Abstract
Few epidemiological data are available after the introduction of the 13-valent pneumococcal vaccine (PCV13) in 2010. We performed repeat nasopharyngeal swabs and evaluated the serotype distribution of Streptococcus pneumoniae (SP) and its association with PCV13 vaccine status in healthy Italian children aged 3–59 months. SP serotypes were assessed by the Quellung reaction. 618 children appropriately (28%) or incompletely (72%) vaccinated for age with PCV13 were available at baseline (T0). 515 were re-evaluated at 6 months from baseline (T6) and 436 at 12 months from baseline (T12). The percentage of appropriately vaccinated subjects at T0, T6 and T12 was 28%, 67% and 92%, respectively. Random effects logistic regression models with robust 95% confidence intervals was used to estimate the time-related changes in SP and PCV13 carriage and marginal probabilities were obtained from such models. The age-corrected probability of SP carriage was 0.31 (95% CI 0.22 - 0.41) at T0, 0.32 (0.24 - 0.40) at T6 and 0.28 (0.20 - 0.35) at T12. The probability of PCV13 serotypes carriage was 0.025 (0.001 - 0.050) at T0, 0.018 (0.001 - 0.039) at T6 and 0.010 (0.001 - 0.023) at T12. A decrease in PCV13 serotypes and a shift in non-PCV13 serotypes colonization was observed. In particular, the 15A serotype accounted for 4%, 8% and 23% of SP isolates at T0, T6 and T12, respectively. In conclusion, the benefits of the PCV13 vaccination on SP carriage increase with increasing coverage rates. The shift of SP isolates toward non-PCV13 serotypes needs to be studied further.
Introduction
Streptococcus pneumonaie (SP) colonizes the nasopharyngeal niche as a part of the commensal flora of the upper respiratory tract. Nasopharyngeal carriage leads the SP spreading within the community and, in some cases, is followed by disease.Citation1 Nasopharyngeal isolates may represent an indicator of invasive disease and potential vaccine coverage. However, certain serotypes and genotypes seem to cause higher rates of invasive disease when corrected for prevalence of nasopharyngeal colonization.Citation1 Therefore, continuous surveillance of invasive pneumococcal diseases (IPDs) and colonization isolates is warranted following the large-scale introduction of pneumococcal vaccination.
The introduction of pneumococcal conjugate vaccines has opened a new era in the prevention of pneumococcal diseases. The pneumococcal 7-valent conjugate vaccine (PCV7), introduced over a decade ago, was highly effective in reducing the incidence of vaccine-type (VT) invasive pneumococcal diseases (IPDs) and nasopharyngeal carriage in countries where it has been included in national immunization programs.Citation2-5
PCV7 has a remarkable herd effect as its impact is seen not only in vaccinated children but also in unvaccinated persons of all ages.Citation6 The introduction of PCV7 caused a shift toward IPDs associated with non-PCV7 serotypesCitation7 and several studies have shown an increase of the prevalence of nasopharyngeal carriage due to non-PCV7 serotypes.Citation8,9 On the basis of such epidemiological findings, a new 13-valent pneumococcal conjugate vaccine (PCV13), including the PCV7 serotypes and 6 additional serotypes (1, 3, 5, 6A, 7F, and 19A), was developed. While concerns are rising about the selective pressure of PCV13, the first epidemiological data on IPD in the PCV13 era are reassuring. A reduction in IPDs, and especially IPDs attributable to PCV13 serotypes, was observed after the introduction of PCV13 in infant immunization schedules in the USA,Citation10,11 Canada,Citation12 UK,Citation13 France,Citation14 Spain,Citation15 GreeceCitation16 and Germany.Citation17 The introduction of PCV13 was associated with a decreased incidence of acute otitis media, community-acquired pneumonia, pneumococcal pneumonia and pleural effusions, especially when PCV13 serotypes were involved.Citation18,19 Carriage studies have likewise shown that the introduction of PCV13 was associated with a reduction in the carriage of PCV13 serotypes, especially in children < 5 yCitation20–27 In the Pneumococcal carriage Milan Study (PNEUmi study) we have recently shown a decrease in nasopharyngeal colonization of PCV13 serotypes and also a shift toward non-PCV13 serotypes in healthy children aged 3–59 months following the introduction of PCV13 in routine practice.Citation28 In the present paper, we report the follow-up of such children, sampled at 6 and 12 months after the enrollment. Our aim is to describe the change in the serotype distribution of SP nasopharyngeal strains and its association with PCV13 vaccination status.
