Abstract
Acinetobacter baumannii (Ab) is a global emerging bacterium causing nosocomial infections such as pneumonia, meningitis, bacteremia and soft tissue infections especially in intensive care units. Since Ab is resistant to almost all conventional antibiotics, it is now one of the 6 top-priorities of the dangerous microorganisms listed by the Infectious Disease Society of America. The development of vaccine is one of the most promising and cost-effective strategies to prevent infections. In this study, we identified potential protective vaccine candidates using reverse vaccinology. We have analyzed 14 on-line available Ab genome sequences and found 2752 homologous core genes. Using information obtained from immuno-proteomic experiments, published proteomic information and the bioinformatics PSORTb v3.0 software to predict the location of extracellular and/or outer membrane proteins, 77 genes were identified and selected for further studies. After excluding those antigens have been used as vaccine candidates reported by the in silico search-engines of PubMed and Google Scholar, 13 proteins could potentially be vaccine candidates. We have selected and cloned the genes of 3 antigens that were further expressed and purified. These antigens were found to be highly immunogenic and conferred partial protection (60%) in a pneumonia animal model. The strategy described in the present study incorporates the advantages of reverse vaccinology, bioinformatics and immuno-proteomic platform technologies and is easy to perform to identify novel immunogens for multi-component vaccines development.
Introduction
Acinetobacter baumannii (Ab) is a gram-negative bacterium responsible for opportunistic nosocomial infections such as pneumonia, bacteremia, and meningitis.Citation1-6 Ab can stick to dry surfaces for a long time especially in hospital intensive care units through ventilator-associated machine and biotic surfaces. Ab accounts for >7,000 hospital-acquired infections (HAI) and over 500 deaths per year in the United States only.Citation3 Since the numbers of multi-drug resistant (MDR) and pan-drug resistant (PDR) strains are increasing,Citation3-6 prevention and treatment of Ab infections have become a significant health care challenge and new strategies against Ab infections are urgently needed. In the absence of efficacious and safe antibiotics, vaccination represents the best strategy to combat MDR pathogens.Citation6,7 So far, several potential Ab antigens have been identified through active and passive immunizations of experimental animals. They include outer membrane vesicles (OMVs), outer membrane protein A (OmpA), auto-transporter (Ata), biofilm-associated protein (Bap), K1 capsular polysaccharide and Poly-N-acetyl-β-(1-6)-glucosamine (PNAG).Citation7-14 The sequence variability of these antigens and their absence in the circulating Ab strains may result poor cross-protective efficacy against HAI caused by new emerging Ab strains that may possibly mutate under selective immunological pressure and down-regulate the target antigens. In our hand, recombinant OmpA and a truncated fragment of Bap have been tested and found no protection in mouse pneumonia challenge studies; only inactivated Ab and/or PNAG-protein conjugate showed good protection in the mouse pneumonia challenge model (Chong et al., unpunished results). Therefore, the identification of highly conserved antigens expressed by the new emerging Ab strains during infections is still warranted.
With the ever increasing information on microbial genomes, reverse vaccinology (RV) has become a promising in silico approach to select vaccine targets.Citation6,15-17 RV integrates comparative analysis of genome sequences and web-based prediction tools for screening highly conserved outer-membrane proteins and soluble secreted proteins. Meningococcal group B vaccine (4CMenB) serves as an example of a successful RV approach.Citation6,16 Combined with other approaches such as in vitro proteomic analysis of OMVs secreted by bacteria, differential expression analysis of virulent strains, in vivo animal model challenge studies, and immuno-proteomics, it provides an important platform to identify novel protective antigens.Citation18-33 Furthermore, through these studies and analyses, we can also deeply understand the pathogenesis of Ab bacteria. In this study, we used an integrated approach combining reverse vaccinology, bioinformatics-based structural analysis and results obtained from published proteomics studies to screen and identify potential protective antigens against Ab infections. As a proof of concept, potential antigens were designed for in vitro/ in vivo vaccine evaluation in a mouse pneumonia challenge model.
Results and Discussion
In silico screening of potential vaccine candidates
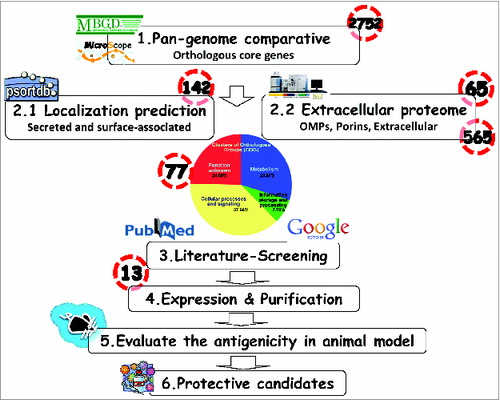
We used the 14 complete genome sequences currently available (levels: gapless chromosome) including 11 multi-drug resistance (MDR) strains from NCBI database for comparative analysis using MBGD program (available on http://mbgd.genome.ad.jp/).Citation34 Three genomes belong to international clade I (IC I), 8 genomes are IC II, 2 genomes are IC III and another one is unclassified indicating the wide coverage of Ab strains. In addition, these clones are characterized for MDR, the year of isolation, geographic location and by their accession number (). As shown in , the phylogenetic lineage of A. baumannii strains analyzed in this study was inferred by the neighbor-joining method performed using the MEGA6 software and the previously published strategy.Citation35,36 Using the MBGD database, 2752 orthologous core genes were identified as novel potential vaccine antigens for these strains. The strain AYE which was used as a reference for annotating was associated with a human clinical isolate from the nationwide outbreak in France in 2001.Citation35,36 In order to predict the localization of proteins in the 14 available genomic sequences, PSORTb web tool was used to define outer membrane or extracellular proteins. This led to the identification of 565 non-redundant antigens as potential vaccine candidates as shown in .
