Abstract
The effect of hepatitis B immunoglobulin (HBIG) on hepatitis B virus (HBV) DNA load and its protective mechanism are not well understood. Twenty-eight hepatitis B surface antigen (HBsAg)–positive pregnant women and their newborns were assigned to an experimental (n = 12) or control group (n = 16) according to whether they received HBIG during pregnancy. HBV DNA load and markers titer of the mothers and newborns were tested. These markers and HBV DNA load in mothers of the experimental group did not fluctuate significantly and were comparable to the control. In the experimental group, there was a positive correlation between mothers and their newborns with regard to hepatitis B surface antibody titer. Immunohistochemical staining of placenta sections showed that HBsAg-positive areas mainly included trophoblastic cells and villous mesenchymal cells without HBIG colocalization, whereas HBIG-positive areas principally included villous capillary endothelial cells and villous mesenchymal cells. Additionally, compared with the control group, the positive rate and mean density of HBIG in the experimental group were remarkably higher. HBIG deposition was seen in Hofbauer cells. Thus, rather than influencing virus replication, HBIG forms an immune barrier between the mother and fetus to prevent HBV transmission.
Introduction
Wide implementation of vaccination program against hepatitis B virus (HBV) in China has significantly decreased the rate of HBV infectionCitation1 Mother-to-child transmission, including intrauterine transmission and transmission during labor, breast-feeding, and daily contact, has become the major route of HBV infectionCitation2,3 With combined hepatitis vaccination and hepatitis B immunoglobulin (HBIG) treatment of newborns, transmission during delivery and the postpartum period can be efficiently prevented.Citation4-6 However, approximately 5–10% of vertical transmission of HBV remains to be prevented because of failure to protect against intrauterine infection.Citation7-11 which is reported to be the bottleneck for further prevention of vertical transmission of HBV worldwide. Considering the largest population and high prevalence of HBV infection in China, there are about 1 million infants born every year facing high risk of HBV infection, which would be serious economic pressure for their families and the society. It is essential to take every effective measure to prevent mother-to-infant transmission.
Unfortunately, there is no standard strategy in gestational period to prevent mother-to-infant intrauterine transmission. The first approved oral nucleoside analog to inhibit HBV replication, lamivudine, is widely used with considerable efficiency and safety in HBV-infected pregnant women to prevent HBV vertical transmission.Citation9,12 Telbivudine and tenofovir are considered class B drugs by the US Food and Drug Administration for use in pregnant women with HBV infection. The following problems with nucleoside and nucleotide analogs require attention: 1) a risk exists for sudden exacerbation of liver disease after withdrawal, 2) the rate of drug resistance may increase in the future, and 3) the long-term impact on children requires further investigation.
Administration of HBIG constitutes another approach for preventing vertical transmission. In a prospective randomized controlled trial, Xu et al. showed that HBIG can interrupt HBV vertical transmission in both hepatitis B e antigen (HBeAg)–positive and –negative pregnant women.Citation13 Other groups have also confirmed protection with HBIG during pregnancy.Citation8,14 Most recently, a meta-analysis of HBIG efficacy emphasized the importance of HBIG in reducing the risk of intrauterine transmission.Citation15 On the contrary, several other studies showed no influence of HBIG on vertical transmission.Citation16 The route and dose of HBIG administration in the individuals investigated may have contributed to this discrepancy.
For more than 20 years, our group has focused on HBV vertical transmission, and a large amount of our data from clinical trials have demonstrated that HBIG is effective in reducing HBV intrauterine infection.Citation17-19 However, its effects on HBV DNA load and the defense mechanism are still poorly understood. In this prospective study, HBV-infected pregnant women were divided into 2 groups, those who received and those who did not receive HBIG during pregnancy. In the mothers, the changes over time in HBV serum markers (hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), HBeAg, hepatitis B core antibody (HBcAb)), and the DNA load were investigated. Immunohistochemical staining with custom-made antibodies against HBIG revealed both the level and distribution of HBIG in placentas.