Results and Discussion
Study population
Six hundred and 18 children appropriately (n = 171, 28%) or incompletely (n = 447, 72%) vaccinated for age with PCV13 were included at baseline (T0).Citation28 Five hundred and 15 (83%) children were re-evaluated at 6 months from baseline (T6) and 436 (70%) at 12 months from baseline (T12). The reasons for loss to follow-up at T6 and T12 were: refusal of consent (36%), unavailable at repeat phone calls (28%), change of residence (18%), unavailability to schedule an appointment (15%), and acute disease (3%). reports the main characteristics of the children at T0, T6 and T12 visits. The number of children appropriately vaccinated for age increased from 28% at T0, to 67% at T6, and to 92% at T12.
Table 1. Main characteristics of the study children
Frequency of SP nasopharyngeal carriage
As compared to the baseline visit, the age-unadjusted odds ratio (OR) for SP carriage was 1.23 (95% CI 0.91 to 1.66, p = 0.18) at T6 and 1.30 (0.96 to 1.78, p = 0.09) at T12 (random effects logistic regression). The corresponding marginal probabilities of SP carriage were 0.25 (95%CI 0.19 to 0.32) at T0, 0.31 (0.24 to 0.39) at T6 and 0.33 (0.25 to 0.42) at T12. Baseline age was directly associated with SP carriage (OR = 1.48, 95%CI 1.12 to 1.97, p = 0.007 for every 12-month increase) (random effects logistic regression). The age-adjusted ORs for SP carriage at T6 and T12 were 1.00 (0.73 to 1.39, p = 0.96) and 0.88 (0.58 to 1.33, p = 0.54), respectively. The corresponding age-corrected probabilities of SP carriage were 0.31 (95%CI 0.22 to 0.41) at T0, 0.32 (0.24 to 0.40) at T6 and 0.28 (0.20 to 0.35) at T12. It should be noted that these estimates were obtained at random effects logistic regression and differ from the crude estimates reported in which do not take into account repeated measures and missing data.
Distribution of SP serotypes.
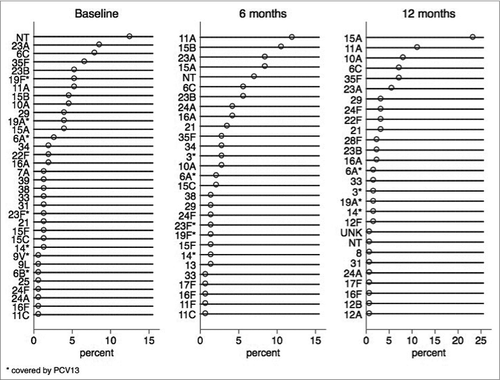
and report the frequency of isolation of SP serotypes at T0 and follow-up visits. Two nasopharyngeal isolates identified as SP died during the passage on plate and therefore the serotype remain unknown. The most frequently isolated SP serotypes were 23A (8.6%), 6C (7.9%) and 35F (6.6%) at T0; 11A (12%), 15B (10.6%), 23A (8.5%) at T6; and 15A (23.4%), 11A (11.3%) and 10A (8.1%) at T12. Serotype 19A accounted for 4.0%, 0.0% and 1.6% of isolates at T0, T6 and T12. Serotype 19F accounted for 5.3%, 1.1% and 0.0% of isolates at T0, T6 and T12. Among the non-PCV13 serotypes, 15A increased during the study, accounting for 4.0%, 8.5% and 23.4% of them at T0, T6 and T12.
Table 2. Frequency of Streptococcus pneumoniae serotype isolation
Frequency of PCV13 nasopharyngeal carriage.
As compared to T0, the odds ratio (OR) for PCV13 carriage was 0.71 (95%CI 0.34 to 1.50, p = 0.37) at T6 and 0.41 (0.17 to 1.05, p = 0.05) at T12 (random effects logistic regression). The corresponding marginal probabilities of PCV13 carriage were 0.025 (0.001 to 0.050) at T0, 0.018 (0.001 to 0.039) at T6 and 0.010 (0.001 to 0.023) at T12. Although these estimates are imprecise because of the low number of positive outcomes (n = 18 at T0, n = 13 at T6 and n = 6 at T12), they go in the expected decreasing direction. It should be noted that these estimates were obtained at random effects logistic regression and differ from the crude estimates reported in , which do not take into account repeated measures and missing data.