Table 1. Complete Ab genomes from NCBI used in this study
Figure 1. Phylogenetic relationship of Acinetobacter baumannii. The phylogenetic lineage of fully sequenced Ab strains analyzed in this study was inferred by the neighbor-joining method performed in MEGA6 using the strategy previously published in.Citation35
Figure 2. Schematic summarizing the flow chart of Reverse Vaccinology used in this study. 1.Comparison of genomic sequences to obtain the orthologous core genes by the in silico web-based tools (MBGD). 2. 2.1 PSORTb 3.0 to predict the cellular localization of each protein. 2.2 Outer membrane and extracellular proteins identification by proteomics analyses/references. 3. Blasting of protein sequences and literature-review to determine if the proteins have ever been used as immunogens. 4. Expression and purification of the consensus sequence of candidate immunogens. 5. Utilization of the mouse pneumonia model to assess vaccine immunogenicity and protective ability against A. baumannii infection. 6. Development of a broadly protective A. baumannii vaccine formulation.
In vitro identification of outer-membrane and secreted proteins
Outer-membrane vesicles (OMVs) secreted by many Gram-negative bacteria can mediate intercellular exchanges, of lipo-polysaccharides (LPS), cell-cell signals, proteins and DNA as well as the transfer of lethal virulence factors.Citation37 A. baumannii secretes OMVs which interact with host cells and then deliver bacterial effectors contributing to bacterial pathogenesis and immuno-pathology.Citation12 To enhance the power of reverse vaccinology, we also integrated functional genomics and proteomic analysis using bioinformatics tools to further refine the screening criteria for protective antigens. We (results to be published elsewhere) and othersCitation17-18,25,28 used HPLC-MS/MS to analyze the proteomic profiles of OMVs and identified 65 potential antigens from A. baumannii ATCC17978 strains (Table S1). Meanwhile, we have also exploited proteomic information on outer membrane and/or secreted proteins predicted from 6 previous experimental in vivo studies.Citation18,22,23,25-28 Taking together the information collected for homologous core genes, in vitro published proteomic information, and in silico predicted secretome profiles, 77 A. baumannii proteins were identified and selected as the most promising vaccine candidates ( & Table S2).
Selection of antigen targets
To minimize the number of vaccine candidates for immunogenicity studies, the selected 77 potential antigens were further analyzed through PubMed and Google Scholar searches to identify those previously used as vaccine candidates against A. baumannii infections. Our combined literature search led to the identification of 13 top vaccine targets based on sequence homologies, prevalence, cellular location and potential to be vaccine candidate in other pathogens (). These potential vaccine antigens are essentially present in all published A. baumannii strains and thus are excellent candidate immunogens to be included in an effective A. baumannii vaccine. According to the definition of Cluster of Orthologous Groups (COG) protein function,Citation36,37 these 13 proteins could fall into 5 different functional groups: 5 could belong to the “cell wall/membrane/envelope biogenesis” group; 4 are in the “posttranslational modification, protein turnover, chaperones” group; 2 are “energy production and conversion” proteins; one is involved in “lipid transport and metabolism;” and one in “nucleotide transport and metabolism” ().
Table 2. Most promising immunogens for an A. baumannii vaccine
ABAYE0130 is homologous with the OmpK of the fish pathogen Vibrio harveyi which has been shown to be effective as a fish vaccine candidate against V. harveyi infactions.Citation38 The Nlp gene of Yersinia pestisnlp D (similar to ABAYE1048) encoding for outer membrane lipoprotein could elicit a protective immunity against Y. pestis.Citation39 ABAYE1583 contains the conserved β-barrel assembly of outer membrane proteins and is similar to Treponema pallidum subspecies Tp92 protein and Pasteurella multocida Oma87 that have previously been identified as potential vaccine candidates.Citation40,41 The ABAYE2931 sequence shares homology with a peptidoglycan-associated lipoprotein related to Aliivibrio salmonicida Pal protein that had been used successfully as immunogen in an A. salmonicida fish vaccine.Citation42 ABAYE3514 which has sequences homology to TolC protein of Samonella paratyphi and belongs to the type I secretion outer membrane channel component could be the vaccine target.Citation43 ABAYE3820 comprises of a rotamase domain that may plays an important role in pathological processesCitation24 and is similar to Haemophilus parasuis FKBP-type peptidyl-prolyl cis/trans isomerase proposed as a vaccine candidate for Glässer's disease in swine.Citation44 ABAYE2564 is similar to a putative alkyl hydroperoxide reductase subunit C that has been identified as a potential target for multivalent anthrax vaccines.Citation45 ABAYE1454 has sequence similarity with the cyclophilin proteins previously been shown to be efficacious vaccines against toxoplasmosis and Leishmania infantum infections.Citation46,47 ABAYE0619 is identified to contain the conserved peroxidase domain that is homology to the thiol-specific-antioxidant (TSA) of Leishmania major that has been used as a vaccine candidate against L. major.Citation48,49 ABAYE0711 has a protein sequence similar to that of the long-chain fatty acid transport protein, Ompp1 which has been considered as a potential vaccine target against Samonella paratyphi and Haemophilus influenza.Citation50,51 ABAYE0776 could be involved in energy product and conversion as a putative succinate dehydrogenase that has been considered as a potential vaccine candidate for Leptospira spp. in an immuno-proteomic study.Citation52 ABAYE0782 contains the highly conserved putative dihydrolipoamide dehydrogenase (Lpd) gene which was depleted in Mycoplasma gallisepticum and tested as a live-attenuated vaccine strain.Citation53 It implies that Lpd protein is virulence factor and could be a possible subunit vaccine target. Finally, ABAYE3267 protein sequence is similar to a highly conserved protein, the nucleoside diphosphate kinase (NDK) that is present in a number of bacteria including Bacillus anthracis, Francisella tularensis and Campylobacter fetus and considered to be a potential virulence target and thus a vaccine candidate.Citation54-56
