics, Population Estimates and Projections, Total Country, 2004) and a retirement age of 60 y in women and 65 in men.
Total costs of the MD epidemic outbreak
Costs of the epidemic outbreak were determined by total number of MD cases per year (1993 to 1999) multiplied by the direct and indirect costs of acute illness, sequelae, and death. Annual discount rate of 6% was used to calculate net present value (year 2013).
Intervention costs
The intervention costs considered a mass vaccination campaign and the hypothetical effectiveness of the vaccine.
Mass vaccination campaign
Costs of the mass vaccination campaign consist of the following: 3 doses at 2, 4 and 6 month of age of 4CMenB vaccine for children under 12 months and one dose for subjects aged 12 months to 25 y Costs of vaccine administration include logistics costs such as transportation, training and snacks; advertising costs (radio, television, posters, meetings, scientific societies) and the cost of cold chain for vaccine preservation. The baseline cost of the vaccination campaign varied according to the unit cost of the vaccine.
Vaccination coverage was estimated based on previous vaccination campaigns implemented in Chile. A one dose vaccination campaign against rubella and measles achieved a coverage of 92%, 95%, and 92% in the age groups 2 months to 2 years, 2 to 16 years, and 16 y and older, respectively.
The total population susceptible for vaccination (from 2 months to 25 y of age) living in Santiago was calculated to be 2.643.388 for the year 2013 using population projections of the National Institute of Statistics. The population between 2 and 11 months was 82.298 and between 1 and 25 years, 2.561.090.
Vaccine effectiveness
The effectiveness of the vaccine was extrapolated from 4CMenB immunogenicity data reported by Vesikari et al.Citation11 in infants and Santolaya et al.Citation12 in healthy adolescents. We used the lowest immunogenicity observed after 3 doses of vaccine in infants (80%)Citation11 and one dose of the vaccine in adolescents (92%).Citation12
Cost-effectiveness analysis
The effectiveness of the vaccination campaign was calculated comparing the number of averted MD cases, MD sequelae, and MD deaths per year to a scenario of no intervention. Expected cases as result of no intervention were calculated based on the observed cases rates per age group registered during the first year of the epidemic outbreak (1993). Costs of the implementation of the vaccination campaign were compared to the costs of non-intervention. The net cost of the vaccination campaign consists of the cost of the intervention (vaccination) plus costs derived from non- prevented MD cases, sequelae and death according to the above mentioned vaccine effectiveness estimate.
Sensitivity analysis was applied to different hypothetical costs of the vaccine. We compared the net cost of vaccination campaing with the costs of MD epidemic outbreak considering an additional year of delay. The assumed costs in this analysis were 0 to 35 USD per vaccine dose, using 3 doses in infants below 1 y and 1 dose in the olders. For the sensitivity analysis we assume a reasonable delay of 12 months from recognition of the outbreak and the start of the vaccination (time to recognize an outbreak, decide to vaccinate the population, obtain vaccine, and vaccinate). The number of cases, sequelae and deaths of this theoretical year of delay was calculated based on the observed cases rates per age group registered during the first year of the epidemic outbreak ().
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Miguel O´Ryan (from the Program of Microbiology, Faculty of Medicine, University of Chile) for the review of the manuscript and enlightening suggestions.
References
- Stephens D, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007; 369:2196-210; PMID:17604802; http://dx.doi.org/10.1016/S0140-6736(07)61016-2
- Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines 2010; 9(3): 285-98; PMID:20218857; http://dx.doi.org/10.1586/erv.10.3
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27(Suppl 2):B51-63; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063
- Halperin S, Bettinger J, Greenwood B, Harrison L, Jelfs J, Ladhani S, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30:B26-36; PMID:22178525; http://dx.doi.org/10.1016/j.vaccine.2011.12.032
- Pan American Health Organization. Informe Regional de SIREVA II: Datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasores, 2010.OPS. Documentos técnicos, Tecnologías Esenciales de Salud 2009. Washington, DC 2011: Pan American Health Organization, Available at: http://new.paho.org/hq/index.php?option=com_content&task=blogcategory&id=3609&itemid=3953;2011. Accessed 12.10.2012.
- Alerta Epidemiológica Meningitis meningocócica(Fiebre cerebroespinal) 24 de abril 2012 http://new.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=17445&Itemid=/ acceded 10.04.2012.