The PNEUmi study showed a lower prevalence of nasopharyngeal PCV13 carriage in appropriately vaccinated for age healthy children aged < 5 yCitation27 In the present one-year follow-up of the PNEUmi cohort, being appropriately vaccinated for age with PCV13 was associated with a reduced probability of nasopharyngeal SP colonization, especially with PCV13 serotypes.
The impact of the PCV13 vaccine on SP nasopharyngeal colonization is presently under debate. Some studies reported a stable pneumococcal colonization after the introduction of PCV13 and suggested that PCV13 immunization does not lead to microbial niches for which other pathogens could compete.Citation25–27 On the contrary, a recent Canadian surveillance study showed a reduction of colonization with any SP after the introduction of PCV13.Citation29 The effect of PCV13 on the carriage of PCV13 serotypes is however more clear. The majority of carriage studies conducted in Europe and USA showed that PCV13 vaccination reduces the carriage of PCV13 serotypes in children < 5 years, in keeping with the results of the present study.Citation21–23,25–27 Because a decreased SP carriage may be predictive of direct protection in vaccinated and not vaccinated individuals (herd immunity), large scale PCV13 immunization may have important implications for the control of IPD.Citation30
Interestingly during the study we observed a trend for decrease in 23A and 23B colonization. This could probably due to the effect on PCV13 on these serotypes, considering that a genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci has been reported for serotypes 23F (included in PCV13), 23A, and 23B. However further study are needed before any definitive conclusion.Citation31
Our study also highlights the importance of receiving an adequate number of PCV13 doses to obtain a protective immune response against SP colonization and vaccine serotypes. We observed that the benefits of vaccination on SP carriage are maximal after completion of the PCV13 vaccine schedule. Recently, a 2-year carriage surveillance study of US children aged < 60 months showed a reduction of PCV13 colonization in immune children (defined as having received a recommended number of PCV13 immunizations) as compared to non-immune children.Citation24 In the US study, the difference in PCV13 serotype colonization between immune and non-immune children disappeared when the community uptake of PCV13 reached 75%. In our population, we were not able to detect this effect even if PCV13 uptake in the study area remained high during the study (80%, personal communication from Regional Health Authorities). A possible reason for this discrepancy may be that the community uptake was estimated in the US study while we obtained it directly from the regional immunization records. Larger population studies are needed to test whether PCV13-induced protection against PCV13 serotypes is associated with herd immunity.
On the other hand, we observed a shift in the SP serotype composition from a mix of PCV13 and non- PCV13 serotypes in incompletely vaccinated children to almost only non-PCV13 serotypes in children appropriately vaccinated for age. Among the non-PCV13 serotypes, we found a substantial increase of 15A from 4.0% at baseline to 23.4% at 12 months. Importantly, the 15A serotype has been associated with SP outbreaks, acute otitis media and IPD since PCV7 introduction.Citation29,32–35 In Lombardy, the 15A serotype was associated with 0.5% of IPDs in the PCV7 era (2007–2010) and with 1.6% of IPDs 3 y after the introduction of PCV13 (personal communication from Regional Health Authorities). Further studies are needed to evaluate the impact of serotype 15A both on carriage and IPDs.
The limitations of PNEUmi study have been discussed in detail elsewhere.Citation28 Such limitations are the choice of a convenience sample of children, the relatively wide age range, and the use of the single-colony method for serotyping.Citation28 The main limitations pertaining to the present longitudinal analysis is the presence of missing data and the low number of events available for some analysis. We analyzed these data with random-effect logistic regression models under the MAR assumption. This is certainly better than restricting the analysis to the non-missing data but will increase the variance of the estimates, especially when few events are available. It should be noted, however, that the unavailability rate was lower than generally reported for similar studies, i.e. Seventeen% at T6 and 30% at T12.
In conclusion, in the PNEUmi cohort study, we observed a decrease in PCV13 nasopharyngeal colonization. The benefits of vaccination on carriage are maximized after completing the vaccine series. Moreover our data documented a shift in non-PCV13 serotypes in children appropriately vaccinated for age with PCV13.