Using the MBGD platform,Citation34 the proteomic data of OMVs obtained from HPLC/MS/MS experiments and bioinformatics prediction of surface-exposed antigens, we have identified 77 antigens as potential vaccine candidates (Table S2). The final list was restricted to 13 putative antigens. Recently, Moriel et al. utilized a similar approach and identified 42 putative vaccine candidates.Citation17 Only one of their potential antigen candidates was identical to ABAYE1048 in our 13 vaccine candidates list and other 6 antigens (ABAYE0360, ABAYE0990, ABAYE1048, ABAYE1129, ABAYE2921, ABAYE3478) can be found in our list of 77 vaccine candidates. Obviously, the analysis of different genomic databases using similar functional genomics and bioinformatics tools could lead to different results. The major difference is the study strategy that we searched for surface-exposed antigens with functional homology to proteins previously identified as vaccine candidates for other pathogens. In order to identify novel antigens; we excluded proteins which had been tested and found to be not protective in our hands. Our current approach may have failed to identify potential vaccine immunogens, such as biofilm associated protein (Bap, ABAYE0792),Citation12 trimeric autotranspoter (Ata),Citation11 and other unknown putative proteins that could play critical role in A. baumannii pathogenesis. In addition, a limitation in our approach is the fact that immuno-proteomic data could be influenced by different culture conditions such as growing the pathogen in clinically relevant media reflecting the infection environment.Citation58
Selection of vaccine immunogens for a pilot study
Ideally, A. baumannii vaccine components should be potential protective immunogens also found in other pathogens and meet the following criteria: (i) expressed on the outer membrane and/or secreted as virulence factors that could interact with host cellular receptors; (ii) contain protein sequences and/or protective epitopes highly conserved among A. baumannii strains; and (iii) play an important role in pathogenesis during infection. The mono-valent vaccine may not meet all the above criteria, so multiple immunogens will very likely be necessary. Furthermore, under the selective immunological pressure generated by vaccination, these immunogens may possibly adapt and mutate to new strains and/or the prevalence of target antigens may be down-regulated. Therefore, 3 potential proteins OmpK, FKIB and Ompp1 corresponding to ABAYE0130, ABAYE3820 and ABAYE0711, respectively which were selected had the highest antigenicity scores 0.73, 0.66 and 0.65, respectively (). These antigens also are potential vaccine candidates in other bacterial pathogensCitation38,44,50,51 and should be evaluated for their protective ability in mouse immunogenicity studies. The protein sequence of each antigen from 14 published A. baumannii genomes was aligned by BlastP to obtain a consensus sequence, and the gene fragment of the identified consensus sequences was synthesized using the codon-optimization for E. coli expression.
Production of recombinant antigens
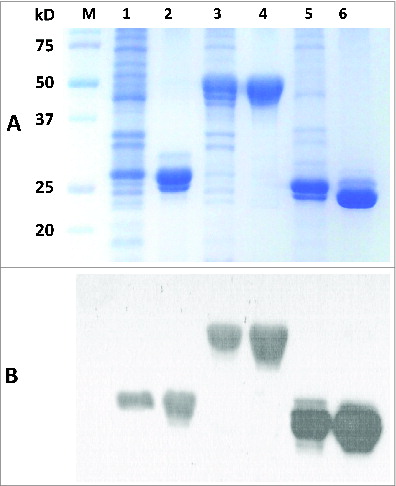
In a pilot study, 3 potential antigens (OmpK, FKIB, Ompp1) were produced and evaluated for their protective activities in a mouse pneumonia model.Citation11 Their respective genes were successfully expressed in E. coli as shown in . After single-step purification using Ni-affinity chromatography, highly-purified OmpK, FKIB, and Ompp1 (>95% purity) were obtained and their purity was confirmed by SDS-PAGE () and western blot analysis (). Trace amounts of degradation fragments were also detected as shown in the protein gel blot (). These degradation products are likely the result of proteolytic digestion during the purification process. In any event, at least 20 mg of highly enriched antigens could be easily obtained from 1 liter of bacterial culture. Most of the E. coli endotoxin (LPS) was removed by passing the antigen preparations through an E membrane; and the residual LPS in the purified antigens was found to be below 30 EU per mL.
Figure 3. 12% SDS-PAGE and Western blot of purified recombinant proteins. The purification process was performed using immobilized-metal affinity chromatography. (A) Lane M-standard protein markers, Lane 1-Whole bacterial lysate after induction of OmpK (27.6 kDa) by IPTG, Lane 2-purified recombinant OmpK protein, Lane 3-Whole bacterial lysate after induction of Ompp1 (50.6 kDa) by IPTG, Lane 4-purified recombinant Ompp1 protein, Lane 5-Whole bacterial lysate after induction of FKIB (25.6 kDa) by IPTG, Lane 6-purified recombinant FKIB protein. (B) Western blot analysis of purified recombinant protein using mouse anti-His antibody.
Mouse immunogenicity studies
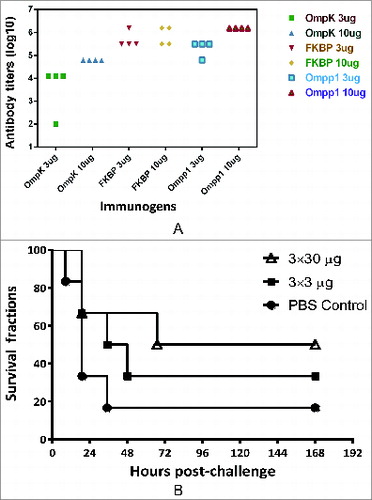
Blood samples from mice immunized with recombinant antigens were collected on day 42 and tested for antibody responses using antigen-specific ELISA. As shown in , individual recombinant antigens (OmpK, FKIB, Ompp1) formulated with CFA/IFA induced strong antigen-specific antibody responses (IgG titers > 104). These results indicate that all 3 recombinant proteins are highly immunogenic since mice vaccinated with 2 × 3 μg of antigens produced very strong IgG antibodies with titer >104 (data not shown).