Results
Characteristics of the mothers
Eventually, 12 samples from experimental group and 16 from control group with complete information were included for final analysis. Based on the final sample size and positive proportion for each group (12/12, 2/16) the actual power of the study is >0.999. presents the demographic and clinical characteristics of mothers in the experimental and control groups. These characteristics were comparable between the 2 groups (P > 0.05)
Table 1. Comparison of the characteristics of the mothers in the experimental and control groups at baseline
Influence of HBIG injection on the mother and neonate
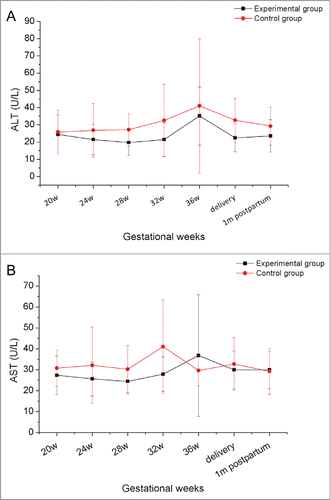
No serious discomfort was reported during pregnancy by the women in our study, whether or not they received HBIG injections. Compared with infants in the control group, no newborns in the experimental group showed differences at birth in terms of weight, length, or Apgar score, and infants grew normally during the first year of follow-up (). Dynamic changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were evaluated during pregnancy in all 28 women. As shown in neither ALT nor AST showed obvious variation, and no significant differences were found between the 2 groups at any time point. All the infants were negative for HBsAg and HBV DNA, in other words, they all were free of HBV infection after 1 y following-up
Table 2. Characteristics of infants from HBV-infected mothers with or without HBIG injection
Effect of HBIG on HBV markers and HBV DNA
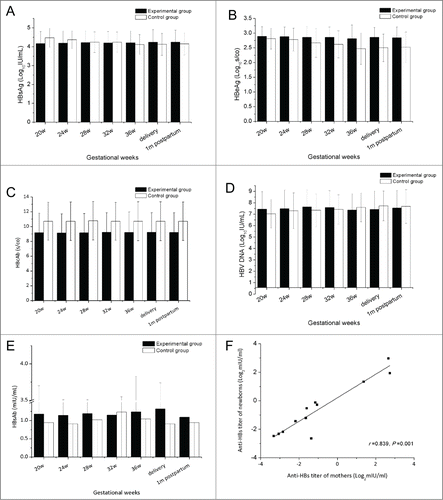
The effect of HBIG on HBV markers and the HBV DNA load during pregnancy was also investigated. HBIG showed no immune regulatory function in previous studiesCitation8, and thus our observation that the titers of HBsAg, HBeAg, and HBcAb were maintained at similar levels during pregnancy was not surprising, and no significant differences between the 2 groups were noted (). The HBV DNA load showed a slight increase after 38 weeks of gestation, consistent with a previous study.Citation20 Similar to the HBV markers, no significant difference in HBV DNA load was observed between the 2 groups ().
Correlation between mothers and newborns regarding HBsAb titer
As a specific immunoglobulin against HBV, HBIG plays a role similar to that of HBsAb. To investigate the influence of HBIG on HBsAb, the HBsAb titer of the 28 mothers was analyzed. Compared with the control group, the titer of HBsAb in mothers in the experimental group was higher at most time points examined () whereas no significant difference was seen between the 2 groups. We further compared the HBsAb titer in the women with their neonates in the experimental group, which revealed a positive correlation (r = 0.839, p = 0.001, ). Infants born from mothers with higher HBsAb titers had higher levels of the protective antibody. Despite all this, titers of HBsAb in most of the mothers and newborns were less than 10 mIU/ml, the established seroprotective threshold of HBsAb.Citation21-23
Histopathological changes in placenta samples from HBV-infected mothers with or without HBIG injection
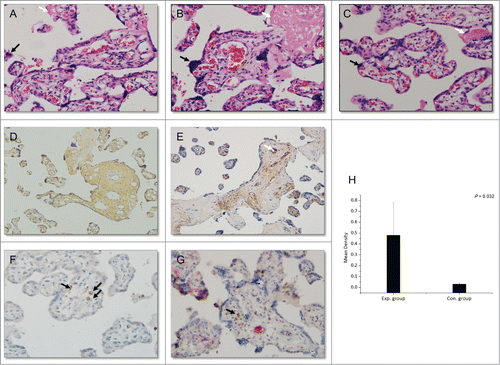
The placenta acts as a barrier separating maternal and fetal blood. Therefore, virus from the mother must pass through the placenta to infect the fetus. To investigate whether HBIG influenced placental morphology, standard hematoxylin-eosin staining of sections was performed. We observed damage in placenta with HBV infection, including stromal fibrosis, syncytial knotting, fibrinoid deposition, fibrinoid necrosis, chorionic hyperemia, and proliferation of capillaries in the villus. HBIG injections did not influence placental morphology compared with the control group (). These changes are also present in the placenta from late pregnancy of healthy women. No pathological typical changes associated with HBV infection were observed in these sections.