- López E, Debbag R. Meningococcal disease: always present. Serogroup changes in the Southern Cone. Rev Chilena Infectol 2012; 29(6):587-94; PMID:23412025; http://dx.doi.org/10.4067/S0716-10182012000700001
- Safadi MAP, Cintra OAL. Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurological Research 2010; 32: 263-71; PMID:20406604; http://dx.doi.org/10.1179/016164110X12644252260754
- Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infectious Disease 2009; 9(7):418-27; PMID:19555901; http://dx.doi.org/10.1016/S1473-3099(09)70132-X
- Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol 1987; 138:4402-07; PMID:3108388
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A; EU Meningococcal B Infant Vaccine Study group. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomized trials. Lancet 2013; 381(9869):825-35; PMID:23324563; http://dx.doi.org/10.1016/S0140-6736(12)61961-8
- Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile. Lancet 2012; 379:617-24; PMID:22260988; http://dx.doi.org/10.1016/S0140-6736(11)61713-3
- Kimura A, Toneatto D, Kleinschmidt A, Wang H, Dull P. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol 2011; 18(3):483-86; PMID:21177912; http://dx.doi.org/10.1128/CVI.00304-10
- Sadarangani M, Pollard A. Serogroup B meningococcal vaccines – an unfinished story. Lancet Infect Dis 2010; 10:112-24; PMID:20113980; http://dx.doi.org/10.1016/S1473-3099(09)70324-X
- Snape M, Dawson T, Oster P, Evans A, John T, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life. a randomized comparative trial. Pediatr Infect Dis J 2010; 29:e71-9; PMID:20844462; http://dx.doi.org/10.1097/INF.0b013e3181faa6be
- Pollard A. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J 2004; 23(12 Suppl):S274-79; PMID:15597069
- Tappero J, Vega J. Estudio colaborativo para el estudio de secuelas de enfermedad meningocócica grupo b. Santiago de Chile 1993. Preliminary results, unpublished.
- Castillo L, Maldonado A, García J, Silva W, Ulloa MT, Valenzuela MT, Bustos R, Valenzuela ME, Gassibe MP. Characterization of Neisseria meningitidis isolated from systemic infections. Chile, 1992-1993. Rev Med Chil 1994; 122(7):760-7; PMID:7732225
- Cruz C, Pavez G, Aguilar E, Grawe L, Cam J, Mendez F, Garcia J, Ruiz S, Vicent P, Canepa I, et al. Serotype-specific outbreak of group B meningococcal disease in Iquique, Chile. Epidemiology and Infection 1990; 105:119-26; PMID:2116973; http://dx.doi.org/10.1017/S0950268800047713
- Tappero J, Lagos R, Maldonado A, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Høiby EA, Holst J, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 1999; 281(16):1520-27; PMID:10227322; http://dx.doi.org/10.1001/jama.281.16.1520
- Snape M, Pollard A. The beginning of the end for serogroup B meningococcus? Lancet 2013; 381:785-7; PMID:23324564; http://dx.doi.org/10.1016/S0140-6736(12)62194-1
- Cofre J. Enfermedad meningocócica: consultas recurrentes. Rev Chil Infectol 2012; 29(6):657-62; PMID:23412036; http://dx.doi.org/10.4067/S0716-10182012000700012
- Advisory Committee on Immunization Practices (ACIP). Licensure of meningococcal conjugate vaccine for children aged 2 through 10 years and updated Booster dose guidance for adolescents and other persons at increased risk for meningococcal disease, 2011. MMWR Morb Mortal Wkly Rep 2001; 60:1018-9; PMID:21814165
- Ema.europa.eu/Find medicine/Human medicines/European public assessment reports. (Acceded 06.05.2013).