Figure 4. Mouse immunogenicity studies of individual antigens. (A) Groups of C57BL/6 mice (n = 4) were intramuscularly immunized with either 3 or 10 μg of individual antigen (OmpK, FK1B and Ompp1) formulated with CFA/IFA adjuvant, or PBS on day 0, 14 and 21. At day 42, blood samples were collected and tested against each immunogen. Serum IgG antibody titers are reported. (B) Survival curves of C57BL/6 mice immunize with Ommp1. Groups of C57BL/6 mice (n = 5) were subcutaneously immunized with either 3 or 30 μg of Ommp1 formulated with CFA/IFA adjuvant, or PBS on day 0, 14 and 21. At day 42, the mice were challenged with 5 × 107 CFU of freshly grown A. baumannii ATCC17978. The survival rates of mice were recorded.
Murine pneumonia challenge model studies
To evaluate the protective efficacy of each vaccine candidate, a murine pneumonia challenge model was established according to previous reports.Citation11,30,31 Because of the low virulence of A. baumannii in mice and porcine mucin was included in the inoculums. Mucin has been shown to decrease phagocytosis and increase bacterial virulence in the mouse challenge model. as shown in Figure S1. The results clearly indicate that A. baumannii can kill 100% of mice within 4 days at a challenge dose of 5 × 107 cfu or within 2 days at a challenge dose of 1 × 108 cfu.
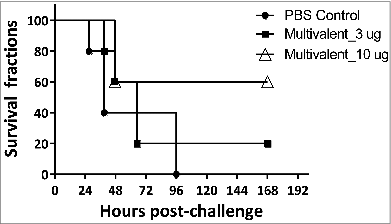
When C57BL/6 mice (n = 5/group) vaccinated 3 times subcutaneously (s.c.) with the purified recombinant immunogens (3 or 30 μg/mouse) formulated with CFA/IFA were challenged with 5 × 107 cfu of freshly grown A. baumannii (ATCC 17978 strain), all mice immunized with either OmpK or FKIP died within 6 days post challenge (data not shown). As shown in , only 50% of mice immunized with 30 μg dose of Ompp1 survived whereas 33% and 20% survival rates were observed in mice immunized with 3 μg dose of Ompp1 and PBS, respectively. When mice vaccinated 3 times s.c. with the mixture of recombinant antigens (3 × 3 or 10 × 3 μg of antigens mixture) formulated in CFA/IFA were challenged with 1 × 108 cfu of freshly grown A. baumannii, the survival rate in the group vaccinated with 10 ug of immunogens was found to be 60%, whereas a 20% survival rate was observed for the group immunized with a 3 μg dose, that was not significantly different from the survival rate in the control group ().
Figure 5. Survival curves of C57BL/6 mice immunized with multivalent vaccines Groups of C57BL/6 mice (n = 5) were subcutaneously immunized with multivalent antigens or PBS on day 0, 14 and 21. At day 42, the mice were challenged with 1.34 × 108 CFU of freshly grown A. baumannii ATCC17978. The survival rates of mice were recorded.
To mimic human disease, we had employed a murine pneumonia model to evaluate the immunogenicity and protective effects of potential vaccine candidates. Although in this pilot study individual purified recombinant antigens (OmpK, FKIB, Ompp1) with high antigenicity scores were shown to be highly immunogenic since mice vaccinated with 3 μg of antigens induced very strong specific IgG responses with titers >104 (), the current results suggest that OmpK and FKIP antigens were not synergistic and did not enhance the protective efficacy of Ompp1 in the mouse pneumonia challenge model. Therefore, in future it is worthy to evaluate more antigens from our top priority list.
In summary, this study provides a comprehensive analysis of A. baumannii genomes for vaccine development, and has identified one antigen, Ompp1 that could potentially be formulated in a novel protective vaccine against A. baumannii since pan-drug resistant A. baumannii are possibly to emerge in near future. The strategy described in the current study incorporates the advantages of reverse vaccinology, bioinformatics and immuno-proteomic platform technologies and is easy to perform to identify novel immunogen for multi-component vaccines.
Materials and Method
Ethics statement
All experiments were conducted in accordance with the guidelines of the Laboratory Animal Center of National Health Research Institutes (NHRI). Animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of National Health Research Institutes (Approved protocol No. NHRI-IACUC-101041-A).
Bacterial strains, growth conditions and OMVs preparation
Genomes of 14 A. baumannii strains were analyzed (). A. baumannii strain ATCC17978 was grown in LB broth medium. Initial OD600nm was set to 0.05 and bacteria were incubated at 37°C with shaking (220 rpm) until OD reached 0.4. OMVs were prepared as previously described.Citation8,9 Briefly, cells were centrifuged at 6,000xg for 10 minutes at 4°C and supernatant was filtered using a 0.22 um (low protein binding) PES filter (Millipore). The flow-through was concentrated using 100 kDa MWCO Amicon Ultracentrifugal concentrators (Millipore, BlossomBio, Taiwan). The precipitate containing OMVs was collected by centrifuging at 100,000xg for 1 hour at 4°C and washed 3 times with PBS. The protein concentration of supernatant was measured by spectro-photometry using a NanoDrop Technologies spectrophotometer (Wilmington, DE). The resulting pellet was submitted to proteomic analysis and the supernatant to secretomic analysis.