Figure 3. Histopathological changes in the placenta (hematoxylin-eosin; magnification, ×200, A–C) and immunohistochemical staining (magnification, ×200, D–F: DAB staining, G: DAB and AP-Red staining). (A) Section of placenta from late pregnancy of a healthy woman. (B) Section of placenta from a woman in the control group (HBV-infected women without HBIG injections). (C) Section of placenta from a woman in the experimental group (HBV-infected women receiving HBIG injections). Black arrows: syncytial knotting; white arrows: fibrinoid necrosis. (D) HBsAg staining. (E) HBIG staining. White arrow: villous capillary endothelial cells. (F) CD68 staining. Black arrows: Hofbauer cells. (G) CD68- and HBIG-double-positive immunohistochemical staining. Black arrow: HBIG in Hofbauer cells. (H) Comparison of HBIG intensity between the groups.
Expression and distribution of injected HBIG in the placenta
The expression of HBV markers in the placenta has been manifested in both in vivo and in vitro experiments from our previous studies.Citation17,24,25 To investigate whether HBIG is deposited in the placenta as HBV markers and to understand its distribution, immunohistochemical staining for HBIG and HBsAg was performed. The staining results are shown in .
HBsAg-positive areas were mainly located in trophoblasts and villous mesenchymal cells, and they were also observed in a few villous capillary endothelial cells (). The distribution was similar to that reported previously.Citation24 The total positive rate of HBsAg among the 28 women was 14.29% (4/28). Interestingly, all of the positive samples were from the control group.
HBIG-positive areas were mainly detected in villous capillary endothelial cells and villous mesenchymal cells of placenta () and a few positive cells were also found in the basal layer of trophoblasts. All 12 placental tissue samples from the experimental group were HBIG positive, whereas only 12.5% (2/16) of control group samples were HBIG positive (P < 0.01). Further considering mother's HBsAb titer before delivery, the 2 HGIB positive samples in control group were from mothers with detective HBsAb, although the titer was low, which may owing to the active enrichment of HGIB at placenta.
Because HBIG is a specific immunoglobulin against HBV, the absence of double-positive staining for HBIG and HBsAg in all samples was not surprising.
Hofbauer cells, the macrophages in the placenta, are mainly present in the interstitial substance and play important roles in mother-to-infant virus transmission and immune defense.Citation26,27 We performed double immunohistochemical staining for HBIG and CD68, the latter of which is a specific marker for Hofbauer cells. As shown in HBIG co-localized with CD68, indicating that HBIG was present in Hofbauer cells.
Finally, the intensity of placental HBIG staining was analyzed with Image-Pro Plus 6 image analysis software, and the mean density was calculated. The intensity was significantly higher in the experimental group than in the control group (, 0.48 ± 0.30 vs 0.03 ± 0.01, p = 0.032).
Discussion
Because of the routine use of combined immunoprophylaxis, which has been implemented extensively for infants from chronic HBV-infected women, intrauterine infection remains the principal route of HBV transmission.Citation28 No standard protocol to efficiently block this transmission route has been established.Citation7
As a specific immunoglobulin against HBV, HBIG has been widely used in liver transplantation to prevent HBV reinfection.Citation29 Consistent with previous reports, no serious side effects on mothers or newborns were observed in the HBIG injection group in the current study, confirming its safety during pregnancy.
Although HBIG has been employed during pregnancy for about 20 years, its efficacy is still debatable. In the late 1990s, Zhu et al. reported that HBV vertical transmission in HBsAg-positive women is reduced with HBIG injections during pregnancy.Citation30,31 A meta-analysis of data from 37 individual research centers in China and other countries also supports this conclusion.Citation15 Zhu and colleagues later demonstrated that HBIG can decrease HBV DNA and thus prevent vertical transmission during pregnancy.Citation8 However, Yuan and other groups obtained opposite results.Citation16 The following reasons may account for this discrepancy: 1) the doses of HBIG used during pregnancy were different; 2) the criteria for HBV infection of newborns were not identical; and 3) the sample sizes of some studies were not sufficiently large. HBIG has been used in our clinical work for over a decade and provides marked protection of the fetus from their HBV-infected mothers.Citation32 In our current study, after 1 y of follow-up, all infants were free of HBV infection whether their mothers had received HBIG or not during pregnancy. Because of the small study population in the current study, determining the difference in the infection rate between the 2 groups was difficult.