Materials and Methods
Study design.
We performed a longitudinal analysis of the PNEUmi cohort to assess the serotype distribution of nasopharyngeal SP carriage isolates in PCV13-vaccinated healthy children aged 3 to 59 months living in the Milan metropolitan area (Milan, Lombardy, Italy). The study design is described in detail elsewhere.Citation28 Briefly, a convenience sample of children aged 3–59 months was recruited from 16 primary care pediatricians working for the Italian National Health System. Reasons for exclusion from the study were: 1) malformation or trauma of the nasopharynx, 2) fever, 3) acute respiratory tract infection, 4) immunological disease, 5) cancer, 6) renal disease, 7) cardiac disease, 8) blood disease, 9) cystic fibrosis, 10) bronchopulmonary dysplasia, and 10) Down syndrome. In the presence of siblings, only one child per family was recruited. The longitudinal study was performed between September 2011 and December 2012. Nasopharyngeal swabs were collected at T0 (baseline, between September and December 2011), at T6 (6 months ± 5 d from baseline), and at T12 (12 months ± 5 d from baseline, between September and December 2012). Children with acute respiratory infections or fever at T6 and T12 had nasopharyngeal swabs taken after disease resolution. The study protocol was approved by the Ethical Committee of Luigi Sacco Hospital (Milan, Italy). Written consent to participate was obtained from the parents or the legal guardians of the children.
Data collection.
Gender and ethnic group were recorded at T0. Time-varying data collected at each visit were: day-care attendance, number of siblings, passive smoking, respiratory infections in the preceding 3 months, antimicrobial use in the previous 3 months, antimicrobial use in the previous 7 d The vaccination status of the children during the study was obtained from the local Public Health authorities. Following the Italian Immunization schedule, the children were considered appropriately vaccinated for age if they had received the recommended (2 + 1), or a catch-up dose at more than one year of age.Citation28 Children were considered incompletely vaccinated for age if they had not completed their age-specific schedule.
Nasopharyngeal swabs.
Nasopharyngeal swabs were collected from children by trained personnel with a nylon flocked flexible sterile Copan Eswab immersed in liquid Amies Transport Medium, following WHO recommendations.Citation36 Specimens were sent to the Regional Reference Laboratory for IPD within 3 hours and processed immediately or stored at 4–8°C and analyzed within 48 hours.
SP identification and serotyping.
Nasopharyngeal swabs were plated on Columbia horse blood agar and on Columbia horse blood agar containing colistin and nalidixic acid. Agar plates were incubated overnight at 35°C in air with 5% CO2. The α-hemolytic suspected hemolytic pneumococcal colonies were plated on Columbia horse blood agar with an optochin disk and incubated overnight at 35°C in air with 5% CO2. On the following day, the pneumococcal colonies were identified by means Gram staining, optochin sensitivity and bile solubility testing. SP serogrouping was performed using latex agglutination (Pneumotest Latex Kit, Statens Serum Institut, Copenhagen, Denmark). The employed kit contains latex particles coated with rabbit antibodies reacting with specific pneumococcal capsular polysaccharide. The identification of pneumococcal serogroups employs a checkerboard system with agglutination in 14 pool suspensions. SP serotypes were determined by the capsular reaction test (Quellung reaction) using specific antisera (Statens Serum Institut, Copenhagen, Denmark). A suspension of the organism was prepared in 0.9% saline solution from well isolated colonies grown on sheep blood agar plates for 18 to 24 h in 5% CO2 at 35°C. One drop of this suspension was mixed with 1 drop of antiserum and, after incubation at room temperature for 10 min, it was examined at 400X magnification at optical microscope. Evidence of capsular swelling with specific antisera and positive Quellung reaction were diagnostic criteria.