Proteome and secretome analyses using HPLC-MS/MS
OMVs preparations as described above were resuspended in ice-cold ethanol and centrifuged at 100,000xg for 1 hour at 4°C. The pellet was dried using a SpeedVac at low drying rate for 20 minutes. Dried pellet was resuspended in 50 μL of resuspension buffer (50 mM ammonium bicarbonate, 3 M urea, 5 mM DTT) and 5 μL of freshly prepared 50 mM ammonium bicarbonate containing 250 mM iodoacetamide were added; and the mixture was incubated for 30 minutes at room temperature in the dark. After incubation, 100 μL of 50 mM ammonium bicarbonate were added to reduce the concentration of urea to 1 M. Digestion was performed using 1 μg/μL trypsin overnight at 37°C. Protein digests were analyzed by online capillary LC-MS/MS (Waters SYNAPT™ HDMS™ System) and peptide fingerprints were analyzed using MassLynx 4.0 software and A. baumannii protein database (NCBI).
Bioinformatics tools
The orthologous core genes were determined by using Microbial Genome Database (MBGD) platformCitation34 which allowed us to do comparative analysis of completely sequenced microbial genomes. PSORTb 3.0.2 was performed to predict the sub-cellular localization of the proteins. The phylogenetic relationship of sequenced A. baumannii strains was inferred with a bootstrapped neighbor-joining analysis using the Molecular Evolutionary Genetics Analysis version 6.0 (MEGA6) based on the nucleotide sequences of 6 of the 7 housekeeping genes in the multi-locus sequence typing (MLST) of A. baumannii.Citation35,36
Cloning and expression of the recombinant antigens: OmpK, Ompp1and FKIB
Consensus protein sequences of OmpK, Ompp1, and FKIB were established using BlastP. Corresponding nucleotide sequences were codon optimized for expression in E. coli, and chemically synthesized (GeneArt, Life technology, Taiwan). The DNA fragments were designed to code for a 6xHis tag attached to the C-terminal of the recombinant protein and cloned into a pET-29a expression vector (Vovagen, Darmstadt, Germany) The resulting plasmids were transformed into the expression host strain E. coli BL21 (DE3) cells (GeneMark, Taiwan).Two-liter cultures were grown in Luria-Bertani (LB) broth containing 30 μg/mL of kanamycin to an optical density (OD) of 0.6 at 600 nm. Protein expression was induced using 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 37°C for 3 h. Cells were harvested by centrifugation at 6,000 rpm for 30 minute and cell pellets were frozen and stored at −20°C.
Purification of the recombinant antigens
Recombinant proteins were purified by nickel-chelated immobilized metal affinity chromatography (IMAC). Briefly, each E. coli pellet was suspended in chilled lysis buffer (50 mM Tris-HCl, 500 mM NaCl, pH 9.0) and the cell suspension was disrupted using a French Press (Constant System, Daventry, UK) at 27 Kpsi. The cell lysate was clarified by centrifugation (32,000 rpm at 4ºC, 45 min) to collect insoluble material. The inclusion body was solubilized with buffer containing 6M urea, 300 mM NaCl and 50 mM sodium phosphate, pH 7.2. After centrifugation at 32,000 rpm at 4ºC for 45 min, the supernatant was collected and filtered through a 0.22 μm MILLEX®GP filtration membrane (Millipore Corp., Ireland) and loaded onto a 10 mL IMAC SepharoseTM High Performance resin (GE Healthcare Bio-Sciences Corp., Sweden) equilibrated with column buffer (50 mM Sodium phosphate pH 7.4, 500 mM NaCl, 5 mM imidazole) and washed 5 × 10 mL of column buffer, then eluted with a 250–500 mM linear imidazole gradient in the column buffer. Fractions containing recombinant proteins (OmpK, Ompp1 and FKIB) were analyzed by SDS-PAGE. Single protein band fractions were pooled and dialyzed against phosphate buffer saline (PBS) and the protein concentrations were determined by bicinchoninic acid (BCA) assay (Thermo Scientific, Pierce, USA). Residual endotoxin content was determined using the Limulus amoebocyte lysate (LAL) assay (Associates of Cape Cod, Inc.., Cape Cod, MA). The final recombinant protein products were frozen and stored at −20°C for further studies.
Mouse immunogenicity studies
Groups of C57BL/6 mice (n = 4) were intramuscularly immunized with either 3 or 10 μg of individual antigen (OmpK, FKIB and Ompp1) formulated with CFA/IFA adjuvant, or PBS on day 0, 14 and 21. On day 42, blood samples were collected and tested against each immunogen. IgG antibody titers were determined using antigen-specific enzyme-linked immunosorbent assay (ELISA).
Murine pneumonia challenge model
Vaccination and murine pneumonia challenge model were performed as previously described.Citation11,30,31 Briefly, mice (n = 5/group) were immunized under isoflurane inhalation anesthesia with recombinant antigens (OmpK, FKIB and Ompp1 at 3 × 3 ug or 3 × 10 μg/mouse) at 2-week intervals on days 0, 14, and 28. The initial vaccination was performed subcutaneously (s.c.) with individual recombinant protein emulsified with complete Freund's adjuvant (CFA) (Sigma), whereas for the next 2 immunizations, immunogens were formulated with incomplete Freund's adjuvant (IFA) (Sigma). Mice in the control group received adjuvant in PBS (n = 5). Blood samples were collected before injections on day 0 and 40 for ELISA to detect antigen-specific immune responses. All the mice were challenged intra-tracheally (IT) on day 42 with a lethal dose (5 × 107 cfu) of freshly grown ATCC 17978 strain, and mice were monitored over 7 days.
Antigen-specific ELISA
ELISA plate wells were coated with 100 ng of either OmpK, or FKIB or Ompp1 overnight, and blocked with 5% nonfat dry milk (w/v) in PBS. Mouse antisera were 2-fold serially diluted with PBS containing 1% BSA (Calbiochem, Darmstadt, Germany), and added to the wells. After incubation at room temperature (RT) for 2 hours, plates were washed 3 times with PBST (PBS containing 0.05% Tween 20), and anti-IgG isotypes (Invitrogen, Carlsbad, CA.) or anti-IgA (Invitrogen, Carlsbad, CA.) HRP-conjugated IgG (KPL, Gaithersburg, MD) specific antibodies diluted in PBS containing 1% BSA were added to the wells and incubated at RT for 1 hour. After washing with 3 × PBST, the plates were treated with TMB peroxidase substrate (KPL) at room temperature in the dark for 20 min. To determine anti-OmpK, anti-FKlB and anti-Ompp1 antibody titers, OD450nm absorbance was measured using a spectrophotometer.