We observed that the level of maternal HBV DNA increased slightly during late pregnancy, which was consistent with a previous report.Citation20 This may be due to the special immune status during the gestational period. Furthermore, the titers of HBV markers (HBsAg, HBeAg, and HBcAb) did not change following HBIG injection. We hypothesize that HBIG may prevent HBV transmission through other mechanisms, rather than serving as a virus inhibitor to block intrauterine transmission.
Studies from as early as 1961 have shown that the placenta can actively transfer IgG to the fetus beginning at ∼12 weeks of gestation.Citation33 The average HBsAb titer increased in mothers of the HBIG-injected group, although no significant difference was observed when comparing with control group. Additionally, we observed a positive correlation when further analyzing HBsAb titer between mothers and neonates from the experimental groups (r = 0.839, p = 0.001). The titer was even higher in some neonates than in their mothers. This may be due to the transfer of HBIG from mother to fetus actively, which may be another mechanism for protecting neonates. Whereas, levels of HBsAb from all mothers were lower than the established seroprotective threshold of HBsAb (10 mIU/mL).Citation21-23 which supported our hypothesis HBIG protect fetus by other mechanism instead of directly neutralizing HBV virus in serum. The response to the vaccine by these neonates with higher HBsAb at birth requires further investigation and a longer follow-up period.
Using immunohistochemical staining and in situ hybridization, Xu et al. determined the distribution of HBsAg, HBcAg, and HBV DNA in the placenta of HBsAg-positive women. In addition, the positive rate of HBV DNA in villus capillary endothelial cells is closely correlated with intrauterine infection (r = 16.15, p = 0.0076).Citation34 Histological staining has shown that HBIG is also deposited in fetal capillary endothelial cells and villous mesenchymal cells. Thus, HBIG may form a protective immunoglobulin “barrier” between the maternal and fetal blood, playing a complementary role to the placental barrier and thus preventing HBV components from permeating the placenta and thereby protecting the fetus from HBV infection.
As the main target cell of virus infection in the placenta, Hofbauer cells comprise 43% of interstitial cells in the placenta Citation26,27 In addition, because the virus preferentially infects Hofbauer cells, these cells may work as ideal transporters for the virus from the mother to fetus.Citation35,36 The data showed the deposition of HBIG in Hofbauer cells in the absence of HBsAg, we hypothesized that HBIG may “neutralize” the virus by an unknown mechanism to prevent intrauterine transmission. Moreover, whether HBIG influences Hofbauer cell function to affect immune regulation and migration requires further study.
The HBIG used in this study was a custom-made polyclonal antiserum that exhibited lower specificity than monoclonal antibodies are expected to display, and this may also explain the positive areas of HBIG staining in sections from the control group. Furthermore, the study population was too small to tell the difference of infection rate of infants between experimental and control groups. Additionally, whether HBIG injection during pregnancy influences HBV mutation or the response of the newborn to HBV vaccination requires additional study.
This prospective study provides further evidence for the safety and efficacy of HBIG use in pregnancy. Rather than influencing virus replication, HBIG may form an “immune barrier” between the mother and fetus to prevent HBV transmission. The effect of HBIG on Hofbauer cells, a “transporter” of virus from mother to fetus, requires further study.
Patients and Motheds
Participants
From May 2012 to May 2013, HBsAg-positive pregnant women who underwent consultation were recruited from the Department of Infectious Diseases, the First Affiliated Hospital of Xi'an Jiaotong University, Shaanxi, China. Mothers willing to receive HBIG administration were assigned to experimental group, others underwent consecutively consultation without HBIG injection were enrolled as control group. The exclusion criteria for participants were: 1) infection with toxoplasmosis, syphilis, parvovirus B19, rubella, cytomegalovirus, herpes, hepatitis C, HIV, or other viruses; 2) obstetric diseases such as pregnancy-related hypertension, placental abruption, threatened miscarriage, and others. All participants in this study gave written informed consent for participation of their infants upon birth. The study protocol was approved by the ethics committee of the First Affiliated Hospital of Xi'an Jiaotong University. Additionally, the protocol has been registrated at ClinicalTrials.gov.