- Christensen H, Hickman M, Edmunds WJ, Trotter CL. Introducing vaccination against serogroup B meningococcal disease: An economic and mathematical modeling study of potential impact. Vaccine 2013; 31(23):2638-46; PMID:23566946; http://dx.doi.org/10.1016/j.vaccine.2013.03.034
- Pouwels KB, Hak E, van der Ende A, Christensen H, van den Dobbelsteen GP, Postma MJ. Cost-effectiveness of vaccination against meningococcal B among Dutch infants: crucial impact of changes in incidence. Hum Vaccin Immunother 2013; 9(5):1-10. Epub ahead of print; PMID:23442580; http://dx.doi.org/10.4161/hv.23888
- Constenla D. First Regional Meningococcal Symposium 2012, 19-20 March, Buenos Aires, Argentina. http://www.sabin.org/sites/sabin.org/files/resources/MeningoccalProcSpan_final.pdf
- Mohammed I, Onyemelukwe GC, Obineche EN, Gupta N, Oyeyinka GO. Control of epidemic meningococcal meningitis by mass vaccination: II. Persistence of antibody four years after vaccination. J Infect 1984; 9:197-202; PMID:6438245; http://dx.doi.org/10.1016/S0163-4453(84)91468-3
- Bovier PA, Wyss K, Au HJ. A cost-effectiveness analysis of vaccination strategies against N.meningitidis meningitis in West Africa: a theorical modeling analysis. Vaccine 2001; 14(25-26):3420-31
- Galloway Y, Stehr-Green P, McNicholas A, O´Hallahan J. Use of an observational cohort study to estimate the effectiveness of the New Zealand Group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol 2009; 38(2):413-8; PMID:18988650; http://dx.doi.org/10.1093/ije/dyn228
- Campell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine 2009; 27S:B20-9; PMID:19477053; http://dx.doi.org/10.1016/j.vaccine.2009.04.067
- Gilca R, Deceuninck G, Lefebvre B, Tsang R, Amini R, Gilca V, Douville-Fradet M, Markowski F, De Wals P. The changing epidemiology of meningococcal disease in Quebec, Canada, 1991–2011: potential implications of emergence of New Strains. PLoS One 2012; 7(11):e50659; PMID:23209803; http://dx.doi.org/10.1371/journal.pone.0050659
- Ministry of Health. Health surveillance, Brazil. Available at: http://portal.saude.gov.br/portal/saude/area.cfm?id_area=962 (May2008)
- Cano R, García C, Mateo S. Enfermedad meningocócica. situación en España en la temporada 1998-1999. Rev Esp Salud Publica [online] 2000; 74(4). ISSN 1135-5727; http://dx.doi.org/10.1590/S1135-57272000000400007
- English P. Vaccination against meningitis B: is it worth it? Drugs Context 2013:212246; PMID:24432035; http://dx.doi.org/10.7573/dic.212246
- Informe de Resultados de Vigilancia de Laboratorio. Enfermedad Invasora Neisseria meningitidis 2012. Instituto de Salud Pública de Chile. Available at: http://www.ispch.cl/sites/default/files/Informe%20N.m%202012.pdf (accessed 03.06.13)
- Davis K, Misurski D, Miller J, Bell T, Bapat B. Cost of acute hospitalization and post-discharge follow-up care for meningococcal disease in the United States. Hum Vaccines 2011; 7(1):96-101; PMID:21278486; http://dx.doi.org/10.4161/hv.7.1.13692
- Levine OS, Shaffer P, Haddix A, Perkins BA. Cost-effectiveness analysis for routine immunization with a quadrivalent meningococcal polysaccharide (A,C,Y,W135) protein conjugate vaccine in the United States. Presented at the Tenth International Pathogenic Neisseria Conference, Baltimore, MD, September 1996.
- Edmond K, Clark A, Korczak V, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10(5):317-28; PMID:20417414; http://dx.doi.org/10.1016/S1473-3099(10)70048-7
- Donnelly J, Medini D, Giuliani MM, et al. Estimating the potential strain coverage in Europe of a multicomponent vaccine targeting serogroup B meningococci. 11th European Meningococcal Disease Society EMGM; Ljubljana, Slovenia; May 18–20, 2011:17-18; http://emgm.eu/meetings/emgm2011/abstracts.pdf (Accessed 01.03.2013)
- Santolaya ME, O'Ryan M, Valenzuela MT, Prado V, Vergara RF, Muñoz A, Toneatto D, Graña G, Wang H, Dull PM. Persistence of antibodies in adolescents 18-24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine. Hum Vaccin Immunother 2013; 9(11):2304-10; PMID:23811804; http://dx.doi.org/10.4161/hv.25505
- Ramsay M, Andrews N, Trotter C, Kaczmarski E, Miller E. Herd immunity from meningococcal serogroup C conjugated vaccination in England: database analysis. BMJ 2003; 326:365-66; PMID:12586669; http://dx.doi.org/10.1136/bmj.326.7385.365
- Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugated vaccination campaing in England and Wales. BMJ 2002; 324(7341):809; PMID:11934772; http://dx.doi.org/10.1136/bmj.324.7341.809
- De Wals P, Erickson L. Economic analysis of the 1992-1993 mass immunization campaing against serogrup C meningococcal disease in Quebec. Vaccine 2002; 20(21-22):2840-4; PMID:12102036; http://dx.doi.org/10.1016/S0264-410X(02)00161-5
- Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008; 197:737-43; PMID:18271745; http://dx.doi.org/10.1086/527401
- Getsios D, Caro I, El-Haidi W, Caro JJ. Assesing the economics of vaccination for Neisseria meningitidis in industrialized nations: a review and recommendations for further research. Int J Technol Asses Health Care 2004; 20(3):280-8; PMID:15446757; http://dx.doi.org/10.1017/S0266462304001096
- International Monetary Fund, April 2011, http://www.imf.org/external/pubs/ft/weo/2011/01/weodata/index.aspx