Statistical analysis
Statistical comparison of survival time per each experimental group was assessed by Kaplan-Meier (Log-Rank) test for dose-response of the survival curve. The difference was considered statistically significant when p-values were less than 0.05 (p < 0.05).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Tables_S1_and_S2.docx
Download MS Word (31.5 KB)Figure_S1.pptx
Download MS Power Point (85.8 KB)Acknowledgements
The authors thank Professor Michel Klein and Miss Lori Chong for their comments on the manuscript.
Funding
This work was supported by the grants from Ministry of Science and Technology (NSC 101-2321-B-400-020-MY2) and National Health Research Institutes, Taiwan, ROC.
References
- Behnia M, Logan S, Fallen L, Catalano P. Nosocomial and ventilator-associated pneumonia in a community hospital intensive care unit: a retrospective review and analysis. BMC Res Notes 2014; 7:7; PMID:24393391
- Boerlin P, Eugster S, Gaschen F, Straub R, Schawalder P. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol 2001; 82:347-59; PMID:11506928; http://dx.doi.org/10.1016/S0378-1135(01)00396-0
- U.S. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. U.S. Centers for Disease Control and Prevention Threat Report.
- Kuo L-CC, Lai C-CC, Liao C-HH, Hsu C-KK, Chang Y-LL, Chang CY, Hsueh PR. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin Microbiol Infect 2007; 13:196-8; PMID:17328733; http://dx.doi.org/10.1111/j.1469-0691.2006.01601.x
- Suetens C, Hopkins S, Kolman J, Högberg LD. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011-2012. Euro Centre Dis Prevent Control 2013:1-5.
- Mishra RP, Oviedo-Orta E, Prachi P, Rappuoli R, Bagnoli F. Vaccines and antibiotic resistance. Curr Opin Microbiol 2012; 15:596-602; PMID:22981392; http://dx.doi.org/10.1016/j.mib.2012.08.002
- Pachón J, McConnell MJ. Considerations for the development of a prophylactic vaccine for Acinetobacter baumannii. Vaccine 2014; 32:2534-6; PMID:24188749; http://dx.doi.org/10.1016/j.vaccine.2013.10.064
- McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, Pachón J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect Immun 2011; 79:518-26; PMID:20974823; http://dx.doi.org/10.1128/IAI.00741-10
- McConnell MJ, Pachón J. Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine 2010; 29:1-5; PMID:21044668; http://dx.doi.org/10.1016/j.vaccine.2010.10.052
- Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, Kim SA, Lee SK, Lee JC. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol 2005; 7:1127-38; PMID:16008580; http://dx.doi.org/10.1111/j.1462-5822.2005.00538.x
- Bentancor LV, Routray A, Bozkurt-Guzel C, Camacho-Peiro A, Pier GB, Maira-Litrán T. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun 2012; 80:3381-8; PMID:22825448; http://dx.doi.org/10.1128/IAI.06096-11
- Fattahian Y, Rasooli I, Mousavi Gargari SL, Rahbar MR, Darvish Alipour Astaneh S, Amani J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb Pathog 2011; 51:402-6; PMID:21946278; http://dx.doi.org/10.1016/j.micpath.2011.09.004
- Bentancor LV, O’Malley JM, Bozkurt-Guzel C, Pier GB, Maira-Litrán T. Poly-N-acetyl-β-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun 2012; 80:651-6; PMID:22104104; http://dx.doi.org/10.1128/IAI.05653-11
- Russo TA, Beanan JM, Olson R, MacDonald U, Cox AD, St Michael F, Vinogradov EV, Spellberg B, Luke-Marshall NR, Campagnari AA. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun 2013; 81:915-22; PMID:23297385; http://dx.doi.org/10.1128/IAI.01184-12
- Bambini S, Rappuoli R. The use of genomics in microbial vaccine development. Drug Dis Today 2009; 14:252-60; PMID:19150507; http://dx.doi.org/10.1016/j.drudis.2008.12.007
- Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity 2010; 33:530-41; PMID:21029963; http://dx.doi.org/10.1016/j.immuni.2010.09.017
- Moriel DG, Beatson SA, Wurpel DJ, Lipman J, Nimmo GR, Paterson DL, Schembri MA. Identification of novel vaccine candidates against multidrug-resistant Acinetobacter baumannii. PloS One 2013; 8:e77631; PMID:24116234; http://dx.doi.org/10.1371/journal.pone.0077631
- Kwon S-OO, Gho YS, Lee JC, Kim SI. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett 2009; 297:150-6; PMID:19548894; http://dx.doi.org/10.1111/j.1574-6968.2009.01669.x
- Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, Lee YC. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PloS One 2013; 8:e71751; PMID:23977136; http://dx.doi.org/10.1371/journal.pone.0071751
- Karlsen C, Espelid S, Willassen N-PP, Paulsen SM. Identification and cloning of immunogenic Aliivibrio salmonicida Pal-like protein present in profiled outer membrane and secreted subproteome. Dis Aquat Organ 2011; 93:215-23; PMID:21516974; http://dx.doi.org/10.3354/dao02302