According to our knowledge, data about the mechanism of HBIG in prevention for intrauterine transmission of HBV was limited, especially histopathological changes in placenta after HBIG administraction. The sample size was calculated based on our preliminary results. Five placenta samples from mothers receiving HBIG injection during pregnancy were collected for preliminary study, all of them showed positive for HBIG, while only 2 of 5 from control group were HBIG positive. Based on this result, 11 samples for each group should be enough to clarify the issue (set α = 0.05, β = 0.1). Considering the withdrawal and loss to follow-up of our participants and immunostaining failing for quality of placenta sample, we collected as many samples as we can.
Data and sample collection
The women enrolled in experimental group willing to receive injections of HBIG (200 IU, S20023028, Hualan Biological Engineering Inc.) during pregnancy beginning at week 20 of gestation (at weeks 20, 24, 28, 32, 34, 36, 38, 39, and 40). The control group only underwent regular examinations without any HBIG treatment during pregnancy. Data were collected from medical records that included complete healthcare information before and after delivery.
Peripheral venous blood (5 ml) was collected at 20, 24, 28, 32, and 36 weeks of gestation and at delivery (prior to HBIG injection in each case) and at 1 month after delivery. Neonatal femoral venous blood (3 ml) was taken prior to combined immunoprophylaxis (HBIG, 200 IU, and the first dose of the hepatitis B vaccine, 5 μg, S19983018, Shenzhen Kangtai Biological Products Co. Ltd.) at different injection sites within 12 h postpartum). Each infant was consecutively followed up after birth, growth index (weight, length and head circumference), feeding patterns and serum level of HBV DNA and viral markers were recorded. Serum was separated for HBV DNA load tests and measurement of the titer of HBV markers (HBsAg, HBsAb, HBeAg, and HBcAb). In most cases, the status of HBsAg is conformity with that of HBV DNA. However, there is exception reported in adults, such as in the case a person shows positive for HBV DNA, while negative for HBsAg. In current study, both HBsAg and HBV DNA had been tested. According to well accepted criteriaCitation19,37-40 vertical transmission was determined by HBsAg or HBV DNA testing from infant peripheral blood at 28 weeks of age.
Placental tissues were collected under sterile conditions immediately after delivery. After washing with normal saline solution to remove maternal blood (the last washing solution was used to check for HBsAg to exclude maternal blood contamination), the tissue was fixed in 4% paraformaldehyde and processed for paraffin embedding. Serial sections (5 μm) were prepared for hematoxylin-eosin staining and immunostaining. Placenta tissue from non–HBV-infected postpartum women was prepared in the same way and used as a control
Virological and immunological assessment
Titers for the HBV markers (HBsAg, HBsAb, HBeAg, and HBcAb) were measured with the Abbott ARCHITECT HBsAg, HBsAb, HBeAg, and HBcAb assays, respectively (Abbott Laboratories, Chicago, IL). The HBV DNA load was quantified using a high-sensitivity fluorescent real-time PCR kit (S20040036, Daangene) and amplified with an ABI 7500 Fast instrument (Applied Biosystems, Foster city, CA). The lower limit of detection for the PCR assay was 1 × 103copies/ml. Liver function was assessed with an automated bioanalyzer (Olympus AU5400, Tokyo, Japan).
Preparation of a polyclonal antiserum against HBIG
HBIG (100 IU/ml, 160 mg/ml) that was injected during pregnancy was used as an antigen to immunize New Zealand white rabbits to prepare a polyclonal antiserum. The rabbits were immunized every other week for 70 d with 1.5 mg HBIG total per rabbit. The detailed immunization procedure was as follows: for the first immunization, a mixture of 3.2 μl HBIG and 497 μl phosphate-buffered saline (PBS) was mixed with 500 μl Freund's complete adjuvant to form a microemulsion that was used for inoculation. The next 4 times, a microemulsion containing 1.6 μl HBIG, 498.5 μl PBS, and 500 μl Freund's complete adjuvant was injected. Rabbit venous blood was collected at the end of the immunization cycle and purified with Tris-phosphate ion-exchange chromatography to yield the anti-HBIG. The antiserum titer was measured with ELISA and was shown to satisfy the requirements for further immunostaining experiments.
Histological staining
Placental tissue sections were dewaxed in xylene and rehydrated in a decreasing alcohol series. After rinsing with double-distilled water, a standard protocol was used for hematoxylin-eosin staining (hematoxylin for 4 min, water rinse, eosin for 30 sec).