Statistical analysis
Categorical variables are reported as counts and percentages. Random effects logistic regression models with robust 95% confidence intervals were used to estimate the time-related changes in SP carriage (0 = no; 1 = yes) and PCV13 carriage (0 = no; 1 = yes).Citation37,38
Such models included discrete time [0 = baseline visit (T0); 1 = 6 month follow-up (T6); 2 = 12 month follow-up (T12)] as predictor and the child as random effect. Point estimates and robust 95% confidence intervals of the frequency of SP carriage and PCV13 carriage during the study were obtained by calculating marginal effects at 0, 6 and 12 months.Citation39,40 For the SP carriage outcome, we evaluated also a random effects logistic regression model including baseline age (continuous, months/12) as predictor together with discrete time. This was done in order to control for the potentially confounding effect of baseline age on the frequency of SP carriage during the study. To test for non-linear associations of baseline age with SP carriage, baseline age was evaluated both as linear and quadratic. As there was no improvement in model fit after the quadratic transformation, baseline age was kept linear in the final model. The point estimates and robust 95% confidence intervals of the frequency of SP carriage were obtained by calculating marginal effects at 0, 6 and 12 months with baseline age fixed at its mean.Citation39,40 The number of events was too low to fit an age-corrected model for the PCV13 outcome. Missing data were handled under the assumption that they were missed at random (MAR) as random effects logistic regression is robust to such assumption.Citation37
Citation35Statistical analysis was performed using Stata version 13.1 (Stata Corp., College Station, TX, US).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Prof Zuccotti and Dr Mameli had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Zuccotti, Mameli, Torresani. Acquisition of data: Mameli, Daprai, Fabiano, Penagini. Analysis and interpretation of data: Zuccotti, Mameli, Bedogni, Torresani, Daprai Garlaschi. Drafting of the manuscript: Zuccotti, Mameli, Bedogni, Daprai. Critical revision of the manuscript for important intellectual content: Zuccotti, Gramegna, Faccini, Torresani. Statistical analysis: Bedogni Study supervision: Zuccotti, Mameli, Torresani, Dilillo. All authors read and approved the final manuscript.
Note
PNEUmi Study Group (PMSG): Emanuela Ballerini, Benincaso, Milena Bonvissuto, Dorella Bricalli, Manuela Brioschi, Cinzia Simona Calloni, Marina Irene Camiletti, Giacomo Colella, Laura De Angelis, Silvia Decarlis, Francesca Di Nello, Massimiliano Dozzi, Erica Galli, Vera Gandini, Maria Grazia Giuliani, Franca Laviola, Barbara Loda, Maddalena Macedoni, Elisabetta Mazzucchi, Maria Gabriella Metta, Anna Moscatiello, Pilar Nannini, Mariangela Petruzzi, Damiano Picicco, Michela Picciotti, Stefania Pisanelli, Norberto Porta, Giulia Ramponi, Francesca Redaelli, Riccardo Rubini, Natascia Sala, Vincenzo Saitta, Rosa Maria Tiso, Mariangela Tomasetto, Matteo Torcoletti, Marta Travaini, Maurizio Valentini.
Funding
PNEUmi was supported by a research grant by the Italian Ministry of Health (“Ricerca finalizzata 2009”) and by an unrestricted grant from Pfizer Italia s.r.l. The sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med 2010; 16:217-25; PMID:20375783
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennet MN, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- Rodenburg GD, Greeff SC de, Jansen AGCS, Melker HE de, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 2010; 16:816-823.
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Active bacterial core surveillance of the emerging infections program network: decline in invasive pneumococcal disease after the introduction of protein polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737-46; PMID:12724479; http://dx.doi.org/10.1056/NEJMoa022823
- Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramsom O, Mendelman PM, Bohidar N, Yagupsky P. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis 1996; 174:1271-1278; PMID:8940218; http://dx.doi.org/10.1093/infdis/174.6.1271
- Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev Vaccines 2009; 8:1351-64; PMID:19803758; http://dx.doi.org/10.1586/erv.09.78
- Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellembrg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007; 26:468-472; PMID:17529860; http://dx.doi.org/10.1097/INF.0b013e31803df9ca
- Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009; 124:e1-11; PMID:19564254; http://dx.doi.org/10.1542/peds.2008-3099
- Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol 2010; 17:325-334; PMID:20107006; http://dx.doi.org/10.1128/CVI.00435-09
- Kaplan SL, Barson WJ,, Lin PL,, Romero JR,, Bradley JS,, Tan TQ,, Hoffman JA,, Givner LB,, Mason EO, Jr. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2013; 32:203-207; PMID:23558320; http://dx.doi.org/10.1097/INF.0b013e318275614b
- Moore M, Link-Gelles R, Farley MM, Schaffner W, Thomas A, Reingold A, Harrison L, Lexau C, Zansky S, Petit S, et al. Impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease, U.S, 2010-11. [ID Week 2012, San Diego, CA, October 17-21, 2012]. 2012. Available at: https://idsa.confex.com/idsa/2012/webprogram/Paper36569.html. Accessed at 15 September 2014.
- Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, Tyrrell GJ, Desai S, Sherrard L, Adam H, et al. Toronto Bacterial Diseases Network; Canadian Public Health Laboratory Network. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010-2012. Can J Microbiol. 2013;59:778-788; PMID:24313450; http://dx.doi.org/10.1139/cjm-2013-0614
- Moore CE, Paul J, Foster D, Mahar SA, Griffiths D, Knox K, Peto TE, Walker AS, Crook DW, on behalf of the Oxford Invasive Pneumococcal Surveillance Group. Reduction of invasive pneumococcal disease three years after the introduction of the 13 valent conjugate vaccine in the Oxfordshire region, England. J Infect Dis 2014; 210:1001-1011; PMID:24719477; http://dx.doi.org/10.1093/infdis/jiu213
- Levy C, Varon E, Picard C, Béchet S, Martinot A, Bonacorsi S, Cohen R. Trends of pneumococcal meningitis in children after introduction of the 13-Valent pneumococcal conjugate vaccine in France. PediatrInfect Dis J 2014; 33:1216-21; PMID:25037044; http://dx.doi.org/10.1097/INF.0000000000000451
- Guevara M, Ezpeleta C, Gil-Setas A, Torroba L, Beristain X, Aguinaga A, García-Irure JJ, Navascués A, García-Cenoz M, Castilla J, Working Group for Surveillance of the Pneumococcal Disease in Navarre. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001-2013. Vaccine 2014; 32:2553-2562; PMID:24674661; http://dx.doi.org/10.1016/j.vaccine.2014.03.054
- Georgakopoulou T, Menegas D, Tzanakaki G, Pipa E, Vernardaki A, Mavraganis P, Theodoridou M, Kremastinou J. Epidemiology of Bacterial Meningitis in Greece, in the Era of Conjugate Vaccines: A 7 Years Review 2005-2011. 52nd ICAAC, San Francisco, CA, USA, September 9-12 2012. Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=d201a978-86b7-4126-b4ebdce82709451f&cKey=fd9b920f-2dad-4c23-9dba-3f0f20b33da8&mKey=%7b6B114A1D-85A4-4054-A83B-04D8B9B8749F%7d. Accessed June 13, 2014.
- van der Linden M, von Kries R, Imohl M. Effects of three years of immunization with higher valent pneumococcal conjugate vaccines on serotype distributions among reported IPD cases in German children and adults. Presented at 52nd ICAAC, September 9-12, 2012, San Francisco. September 10, 2012. Available at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=becf1b8c-545a-4792-ac02-4a9cb31fdf7b&cKey=bb809851-e003-4b87-8957-043e6ed60add&mKey=%7b6B114A1D-85A4-4054-A83B-04D8B9B8749F%7d. Accessed June 8, 2014.
- Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, at al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis 2014; 58:918-924; PMID:24532543; http://dx.doi.org/10.1093/cid/ciu006
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near elimination of otitis media caused by the PCV13 serotypes in Southern Israel shortly after sequential introduction of PCV7/PCV13. Clin Infect Dis 2014; 59:1724-32; PMID:25159581; http://dx.doi.org/10.1093/cid/ciu683
- Azzari C, Martinón-Torres F, Schmitt HJ, Dagan R. Evolving role of 13-Valent pneumococcal conjugate vaccine in clinical practice. Pediatr Infect Dis J 2014; 33:858-864; PMID:24618937; http://dx.doi.org/10.1097/INF.0000000000000328
- Camilli R, Daprai L, Cavrini F, Lombardo D, D'Ambrosio F, Del Grosso M, Vescio MF, Landini MP, Pascucci MG, Torresani E, et al. Pneumococcal carriage in young children one year after introduction of the 13-valent conjugate vaccine in Italy. PLoS One 2013;8:e76309; PMID:24124543; http://dx.doi.org/10.1371/journal.pone.0076309
- Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013; 57:952-62; PMID:23804191; http://dx.doi.org/10.1093/cid/cit428
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J 2012; 31:297-301; PMID:22330166; http://dx.doi.org/10.1097/INF.0b013e318247ef84
- Loughlin AM, Hsu K, Silverio AL, Marchant CD, Pelton SI. Direct and indirect effects of PCV13 on nasopharyngeal carriage of PCV13 unique pneumococcal serotypes in massachusetts' children. Pediatr Infect Dis J 2014; 33:504-510; PMID:24670957; http://dx.doi.org/10.1097/INF.0000000000000279
- van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014; 32:4349-4355; PMID:24657717; http://dx.doi.org/10.1016/j.vaccine.2014.03.017
- Lee GM, Kleinman K, Pelton SI, Hanage W, Huang SS, Lakoma M, Dutta-Linn M, Croucher NJ, Stevenson A, Finkelstein JA. Impact of 13-valent pneumococcal conjugate caccination on streptococcus pneumoniae carriage in young children in Massachusetts. J Pediatric Infect Dis Soc 2014; 3:23-32; PMID:24567842; http://dx.doi.org/10.1093/jpids/pit057
- Gounder PP, Bruce MG, Bruden DJ, Singleton RJ, Rudolph K, Hurlburt DA,, Hennessy TW, Wenger J. Effect of the 13-Valent pneumococcal conjugate vaccine on nasopharyngeal colonization by streptococcus pneumoniae-Alaska, 2008-2012. J Infect Dis 2014; 209:1251-1258; PMID:24273178; http://dx.doi.org/10.1093/infdis/jit642
- Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, Faccini M, Gramegna M, Torresani E; PneuMi Study Group (PMSG), et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 2014; 32:527-534; PMID:24342249; http://dx.doi.org/10.1016/j.vaccine.2013.12.003
- Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, Demczuk WH, Kellner JD; Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) investigators. Trends in asymptomatic nasopharyngeal colonization with streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J 2014; 33:724-30; PMID:24463806; http://dx.doi.org/10.1097/INF.0000000000000267
- Bogaert D, de Groot R, Hermans PW. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis 2004; 4:144-54; PMID:14998500; http://dx.doi.org/10.1016/S1473-3099(04)00938-7
- Mavroidi A, Aanensen DM, Godoy D, Skovsted IC,, Kaltoft MS, Reeves PR, Bentley SD, Spratt BG. Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci. J Bacteriol 2007; 189:7841-55; PMID:17766424; http://dx.doi.org/10.1128/JB.00836-07
- Steens A, Bergsaker MA, Aaberge IS, Rønning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013; 31:6232-6238; PMID:24176490; http://dx.doi.org/10.1016/j.vaccine.2013.10.032
- Fleming-Dutra K, Mbaeyi C, Link-Gelles R, Alexander N, Guh A, Forbes E, Beall B, Winchell JM, Carvalho Mda G, Pimenta F, Kodani M, et al. Streptococcus pneumoniae serotype 15A in psychiatric unit, Rhode Island, USA, 2010-2011. Emerg Infect Dis 2012; 18:1889-93; PMID:23092658; http://dx.doi.org/10.3201/eid1811.120454
- Parra EL, De La Hoz F, Díaz PL, Sanabria O, Realpe ME, Moreno J. Changes in Streptococcus pneumoniae serotype distribution in invasive disease and nasopharyngeal carriage after the heptavalent pneumococcal conjugate vaccine introduction in Bogotá, Colombia. Vaccine 2013; 31:4033-4038; PMID:23680440; http://dx.doi.org/10.1016/j.vaccine.2013.04.074
- Martin JM, Hoberman A, Paradise JL, Barbadora KA, Shaikh N, Bhatnagar S, Shope T, Block SL, Haralam MA, et al. Emergence of streptococcus pneumoniae serogroups 15 and 35 in nasopharyngeal cultures from young children with Acute Otitis Media. Pediatr Infect Dis J 2014; 33:e286-90; PMID:24911895
- O’Brien KL, Nohynek H, World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J 2003; 22:133-40; PMID:12586987
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, 3rd Edition. College Station: Stata Press; 2012.
- Skrondal A, Rabe-Hesketh S. Generalized latent variable modeling: multilevel, longitudinal, and structural equation models. Boca Raton: Chapman & Hall; 2004.
- Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 2012; 12:308-331.
- Cummings P. Methods for estimating adjusted risk ratios. Stata J 2009; 9:175-96.