- King LB, Pangburn MK, McDaniel LS. Serine protease PKF of Acinetobacter baumannii results in serum resistance and suppression of biofilm formation. J Infect Dis 2013; 207:1128-34; PMID:23303803; http://dx.doi.org/10.1093/infdis/jis939
- Mendez JA, Soares NC, Mateos J, Gayoso C, Rumbo C, Aranda J, Tomas M, Bou G. Extracellular proteome of a highly invasive multidrug-resistant clinical strain of Acinetobacter baumannii. J Proteome Res 2012; 11:5678-94; PMID:22966805
- Moganty R, Vashistt J, Tiwari V, Kapil A. Differential expression of Outer membrane proteins in early stages of meropenem-resistance in Acinetobacter baumannii. J Integrat OMICS 2011; 1(2):281-287; http://dx.doi.org/10.5584/jiomics.v1i2.67
- Ponomarenko EA, Lisitsa AV, Petrak J, Moshkovskii SA, Archakov AI. Identification of differentially expressed proteins using automated meta-analysis of proteomic articles. Biomed Khim 2009; 3:10-16; PMID:19351029; http://dx.doi.org/10.1134/S1990750809010028
- Siroy A, Cosette P, Seyer D, Lemaître-Guillier C, Vallenet D, Van Dorsselaer A, Boyer-Mariotte S, Jouenne T, Dé E. Global comparison of the membrane subproteomes between a multidrug-resistant Acinetobacter baumannii strain and a reference strain. J Proteome Res 2006; 5:3385-98; PMID:17137340; http://dx.doi.org/10.1021/pr060372s
- Vallenet D, Nordmann P, Barbe V, Poirel L, Mangenot S, Bataille E, Dossat C, Gas S, Kreimeyer A, Lenoble P, et al. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PloS One 2008; 3:e1805; PMID:18350144; http://dx.doi.org/10.1371/journal.pone.0001805
- Vashist J, Tiwari V, Kapil A, Rajeswari MR. Quantitative profiling and identification of outer membrane proteins of beta-lactam resistant strain of Acinetobacter baumannii. J Proteome Res 2010; 9:1121-8; PMID:20041708; http://dx.doi.org/10.1021/pr9011188
- Yun S-HH, Choi C-WW, Kwon S-OO, Park GW, Cho K, Kwon KH, Kim JY, Yoo JS, Lee JC, Choi JS, et al. Quantitative proteomic analysis of cell wall and plasma membrane fractions from multidrug-resistant Acinetobacter baumannii. J Proteome Res 2011; 10:459-69; PMID:21053951; http://dx.doi.org/10.1021/pr101012s
- Li N, Yang Z, Bai J, Fu X, Liu L, Shi C, Wu S. A shared antigen among Vibrio species: outer membrane protein-OmpK as a versatile Vibriosis vaccine candidate in Orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immun 2010; 28:952-6; PMID:20170736; http://dx.doi.org/10.1016/j.fsi.2010.02.010
- Kuolee R, Harris G, Yan H, Xu HH, Conlan WJ, Patel GB, Chen W. Intranasal immunization protects against Acinetobacter baumannii-associated pneumonia in mice. Vaccine 2015; 33(1):260-7; PMID:24699469; http://dx.doi.org/10.1016/j.vaccine.2014.02.083. [Epub ahead of print]
- Harris G, KuoLee R, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother 2013; 57:3601-13; PMID:23689726; http://dx.doi.org/10.1128/AAC.00944-13
- Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PloS One 2012; 7:e29446; PMID:22253723; http://dx.doi.org/10.1371/journal.pone.0029446
- McConnell MJ, Rumbo C, Bou G, Pachón J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 2011; 29:5705-10; PMID:21679737; http://dx.doi.org/10.1016/j.vaccine.2011.06.001
- Uchiyama I, Mihara M, Nishide H, Chiba H. MBGD update 2013: the microbial genome database for exploring the diversity of microbial world. Nucl Acids Res 2013; PMID:23118485; http://dx.doi.org/10.1093/nar/gks1006
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evolut 2013; 30:2725-9; PMID:24132122; http://dx.doi.org/10.1093/molbev/mst197
- Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucl Acids Res 2000; 28:33-6; PMID:10592175; http://dx.doi.org/10.1093/nar/28.1.33
- Farrugia D, Elbourne L, Hassan K, Eijkelkamp B, Tetu S, Brown MH, Shah BS, Peleg AY, Mabbutt BC, Paulsen IT. The complete genome and phenome of a community-acquired Acinetobacter baumannii. PloS One 2013; 8:e58628; PMID:23527001; http://dx.doi.org/10.1371/journal.pone.0058628
- Ningqiu L, Junjie B, Shuqin W, Xiaozhe F, Haihua L, Xing Y, Cunbin S. An outer membrane protein, OmpK, is an effective vaccine candidate for Vibrio harveyi in Orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol 2008; 25(6):829-833; PMID:18854216; http://dx.doi.org/10.1016/j.fsi.2008.09.007
- Tidhar A, Flashner Y, Cohen S, Levi Y, Zauberman A, Gur D, Aftalion M, Elhanany E, Zvi A, Shafferman A, et al. The NlpD lipoprotein is a novel Yersinia pestis virulence factor essential for the development of plague. PloS One 2009; 4:e7023; PMID:19759820; http://dx.doi.org/10.1371/journal.pone.0007023
- Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 2000; 181(4):1401-1413; PMID:10762571; http://dx.doi.org/10.1086/315399
- Mitchison M, Wei L, Kwang J, Wilkie I, Adler B. Overexpression and immunogenicity of the Oma87 outer membrane protein of Pasteurella multocida. Vet Microbiol 2000; 72:91-6; PMID:10699506; http://dx.doi.org/10.1016/S0378-1135(99)00190-X