For immunohistochemical staining, after dewaxing and rehydration, sections were immersed in 0.01 M sodium citrate (pH 6.0) to inactivate the endogenous peroxidases. Then, the samples were blocked with 5% bovine serum albumin for 20 min and incubated for 45 min at room temperature with a primary antibody against HBsAg (mouse, 1:50, ZM-0122, Beijing Zhongshan Golden Bridge Biotechnology Co.), HBIG (rabbit, 4.7 mg/ml, 1:2500, prepared as described above), or CD68 (mouse, 1:25, ab955, Abcam). After rinsing with PBS, the tissue sections were proceeded with the mouse/rabbit PowerVision™ Two-Step according to the manufacturer's instructions, respectively (PV-9002, Beijing Zhongshan Golden Bridge Biotechnology Co.), and color was developed with a DAB detection kit (DAB-0031/1031, Maixin Bio.). After substrate reaction, stained tissues were briefly counterstained with hematoxylin (ZLI9609, Beijing Zhongshan Golden Bridge Biotechnology Co.). Slides were dehydrated in an ascending ethanol series and mounted in neutral resins (ZLI9555, Beijing Zhongshan Golden Bridge Biotechnology Co.). For negative controls, the primary antibody was omitted. All immunostaining was performed at least 3 independent times to confirm reproducibility.
For double immunohistochemical staining, sections were co-incubated with antibodies against HBsAg + HBIG or CD68 + HBIG, and the other procedures were the same as above. The sections were stained with DAB and alkaline phosphatase Red (AP-Red, ZLI9042, Beijing Zhongshan Golden Bridge Biotechnology Co.) for color development. Stained sections were analyzed and photographed under a light microscope (DM 4000 B, Leica, Wetzlar, Germany) at 200× magnification.
Yellow-brown particles visible in placental tissue were considered positive staining. All images were examined by 2 experienced pathologists independently, who were blind to the patient interventions and all clinicopathological findings. The quantification of the intensity of HBIG staining was analyzed with Image-Pro Plus 6 image analysis software (Media Cybernetics, Inc.., Silver Spring, MD, USA) according to manufacturer's instructions. Areas of specific staining from various images were chosen to determine the positivity discrimination plane, minimizing the possible visual variation in the detection of immunostaining areas. The discrimination plane was set at 030 in the H channel and 0255 in the S and I channels. Identical settings were performed for each field. A total of 10 fields (200X objective lens) were randomly selected per slide. The integrated optical density (IOD) from all the positive staining in each field was measured to evaluate the area and intensity of the positive staining, as well as area of interest (AOI). The average value (mean density, IOD/AOI) represented the intensity value of HBsAg or HBIG expression per unit area.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 statistical software (SPSS Inc.., Chicago, USA). Data were expressed as the mean ± standard deviation (SD). The data were analyzed with the Shapiro-Wilk test and the Levene statistic for normality and homogeneity of variance, respectively. The difference between 2 quantitative groups was compared with an independent-sample t-test or the Mann-Whitney U-test as appropriate, and correlations were analyzed with the Pearson or Spearman correlation test. The chi-square test or Fisher's exact test was used to compare the proportions of the 2 groups. All tests were 2-tailed with the risk set at 5%, and the statistical significance was set as P < 0.05. The chi-square test with SAS (SAS Institut Inc., Cary, NC, USA) was used to calculated the study sample size and the actual power of the study, which was also assumed as 2-tailed test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors appreciate all the mothers and children who participated. We appreciate the guidance provided by Professor Shulin Zhang of the Medical College of Xi'an Jiaotong University.
Funding
This work received financial support from the National Science and Technology Projects on Major Infectious Diseases (12th Five Year, China) (Project No. 2012ZX10002007-001-001 and 2012ZX10002007-002-007) and the National Natural Science Fund (Project No. 31100129).