- Karlsen C, Espelid S, Willassen N-PP, Paulsen SM. Identification and cloning of immunogenic Aliivibrio salmonicida Pal-like protein present in profiled outer membrane and secreted subproteome. Dis Aquat Organ 2011; 93(3):215-223; PMID:21516974; http://dx.doi.org/10.3354/dao02302
- Doyle CR, Pan J-AA, Mena P, Zong W-XX, Thanassi DG. TolC-dependent modulation of host cell death by the Francisella tularensis live vaccine strain. Infect Immun 2014; 82:2068-78; PMID:24614652; http://dx.doi.org/10.1128/IAI.00044-14
- Martínez-Martínez S, Frandoloso R, Rodríguez Ferri EF, Gil C, Hernández-Haro C, Yubero S, Gutiérrez Martín CB. Immunoproteomic analysis of the protective response obtained with subunit and commercial vaccines against Glässer's disease in pigs. Vet Immunol Immunopathol 2013; 151(3-4):235-247; PMID:23266097; http://dx.doi.org/10.1016/j.vetimm.2012.11.014
- Kim YH, Kim KA, Kim Y-RR, Choi MK, Kim HK, Choi KJ, Chun JH, Cha K, Hong KJ, Lee NG, et al. Immunoproteomically identified GBAA_0345, alkyl hydroperoxide reductase subunit C is a potential target for multivalent anthrax vaccine. Proteomics 2014; 14(1):93-104; PMID:24273028; http://dx.doi.org/10.1002/pmic.201200495
- Santos-Gomes GM, Rodrigues A, Teixeira F, Carreira J, Alexandre-Pires G, Carvalho S, Santos-Mateus D, Martins C, Vale-Gato I, Marques C, et al. Immunization with the Leishmania infantum recombinant cyclophilin protein 1 confers partial protection to subsequent parasite infection and generates specific memory T cells. Vaccine 2014; 32:1247-53; PMID:24486368; http://dx.doi.org/10.1016/j.vaccine.2014.01.024
- Yu Q, Huang X, Gong P, Zhang Q, Li J, Zhang G, Yang J, Li H, Wang N, Zhang X. Protective immunity induced by a recombinant BCG vaccine encoding the cyclophilin gene of Toxoplasma gondii. Vaccine 2013; 31:6065-71; PMID:24176493; http://dx.doi.org/10.1016/j.vaccine.2013.10.015
- Ghaffarifar F, Jorjani O, Sharifi Z, Dalimi A, Hassan ZM, Tabatabaie F, Khoshzaban F, Hezarjaribi HZ. Enhancement of immune response induced by DNA vaccine cocktail expressing complete LACK and TSA genes against Leishmania major. APMIS 2013; 121:290-8; PMID:23030526; http://dx.doi.org/10.1111/j.1600-0463.2012.02968.x
- Sangpairoj K, Changklungmoa N, Vanichviriyakit R, Sobhon P, Chaithirayanon K. Analysis of the expression and antioxidant activity of 2-Cys peroxiredoxin protein in Fasciola gigantica. Exp Parasitol 2014; 140:24-32; PMID:24594261; http://dx.doi.org/10.1016/j.exppara.2014.02.017
- Mes TH, van Putten JP. Positively selected codons in immune-exposed loops of the vaccine candidate OMP-P1 of Haemophilus influenzae. J Mol Evol 2007; 64:411-22; PMID:17479342; http://dx.doi.org/10.1007/s00239-006-0021-2
- Yang T-CC, Ma X-CC, Liu F, Lin L-RR, Liu L-LL, Liu GL, Tong ML, Fu ZG, Zhou L. Screening of the Salmonella paratyphi A CMCC 50973 strain outer membrane proteins for the identification of potential vaccine targets. Mol Med Rep 2012; 5:78-83; PMID:21922141
- Sakolvaree Y, Maneewatch S, Jiemsup S, Klaysing B, Tongtawe P, Srimanote P, Saengjaruk P, Banyen S, Tapchaisri P, Chonsa-nguan M, et al. Proteome and immunome of pathogenic Leptospira spp. revealed by 2DE and 2DE-immunoblotting with immune serum. Asian Pac J Allergy Immunol 2007; 25(1):53-73; PMID:17891922
- Gates AE, Frasca S, Nyaoke A, Gorton TS, Silbart LK, Geary SJ. Comparative assessment of a metabolically attenuated Mycoplasma gallisepticum mutant as a live vaccine for the prevention of avian respiratory mycoplasmosis. Vaccine 2008; 26:2010-9; PMID: 18342996; http://dx.doi.org/10.1016/j.vaccine.2008.02.010
- Whiting GC, Rijpkema S, Adams T, Corbel MJ. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine 2004; 22:4245-4251; PMID:15474715; http://dx.doi.org/10.1016/j.vaccine.2004.04.036
- Havlasová J, Hernychová L, Brychta M, Hubálek M, Lenco J, Larsson P, Lundqvist M, Forsman M, Krocová Z, Stulík J, et al. A Proteomic analysis of anti-Francisella tularensis LVS antibody response in murine model of tularemia. Proteomics 2005; 5:2090-103; PMID:15892173; http://dx.doi.org/10.1002/pmic.200401123
- Ali A, Soares SC, Santos AR, Guimarães LC, Barbosa E, Almeida SS, Abreu VA, Carneiro AR, Ramos RT, Bakhtiar SM, et al. Campylobacter fetus subspecies: comparative genomics and prediction of potential virulence targets. Gene 2012; 508(2):145-156; PMID:22890137; http://dx.doi.org/10.1016/j.gene.2012.07.070
- Perez F, Bonomo RA. Vaccine for Acinetobacter baumannii: Thinking "out of the box". Vaccine 2014; 32(22):2537-9; PMID:24662709; http://dx.doi.org/10.1016/j.vaccine.2014.03.031
- Umland TC, Schultz LW, MacDonald U, Beanan JM, Olson R, Russo TA. In vivo-validated essential genes identified in Acinetobacter baumannii by using human ascites overlap poorly with essential genes detected on laboratory media. mBio 2012; 3(4):e00113-12; PMID: 22911967; http://dx.doi.org/10.1128/mBio.00113-12