References
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27:6550-7; PMID:19729084; http://dx.doi.org/10.1016/j.vaccine.2009.08.048
- Hamdani-Belghiti S, Bouazzaou NL. Mother-child transmission of hepatitis B virus. State of the problem and prevention. Arch Pediatr 2000; 7:879-82; PMID:10985190; http://dx.doi.org/10.1016/S0929-693X(00)80199-2
- Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, Sun W, Yang X, Zhang L, Lu W, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med 2011; 165:837-46; PMID:21536948; http://dx.doi.org/10.1001/archpediatrics.2011.72
- Tajiri H, Nose O, Shimizu K, Ida S, Miki K, Kimura S, Yabuuchi H. Prevention of neonatal HBV infection with the combination of HBIG and HBV vaccine and its long-term efficacy in infants born to HBeAg positive HBV carrier mothers. Acta Paediatr Jpn 1989; 31:663-8; PMID:2533788; http://dx.doi.org/10.1111/j.1442-200X.1989.tb01376.x
- Monna T, Kuroki T, Oka H, Yamamoto S, Murata R, Suyama I, Issiki G, Nakao M. Prevention of vertical transmission of HBV by administration of hepatitis B vaccine combined with HBIG and long-term follow-up of HBsAb titer. Osaka City Med J 1988; 34:9-17; PMID:3419810
- Zanetti AR, Dentico P, Del Vecchio Blanco C, Sagnelli E, Villa E, Ferroni P, Bergamini F. Multicenter trial on the efficacy of HBIG and vaccine in preventing perinatal hepatitis B. Final report. J Med Virol 1986; 18:327-34; PMID:2940333; http://dx.doi.org/10.1002/jmv.1890180405
- Shao ZJ, Zhang L, Xu JQ, Xu DZ, Men K, Zhang JX, Cui HC, Yan YP. Mother-to-infant transmission of hepatitis B virus: a Chinese experience. J Med Virol 2011; 83:791-5; PMID:21360547; http://dx.doi.org/10.1002/jmv.22043
- Zhu Q, Yu G, Yu H, Lu Q, Gu X, Dong Z, Zhang X. A randomized control trial on interruption of HBV transmission in uterus. Chin Med J 2003; 116:685-7; PMID:12875680
- Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol 2010; 116:147-59; PMID:20567182; http://dx.doi.org/10.1097/AOG.0b013e3181e45951
- Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM. High prevalence of occult hepatitis B virus infection in children born to HBsAg-positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J Hepatol 2012; 57:515-21; PMID:22617152; http://dx.doi.org/10.1016/j.jhep.2012.04.021
- Bedimo RJ, Drechsler H, Jain M, Cutrell J, Zhang S, Li X, Farukhi I, Castanon R, Tebas P, Maalouf NM. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PloS one 2014; 9:e106221; PMID:25170938; http://dx.doi.org/10.1371/journal.pone.0106221
- Yi W, Liu M, Cai HD. Safety of lamivudine treatment for chronic hepatitis B in early pregnancy. World J Gastroenterol 2012; 18:6645-50; PMID:23236240; http://dx.doi.org/10.3748/wjg.v18.i45.6645
- Xu Q, Xiao L, Lu XB, Zhang YX, Cai X. A randomized controlled clinical trial: interruption of intrauterine transmission of hepatitis B virus infection with HBIG. World J gastroenterol 2006; 12:3434-7; PMID:16733865
- Xiao XM, Li AZ, Chen X, Zhu YK, Miao J. Prevention of vertical hepatitis B transmission by hepatitis B immunoglobulin in the third trimester of pregnancy. Int J Gynaecol Obstet 2007; 96:167-70; PMID:17296201; http://dx.doi.org/10.1016/j.ijgo.2006.11.011
- Shi Z, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int J Infect Dis 2010; 14:e622-34; PMID:20106694; http://dx.doi.org/10.1016/j.ijid.2009.09.008
- Yuan J, Lin J, Xu A, Li H, Hu B, Chen J, Yao J, Dong H, Jiang M. Antepartum immunoprophylaxis of three doses of hepatitis B immunoglobulin is not effective: a single-centre randomized study. J Viral Hepat 2006; 13:597-604; PMID:16907846; http://dx.doi.org/10.1111/j.1365-2893.2006.00738.x
- Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol 2004; 10:437-8; PMID:14760774
- Zhang SL, Han XB, Yue YF. Relationship between HBV viremia level of pregnant women and intrauterine infection:neated PCR for detection of HBV DNA. World J Gastroenterol 1998; 4:61-3; PMID:11819234
- Chen T, Wang J, Feng Y, Yan Z, Zhang T, Liu M, Bai Y, Song H, Liu H, Yang Y, et al. Dynamic changes of HBV markers and HBV DNA load in infants born to HBsAg(+) mothers: can positivity of HBsAg or HBV DNA at birth be an indicator for HBV infection of infants? BMC Infects Dis 2013; 13:524; PMID:24195671; http://dx.doi.org/10.1186/1471-2334-13-524
- Soderstrom A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis 2003; 35:814-9; PMID:14723355; http://dx.doi.org/10.1080/00365540310016547
- Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J 2001; 20:63-75; PMID:11176570; http://dx.doi.org/10.1097/00006454-200101000-00013
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet 2000; 355:561-5; PMID:10683019; http://dx.doi.org/10.1016/S0140-6736(99)07239-6
- Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, Duval B, Jilg W, Böcher WO, Lu SN, et al. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine 2005; 23:4158-66; PMID:15964484; http://dx.doi.org/10.1016/j.vaccine.2005.03.017
- Chen Y, Wang L, Xu Y, Liu X, Li S, Qian Q, Hu B, Zhou A, Chen T, Zhao Y. Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microbes Infect 2013; 15:409-15; PMID:23500187; http://dx.doi.org/10.1016/j.micinf.2013.02.008
- Jin Y, Ye F, Shi J, Qiu H, Zhao Y, Lin S, Chen T, Liu M, He Y, Zhang S. Hepatitis B virus infection and replication in primary cultured human granulosa cells. Arch Virol 2011; 156:1-7; PMID:20878429; http://dx.doi.org/10.1007/s00705-010-0808-8
- Johnson EL, Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012; 9:101; PMID:23217137; http://dx.doi.org/10.1186/1742-4690-9-101
- Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 2006; 168:1210-26; PMID:16565496; http://dx.doi.org/10.2353/ajpath.2006.050482
- Borgia G, Carleo MA, Gaeta GB, Gentile I. Hepatitis B in pregnancy. World J Gastroenterol 2012; 18:4677-83; PMID:23002336; http://dx.doi.org/10.3748/wjg.v18.i34.4677
- Terrault N. Prophylaxis in HBV-infected liver transplant patients: end of the HBIG era? Am J Gastroenterol 2013; 108:949-51; PMID:23735916; http://dx.doi.org/10.1038/ajg.2013.122
- Zhu QR, Lu Q, Gu XH, Xu HF, Duan SC. A preliminary study on interruption of HBV transmission in uterus. Chin Med J 1997; 110:145-7; PMID:9594288
- Zhu Qirong, Lu Qing, Gu Xinhuan ea. A preliminary study on interrup-tion of hbv transmission in utero. Chinese J Pediatr 1995; 33:93-4; PMID:9594288
- Yue Y, Yang X, Zhang S. Prevention of intrauterine infection by hepatitis B virus with hepatitis B immune globulin: efficacy and mechanism. Chin Med J 1999; 112:37-9; PMID:11593638
- Dancis J, Oratz M, Lind J, Vara P, Smolens J. Placental transfer of proteins in human gestation. Am J Obstet Gynecol 1961; 82:167-71; PMID:13719557
- Xu D, Yan Y, Xu J. A molecular epidemiology study on risk factors and mechanism of HBV intrauterine transmission. Zhonghua Yi Xue Za Zhi 1999; 79:24-7; PMID:11601001
- Liu Y, Zhang J, Zhang R, Li S, Kuang J, Chen M, Liu X. Relationship between the immunohistopathological changes of hepatitis B virus carrier mothers' placentas and fetal hepatitis B virus infection. Zhonghua Fu Chan Ke Za Zhi 2002; 37:278-80; PMID:12133400
- Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol 2004; 35:536-45; PMID:15138926; http://dx.doi.org/10.1016/j.humpath.2004.01.015
- Jiang HX, Han GR, Wang CM, Yue X, Wang GJ. Abstract efficacy of combined vaccine for the prevention of HBV transmission in highly viremic HBeAg+ mothers and the HBV markers' dynamic change of babies in follow-up. Zhonghua Gan Zang Bing Za Zhi 2011; 19:818-22; PMID:22433302
- Yi W, Li MH, Hu YH, Liu F, Zhang YL, Liu XJ, Hao HX, Song SJ, Liu Y, Li XH, et al. Predictive study of HBsAg in different stages of neonatal venous blood on failure of blocking HBV mother to infant transmission. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011; 25:338-41; PMID:22338218
- Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, Yue X, Wang GJ, Tang X, Fang ZX. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol 2011; 55:1215-21; PMID:21703206; http://dx.doi.org/10.1016/j.jhep.2011.02.032
- Sun KX, Li J, Zhu FC, Liu JX, Li RC, Zhai XJ, Li YP, Chang ZJ, Nie JJ, Zhuang H. A predictive value of quantitative HBsAg for serum HBV DNA level among HBeAg-positive pregnant women. Vaccine 2012; 30:5335-40; PMID:22749833; http://dx.doi.org/10.1016/j.vaccine.2012.06.036